Abstract
The water-soluble plant polyphenols rutin, (+)-catechin, and (−)-epigallocatechin gallate were each found to be efficient quenchers for the triplet-excited state of riboflavin in aqueous solution using nano-second laser flash photolysis. The deactivation followed second-order kinetics with rates close to the diffusion control: (−)-epigallocatechin gallate (1.70±0.2×109 l mol−1 s−1), (+)-catechin (1.44±0.04×109 l mol−1 s−1), and rutin (9.7±0.2×108 l mol−1 s−1) at 25 °C and pH = 6.8. No synergetic effects for mixtures of the phenolic compounds were found. Hydrogen atom transfer is calculated to be more exergonic than electron transfer, suggesting the hydrogen atom transfer mechanism to be operative. The efficient deactivation by the plant polyphenols makes this type of compounds of importance as protectors against light-induced oxidation in flavored milk-based beverages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to light is known to result in development of off-flavors in dairy products [1]. Riboflavin (Vitamin B2) present in milk is an efficient photosensitiser, which upon absorption of visible light forms excited-singlet states, which by intersystem crossing yields the very reactive triplet-excited state [2]. In milk and cheeses, the triplet riboflavin may oxidise proteins forming low-molecular weight sulphur compounds like dimethyldisulfide resulting in a “burnt-feather” off-flavour of the product [1, 3]. Water-soluble antioxidants such as uric acid present in milk have been shown experimentally and by ab initio calculations to quench triplet riboflavin by electron-transfer rather than by the hydrogen-atom transfer mechanism in competition with protein oxidation [4].
New types of milk-based beverages are being introduced in the market with natural flavourings based on chocolate, various herbs and fruits. Such products will contain plant polyphenols like catechin from chocolate, rutin from fruits and (−)-epigallocatechin gallate (EGCG) from tea, which are known as antioxidants in lipid oxidation [5, 6]. Such compounds could also be effective as quenchers of triplet riboflavin and protect milk whey proteins against degradation and consequently against formation of off-flavours. We have accordingly selected three plant polyphenols important in such flavourings and studied their reaction with triplet riboflavin alone and in combination. The results presented are based on real-time kinetic methods using nano-second laser flash photolysis combined with transient absorption spectroscopy set up to generate the excited-state of riboflavin and for detection of the reaction intermediates.
Experimental section
Chemicals
Riboflavin, rutin hydrate, (+)-catechin and (−)-(EGCG) of analytical grade were purchased from Sigma-Aldrich (Steinheim, Germany) and used as received. Coumarin 120, spectroscopic grade, was obtained from Lambda Physic (Göttingen, Germany). All solvents were of HPLC grade supplied by Lab Scan Analytical Sciences (Dublin, Irland) and used without further purification.
Sample preparation
Riboflavin and the antioxidants were dissolved in a solution (1:1, v/v) of acetonitrile:phosphate buffer (I=0.01 M; pH 6.8). The samples consisted of a mixture of 125 μM riboflavin and 125, 250, 500, and 1000 μM of the antioxidant. For antioxidant combinations equimolar concentrations of each pair of antioxidants were added to the riboflavin solution obtaining the same final concentration of 125, 250, 500, and 1000 μM. The samples were purged with high purity nitrogen in curvets closed by rubber septa for 10 min. All solutions were protected from light prior to experiments.
Laser flash photolysis kinetic experiments
Laser flash photolysis experiments were carried out with an LKS.50 spectrometer from Applied Photophysics Ltda (Leatherhead, UK). The third harmonic at 355 nm of a pulsed Q-switched Nd-YAG laser was used to pump a dye laser Spectron Laser System (Rugby, UK) using Coumarin 120 which has an emission peak at 440 nm. The intensity of the laser pulse was approximately 2.7 mJ cm−2. A R928 photomultiplier tube from Hamamatsu (Hamamatsu, Japan) was used to detect the transient absorption (300–800 nm). Appropriate UV cut-off filters were used to minimise the sample degradation by the monitoring light. The samples were excited in 1.0 cm×1.0 cm fluorescence curvets from Hellma (Mulheim, Germany). All samples were prepared using fresh solutions thermostated at 25±0.5 °C and purged with N2 before experiment.
Results
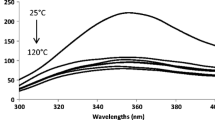
Following excitation with 440 nm light-pulses of 8 ns, aqueous solution of riboflavin were found to form triplet riboflavin as evidenced by the transient absorption spectra recorded after 1 μs of the laser-pulse in the 300–800 nm spectral region. The transient spectra were similar to those previously reported using the same experimental set up and with a comparable half-life time of 15 μs in the absence of quencher [3].
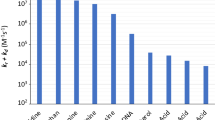
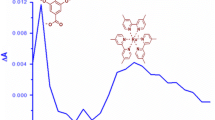
Repeating the experiments with rutin present together with riboflavin gave the transient spectra reported in Fig. 1. Immediately after excitation, 0.1 μs, a riboflavin ground state bleaching centered around 445 nm was evident together with the triplet-triplet absorption with a maximum at 720 nm. After 50 μs an absorption band with a maximum around 480 nm appears which is not seen in the absence of rutin. This absorption maximum was found to increase in intensity concomitant with the decay of the triplet riboflavin absorption monitored at 720 nm. Based on the transient absorption spectra of rutin obtained by pulse radiolysis [8], this transient spectrum was assigned to the rutin neutral radical. From the time profile up to 90 μs observed for the riboflavin triplet-triplet absorption at 720 nm and presented in Fig. 2, the decay rate constant was calculated using exponential fitting to the absorption time traces. The decay of triplet riboflavin was in each experiment fully described by single exponential decay as tested statistically, and the pseudo-first order rate constant is plotted in Fig. 3 as a function of varying rutin concentration. From Fig. 3 it is further seen that the pseudo-first order rate constant for triplet riboflavin deactivation is linearly dependent on rutin concentration for excess of rutin, and the second-order rate constant for the bimolecular deactivation of triplet riboflavin by rutin was calculated by linear regression and is presented in Table 1. It should be noted that only a small fraction of the total riboflavin concentration is in the triplet state, however, the observed linearity confirms the pseudo-first order condition for the triplet decay.
Similar procedures were used for (+)-catechin and (−)-EGCG. The transient absorption spectra obtained for (+)-catechin are presented in Fig. 4, which are similar to the spectra obtained for (−)-EGCG (data not shown). For (+)-catechin and (−)-EGCG the spectral resolution is less clear due to an overlap of the phenoxyl radical absorption and triplet riboflavin absorption, as was evident from a lower total change in transient absorption. The concentration dependence for (+)-catechin and (−)-EGCG is also linear as is seen in Fig. 3. The second-order rate constant for the three plant polyphenols studied is reported in Table 1 and each is a mean value of the slope from two or three plots like those shown in Fig. 3.
In order to explore a synergistic effect among these three plant polyphenols, they were combined two and two in three independent series of experiments as those described above. The apparent second-order rate constant for these combinations are also included in Table 1 and it is seen that values are merely interpolations between the values of the specific rate constants for the individual compounds, indicating a lack of synergism [6]. In addition, the transient spectra, Fig. 5, obtained for the combination of (+)-catechin and rutin did not show the formation of the rutin radical which is expected in case of a synergic regeneration of the (+)-catechin.
Aiming to establish a detailed mechanism for the triplet deactivation, changes in free energy for an electron transfer, \(\Delta G_{\text{ET}}^\theta\), and for hydrogen atom transfer, \(\Delta G_{\text{HT}}^\theta\), were calculated from riboflavin triplet-state excited energy, ΔE 0,0, and E θ and pK a for the involved species, respectively:
The values for the two types of deactivation mechanism for rutin and catechin calculated using the Rehm-Weller equation given above [9], are reported in Table 1 inviting further speculation. All thermodynamic parameters for a similar calculation for (−)-EGCG are not available.
Discussion
The finding of an almost diffusion controlled deactivation of triplet riboflavin by (+)-catechin, rutin and (−)-EGCG is important when considering the resistance of several types of beverages and foods against light exposure. Riboflavin absorbs light forming the very reactive triplet-excited state by intersystem crossing from the initially populated singlet-excited state [1, 2]. Riboflavin is present in a wide variety of products, where it accordingly may act as a photosensitiser inducing protein oxidation as in milk and beer or inducing lipid oxidation as in cheese [1, 2, 7].
Riboflavin is a water-soluble vitamin and long lived triplet-excited state reacts with others compounds present in the aqueous phase by electron transfer (Eqs. (3), (4)) or by hydrogen-atom transfer (Eqs. (5), (6)) following excitation and intersystem crossing (Eqs. (1), (2)) as shown in the following reaction sequence valid for aerobic conditions and which includes subsequent generation of superoxide anion radical (Eq. (6)):
ROH represent a phenolic compound which may reduce the triplet riboflavin,3Rib*, as shown in Eq. (3) or deactivate3Rib* by transfer of a hydrogen atom to form2RibH•, as shown in Eq. (5). For anaerobic conditions the reaction of Eq. (6) will be replaced by reaction with another electron acceptor. For the aqueous solution used in the present study the change in free energy both for the electron transfer Eq. (3) and for the H-atom transfer Eq. (5) mechanism were calculated based on E θ, the standard reduction potential for one-electron reduction of the plant phenoxyl radical (RO•), and the bond energy for oxygen-hydrogen found in the phenolic compound, respectively. Establishment of a linear free energy relationship (LFER) in order to distinguish between various mechanism normally requires larger series of compounds as in the study of reduction of ferrylmyoglobin by 14 structurally related flavanoid aglycones and the glycoside rutin [8] or if only fewer compounds are being studied, in very large energy differences as for purine derivatives deactivation of triplet riboflavin [4]. However, the magnitude for the \(\Delta G_{\text{ET}}^{^\theta }\) for electron transfer and \(\Delta G_{\text{HT}}^{^\theta }\) for the hydrogen atom transfer with \(\Delta G_{\text{HT}}^{^\theta }\) being more negative, points toward a hydrogen atom transfer mechanism, Table 1, with the same ordering of the free energy of activation, ΔG ≠, calculated from the rate constants determined in the present study as for \(\Delta G_{\text{HT}}^{^\theta }\). More compounds of similar structure will have to be included in the studies before more definitive mechanistic assignment can be made. Such studies should, moreover, include the effect of pH, as a shift in the mechanism from the electron transfer under basic conditions to hydrogen atom transfer in acidic medium was observed for triplet riboflavin deactivation by cystein [3]. It should also be noted, that (−)-EGCG is the more efficient reactant towards triplet excited state riboflavin followed by (+)-catechin and the glycosid rutin. A similar ordering of polyphenols has been observed for reaction with free radicals like the superoxide anion radical [6].
In conclusion, the plant polyphenol present in various flavourings used for milk-based beverages are efficient quenchers of triplet riboflavin responsible for light-induced off-flavours. Independent of the detailed mechanism such compounds may be important for flavour-stability under light-exposure. Notably, any superoxide anion radical subsequently formed from the reduced doublet-state of riboflavin in Eq. (6) may also react with the plant polyphenols showing a dual role of these polyphenols both as triplet quenchers and as radical scavengers.
References
Skibsted LH (2000) Bull Int Dairy Found 346:4–9
Li T, King JM, Min DB (2000) J Food Biochem 24:477–492
Cardoso DR, Franco DW, Olsen K, Andersen ML, Skibsted LH (2004) J Agric Food Chem 52:6602–6606
Cardoso DR, Homem-de-Mello P, Olsen K, da Silva AB, Franco DW, Skibsted LH (2005) J Agric Food Chem 53:3679–3684
Chipault JR (1962) In: Lundberg WO (ed) Autoxidation and antioxidants. Wiley, New York, pp 477–542
Becker EM, Nielsen LR, Skibsted LH (2004) Eur Food Res Technol 219:561–567
Mortensen G, Sørensen J, Stapelfeldt H (2002) J Agric Food Chem 50:4364–4370
Jørgensen LV, Skibsted LH (1998) Free Rad Res 28:335–351
Rehm D, Weller A (1970) Israel J Chem 8:259–271
Acknowledgments
This research is part of the research programme New Antioxidants Strategies for Food Quality and Consumer Health (FOODANTIOX) supported by the Committee for Research and Development in Öresund region (Öforsk) and the Danish Dairy Research Foundation. Daniel R. Cardoso thanks CAPES for the PDEE fellowship (BEX 2476/01-9)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Becker, E.M., Cardoso, D.R. & Skibsted, L.H. Deactivation of riboflavin triplet-excited state by phenolic antioxidants: mechanism behind protective effects in photooxidation of milk-based beverages. Eur Food Res Technol 221, 382–386 (2005). https://doi.org/10.1007/s00217-005-1184-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-1184-6