Abstract
Antioxidants act as quenchers or scavengers of oxidative free radicals and singlet oxygen (1O2); these compounds are effective in the treatment of diseases associated with oxidative stress and food preservation. We studied the antioxidant activity of hesperidin which was isolated from mandarin (Citrus reticulata) fruit peels against singlet oxygen (1O2). High-performance liquid chromatography with diode array detection was used for chemical characterization. The total singlet oxygen quenching rate constants of the hesperidin was measured by using the Stern–Volmer model. Results showed an overall quenching rate constant with a value of 6.43 × 107 M−1 s−1 for 1O2, which for the best of our knowledge has not been reported before. Furthermore, quantum chemical calculations were performed to get insight into the molecular and electronic structure properties of this antioxidant, which confirms that the quenching mechanism through energy transfer is rarely probable and that 1O2 could interact with the methoxy phenyl charge rich fragment of the molecule.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world population is growing every year at an exponential rate, likewise, the production of waste, especially from agricultural and urban activities (Marzo et al. 2019; Senthilkumar et al. 2020). In this sense, the recycle of agro-industrial waste represents an option to transform it into new raw materials that is emerging as an alternative to obtain compounds with beneficial properties for human health. The valorization of agro-industrial waste and the subsequent production of antioxidant substances is of great importance due to the growing demand for natural antioxidant products, while at the same time, a sustainable and environmentally friendly alternative for waste treatment is being developed (Larios-Cruz et al. 2019). The chemical and biological characteristics of agro-industrial waste depend on the transformation process and the raw material used in the initial process. For fruits, as well as their residues, it is known as a rich composition in phenolic compounds and flavonoids (Mannino et al. 2020; Pereira et al. 2018).
In an oxidation reaction, a reactive oxygen species cause several chain reactions, which may disrupt biomolecules (Bonnefont-Rousselot et al. 2011; Buonocore et al. 2010). An antioxidant is able to delay the oxidation of many compounds (e.g., carbohydrates, proteins and lipids) (Papas 1999; Neha et al. 2019; Sindhi et al. 2013). Natural antioxidants are used routinely in food to protect it against oxidation (Xu et al. 2017; Jayathilakan et al. 2007). The synthetic antioxidants (e.g., hydroxytoluene, butylated hydroxyanisole) act as quenchers of singlet oxygen (1O2) (Fatima et al. 2016; Bisbyb et al. 1999). However, synthetic antioxidants have health concerns due to their potential carcinogenic activity (Augustyniak et al. 2010). Currently, there is a substantial motivation to obtain and utilize antioxidants from natural sources because they are presumed to be safe from different points of view (Lourenço et al. 2019). 1O2 can react with a large number and important biological molecules (e.g., proteins, DNA and lipids) (Miyamoto et al. 2014), thus, research on quenching singlet oxygen generation is a constant topic of interest. Different reports verified that flavonoids and their derivatives could effectively act as scavengers of 1O2. The physical and chemical quenching are the two types of mechanisms for the 1O2 quenching (Racine and Auffray 2005), each mechanism has a specific rate constant value (kq = physical quenching, kr = and chemical reaction). Conventional measurement of global quenching rates kQ (kQ = kq + kr) are reported in the literature (Diaz-Uribe et al. 2015a):
where the former (kq) mechanism results in energy transfer but no chemical change in the energy acceptor. The (kr) results in modification of the antioxidant. (Ray et al. 2013; Sjöberg et al. 2016; Davies 2003). The 1O2 (1∆g) reactivity toward a certain biological objective will depend on the cell's ability to cope with oxidative damage and the concentration of antioxidants present. Only those electron-rich antioxidant substrates can compete with the solvent deactivation pathway since the 1O2 (1∆g) lifetime within the cell is too small. This means that global values of quenching rates (kQ = kr + kq) should be much higher than 106 M−1 s−1 value. Figure 1 compares the global rate constants (kQ) of some synthetic and natural compounds that act as antioxidants.
Hesperidin is a flavanone glycoside, see Fig. 2 (Goliomytis et al. 2014). It is an abundant and inexpensive natural product widely found in different citrus species (Devi et al. 2015).
Over time, hesperidin has become relevant because a large number of studies describe new pharmacological activities, with molecular objectives and mechanisms of action, it has anti-carcinogenic, anti-allergic and antioxidant properties. Matias et al. reported the effects of hesperidin as neuroprotective agent; this study confirmed its protective effects by normalizing oxidative stress and inflammation (Matias et al. 2017). The antioxidant activity and mechanisms of scavenging reactive oxygen species by hesperidin has been reported by different authors (Balakrishnan and Menon 2007; Bigoniya and Singh 2014; Pari et al. 2015). However, there is no information on the mechanisms of interaction of this molecule with singlet oxygen, as well as their kinetic parameters. In the present work, we studied the relative singlet oxygen quenching abilities of hesperidin isolated from Citrus reticulata peels and besides, the first singles and triplet states (T1) of hesperidin were determined using DFT and TD-DFT approaches.
Experimental
Reagents and standards
All reagents used in this work were commercially acquired, ACS grade, such as acetic acid glacial (≥ 99.7%, Sigma-Aldrich), acetone (≥ 99.5%, Sigma-Aldrich), methanol (≥ 99.8%, Sigma-Aldrich), phosphoric acid (≥ 85%, Sigma-Aldrich), Rubrene (99.99%, Sigma-Aldrich), Sodium Molybdate dihydrate (99.9%, Sigma-Aldrich). Besides, certified standards were used: hesperidin (97%, Sigma-Aldrich). UV–Vis and FT-IR (KBr) spectra were obtained by Hewlett-Packard 8453 spectrophotometer, Bruker Tensor 27 spectrometers.
Isolation and characterization of hesperidin
The peels of the C. reticulata fruit previously washed and dehydrated (60 °C, 24 h) were cut into small pieces (ca. 4 mm2), which (50 g) were defatted with petroleum ether (peels:solvent ratio, 1:3) using the Soxhlet method (reflux, 18 h). Once defatted peels was dried, they were subjected to ultrasound-assisted extraction (three times) by means of ultrasonic bath (Ney 57H ULTRAsonik, 330 W 48 kHz) at 60 °C during 60 min (at 30 min intervals), with ethanol (1:3 ratio), to obtain a flavonoid-rich fraction (Bousbia et al. 2009; Ghafoor 2009). This fraction was dried in vacuum to dryness. From the flavonoid-rich fraction, hesperidin was isolated using a modified method based on the procedures described by Dickson, and Nipornram et al. (Nipornram et al. 2018; Singanusong et al. 2015). In short, 1 g of ethanol extract was mixed/completely dissolved in 10% acetic acid by sonication at 50 °C (10 min). After that, hesperidin precipitated as a pale yellowish powder, which was centrifuged to separate it, and then the powder was washed with hot acetone and recovered as a white solid, which was structurally characterized (m.p., UV, IR). Subsequently, the purity of the isolated hesperidin was determined by means of liquid chromatography and by comparison with the certified standard of hesperidin. The chemical analysis was carried out in a RP-HPLC–DAD (UHPLC Ultimate 3000, Dionex—Thermo Fisher Scientific, Inc.; DAD, UV/Vis) equipped with a Capcell-Pak® C18 UG120 S-5 column (C18-bonded silica gel, 120 Å, 5 μm, 250 mm × 4.6 mm I.D, Shiseido Co, Tokyo); for the elution, a mobile phase (flow: 1 mL/min) constituted by methanol (solvent A—20–95%) and 0.25% phosphoric acid (solvent B—80–5%) programmed as a linear gradient, was used. The selected wavelengths (λmax) for detection were 250 nm, 285 nm, 320 nm, and 355 nm. Data acquisition and processing were supported with the Chromeleon® 7 Chromatography Data System software (Version 7.2.1.5833, Thermo Fisher Scientific, Inc.).
Singlet oxygen generation and kinetic study
Singlet oxygen was generated by using methodology proposed by Aubry and Bouttemy (Nardello et al. 1999). In short, the overall rate constant kQ = (kq + kr) for the reaction of 1O2 with hesperidin was determined in ethanol solution at 25 °C through competition reaction method using Rubrene as standard compound and analyzing the first-order rate constant (S) of the decay curve of Rubrene. 1O2 was induced from dark chemical reaction of Sodium Molybdate and hydroxide peroxide. The overall rate constant was determined using a Stern–Volmer plot derived from steady state kinetics. The reciprocal life times were represented as a function of the hesperidin concentration and the bimolecular rate constants were determined from the slope of the linear plots. Rubrene oxidation with a chemical source of singlet oxygen in microemulsion was performed to check singlet oxygen quenching activity (Lee and Jung 2010). The microemulsions was prepared at temperature (298 K) by adding an aqueous solution of 0.2 M Na2MoO4.2H2O (290.4 mg in 6 mL of water) dropwise to a magnetically stirred slurry solution of SDS (4.7 g), 1-Butanol (9.4 g), and methylene chloride (60 mL). After a few minutes, the turbid suspension was converted into a mobile and transparent liquid. Then, 2.0 × 10−4 mol of rubrene was introduced into a small Erlenmeyer flask plus 15 mL of microemulsion. The medium was magnetically stirred for 10 min and stored in darkness to prevent the autosensitized photo oxidation of Rubrene. After that, 50 mmol of H2O2 were added to the red solution and the reaction medium was stirred with a microscale magnetic bar at room temperature. The samples solutions also contained PAPs (as quenchers, 0–3.0 × 10–3 M). The oxidation of Rubrene was monitored using visible spectroscopy at 522 nm (Diaz-Uribe et al. 2015a).
Quantum chemical calculations
Geometry optimization of the electronic ground states of hesperidin was performed using the B3LYP functional (Becke 1998,1993; Lee 1988) and the 6–31 + g(d,p) basis set as implemented in the Gaussian 09 package (Frisch et al. 2009). The minima of the first singlet and triplet states (T1) of hesperidin were determined using the time-dependent DFT (TD-DFT) approaches. Corrections to the dispersion energy were taken into account in all geometry optimizations using the Grimme approaches (DFT-D3) (Grimme and Waletzke 1999). Harmonic vibrational frequencies were computed numerically to establish the structures as minima points in the potential energy surface. The adiabatic excitation energy of the first T1 state of hesperidin was obtained from geometry optimization computation. The vertical excitation energies were computed in the framework of TD-DFT method. Implicit solvation effects were incorporated using the polarized continuum model (PCM, ε = 24.5) for ethanol (Schäfer et al. 2000; Klamt and Schüürmann 1993; Reichardt and Welton 2010). The nature of the electronic transitions has been elucidated in terms of the electron density difference maps (EDDMs), which were computed and drawn with GaussSum (v. 2.2.6) (O’Boyle et al. 2008).
Results and discussion
Characterization of isolated hesperidin
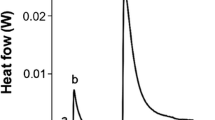
Once the ethanol extract and hesperidin was obtained from the tangerine peels by sonication, the yield and/or content (flavonoid) was calculated. As a result, the flavonoid-rich extract presented a yield of 7.4 ± 0.4% (on a dry basis), and the content of isolated hesperidin (mg) per gram of dry extract was 98 ± 0.4 mg/g. The qualitative chemical analysis by HPLC–DAD (λ: 285 nm) of the isolated hesperidin (> 99%) is shown in Fig. 3, where the chromatogram spectrum of the ethanolic extract (Fig. 3a) and the chromatogram and UV absorption spectrum of hesperidin (Fig. 3b) isolated from mandarin fruit peels are compared. The retention times obtained for (Rt: 16.55 min) and UV absorption spectra (main λmax: 230 nm, 284 nm, 328 nm) of both samples match with the reported for the commercial standard. Likewise, the IR spectra for the two samples were corresponding to each other; i.e., the characteristic absorption bands [ν: 3420–3381 cm−1 and 1356 cm−1 (–OH); 2938–2916 cm−1 (–CHalip) and 1605–1516 cm−1 (–C = C–CH–); 1645 cm−1 (–C = O); 1277–1242 cm−1 (–CAr–O–); 1130–1094 cm−1 (–Calip–O–); 1065–1049 cm−1 (–OCH3)] were similar.
In addition, the melting points measured for the two samples was 248–250 °C (isolated hesperidin) and 250–252 °C (certified standard of hesperidin), respectively; these values are included in the range (250–262 °C) reported in the consulted literature for other reports(Book and Hesperidin 2019; Hassan et al. 2018).
k Q value for the quenching of 1 O 2 by Hesperidin
Figure 4a shows the kinetics results for the chemical trapping of rubrene with singlet oxygen in the presence and absence of hesperidin. In all cases, the reactions were fitted to the pseudo-first-order kinetics. Results show direct proportionality between hesperidin concentration and its antioxidant capacity to act as a singlet oxygen quencher.
The kQ value for the reaction of singlet oxygen with hesperidin in ethanol were determined according to (1) (Hocman 1988):
S0 and SS correspond to the slope of the disappearance of singlet oxygen (acceptor) first-order plots, in the absence and presence of hesperidin, see Fig. 4a. The rate of deactivation of singlet oxygen (kd) in ethanol has a value of 8.3 × 104 s−1 (Merkel and Kearns 1972). Figure 4b shows the plot of S0/SS vs concentration of hesperidin. Applying this methodology, the kQ value obtained for hesperidin was 6.43 × 107 M−1 s−1, furthermore Table 1 lists the kQ values for previously reported antioxidants.
The herein obtained results indicate that hesperidin rate constants (kQ) is similar to the values reported for flavonoids in ethanol like chrysin (2.01 × 107 M−1 s−1), apigenin (2.84 × 107 M−1 s−1), catechins (1.09 × 107 − 1.47 × 108 M−1 s−1). Hesperidin has similar singlet oxygen quenching activity as previously reported synthetic antioxidants like tert-butyl-hydroxyanisol (3.37 × 107 M−1 s−1) and tert-di-Butyl-hydroxytoluene (4.26 × 106 M−1 s−1). Reports of kQ for similar antioxidants such as naringenin (where the glycoside rings are replaced by Hydroxyl groups) show smaller kQ values, as the ones reported in this work (see Table 1). The herein obtained result indicate that the quenching rate increases with increasing number of –OH substituents (over the glycoside rings), that is, the electron-donating capacity of these flavonoids (Diaz-Uribe et al. 2016). Other similar structure to hesperidin is naringin, differentiating between them due to the presence of a methoxy group and the location of the hydroxyl group in the B-ring into the hesperidin structure, a value of 2.1 × 107 M−1 s−1 was previously reported for the kQ for naringin. Thus, the overall rate constants kQ for hesperidin is three times larger than the kQ value reported for naringin. These results suggest that besides of the substituents into the flavone skeleton, the substituents in the B-ring directly affects the quenching activity of the hesperidin, thus the existence of methoxy group in the B-ring increased the antioxidant activity. Currently, research has been focused on the analysis of the antioxidant capacity of extracts from agro-industrial wastes due to their content of secondary metabolites (e.g. ,phenols, flavonoids, anthocyanins, and carotenes).
The exponential growth of the world population implies continuous increasing in the production agro-industrial wastes, especially from agricultural and urban activities. This situation presents an important opportunity for natural products (such as the residues of the mandarin peel) as a secondary source of compounds with high added-value (Lemes et al. 2016; Fierascu et al. 2019; Sadh et al. 2018). The agro-industrial waste represents an option to transform it into new raw materials that are emerging as an alternative to obtain compounds with beneficial properties for human health. The valorization of agro-industrial waste for the production of antioxidant substances is of utmost importance due to the growing demand for natural antioxidant products, while providing a sustainable and environmentally friendly alternative for waste treatment (Castro-Vargas et al. 2019; Toop et al. 2017).
Computational study
The optimized ground S0 and T1 state minima of hesperidin at the B3LYP-D3/6–31 + G(d,p) level are presented in Fig. 5. Both geometries, S0 and T1 show the sugar group and substituted phenyl group out of the plane of the fused rings fragment. As can be observed in Fig. 5, the dihedral angles indicated by yellow and green circles show differences of around 10 and 5 degrees, respectively. The computations results provide adiabatic energy value of the T1 state of 2.91 eV, which is a value above of the reported energy for singlet oxygen of 0.98 eV (Diaz-Uribe et al. 2015b).
This energy difference represents a pronounced endergonic process which indicates that energy transfer is not able to occur due to the very high barrier. Therefore, it is expected that the mechanism of quenching of the reactive oxygen species generation be through a chemical reaction process. On the other hand, the chemical reactivity indexes were calculated in the framework of Koopmans theory. The estimated chemical potential (μ) is − 4.02 which represents the infinitesimal change of energy, when electronic charge is added to a molecular system at a constant external potential of the nuclei (v(r)), fact closely related with its electronegativity (χ). Chemical hardness (η), and electrophilicity (υ) observed values are 2.32 and 3.48, respectively. The HOMO and LUMO energies for this compound are − 6.33 and − 1.70 eV. These magnitudes show the same tendency than others efficient quenchers reported by our group (Diaz-Uribe et al. 2015b), which have shown similar antioxidant properties as flavonoids acting as source of electrons which enhance their antioxidant activity. To go further in the electronic structure description of hesperidin, we also analyzed the condensed Fukui functions. Figure 6 shows the wave functions for nucleophilic (f+) and electrophilic (f−) attacks. Considering the information provided by the Fukui functions, the singlet oxygen which preferable attacks high electron density sites, could interacts with the ring holding the methoxy group which is the fragment for electrophilic attack, being thus a feasible pathway for chemical quenching process.
On the other hand, the theoretical characterization of the corresponding excited states involved in the absorption bands of the UV–Vis spectrum was carried out. The results provided by the simulations are in great agreement with the experimental reported data, as the wavelengths show slight shifts as can be found in Table 2. Another worthy fact to mention is that the oscillator strengths (f), which is related to the probability of this transition to occurs, which exhibit the same tendency as the intensities of the bands in the spectrum profile.
The molecular orbitals (MOs) that form the configurations for these transitions are mainly HOMO, HOMO-1, HOMO-2, LUMO, among others. In this sense, to show the changes in the electron density localization involved in the excitations, the electron density difference maps (EDDMs) were plotted (Fig. 7). From these maps it is possible to observe more charge migration upon photo absorption in the electron excitation of band 3 followed by band 1.
Conclusions
The antioxidant activity of hesperidin isolated from mandarin (Citrus reticulata) fruit peels as a source of natural antioxidant against singlet oxygen (1O2) was studied. The kQ value for 1O2 quenching reported for hesperidin was 6.43 × 107 M−1 s−1, this value is suitable compared to develop practical applications to both synthetic and natural antioxidants. The DFT simulations i.e., the electronic ground and excited states calculations of hesperidin, suggested that there is a high energetic barrier to lead a physical quenching of 1O2 so, the mechanism could mostly follow the chemical quenching pathway. In this regard, it was found that the 1O2 could be able of attacking the ring holding the methoxy group of the hesperidin compound.
These results may contribute to the development of natural products (including food waste and residues) with potential applications in reducing oxidative damage involving reactive oxygen species in living organisms and in food preservation.
References
Alarcón E, González-Béjar M, Gorelsky S, Ebensperger R, Lopez-Alarcón C, Netto-Ferreira JC, Scaiano JC (2010) Photophysical characterization of atorvastatin (Lipitor®) ortho-hydroxy metabolite: role of hydroxyl group on the drug photochemistry. Photochem Photobiol Sci 9:1378–1384. https://doi.org/10.1039/c0pp00102c
Augustyniak A, Bartosz G, Čipak A, Duburs G, Horáková L, Łuczaj W, Majekova M, Odysseos AD, Rackova L, Skrzydlewska E, Stefek M, Štrosová M, Tirzitis G, Venskutonis PR, Viskupicova J, Vraka PS, Žarković N (2010) Natural and synthetic antioxidants: an updated overview. Free Radic Res 44:1216–1262. https://doi.org/10.3109/10715762.2010.508495
Balakrishnan A, Menon VP (2007) Effect of hesperidin on matrix metalloproteinases and antioxidant status during nicotine-induced toxicity. Toxicology 238:90–98. https://doi.org/10.1016/j.tox.2007.04.022
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Becke AD (1998) Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J Chem Phys 104:1040. https://doi.org/10.1063/1.470829
Bigoniya P, Singh K (2014) Ulcer protective potential of standardized hesperidin, a citrus flavonoid isolated from citrus sinensis. Braz J Pharmacogn 24:330–340. https://doi.org/10.1016/j.bjp.2014.07.011
Bisbyb RH, Morgan CG, Hamblett I, Gorman AA (1999) Quenching of singlet oxygen by Trolox C, ascorbate, and amino acids: effects of pH and temperature. J Phys Chem A 103:7454–7459. https://doi.org/10.1021/jp990838c
Bonnefont-Rousselot D, Collin F, Jore D, Gardès-Albert M (2011) Reaction mechanism of melatonin oxidation by reactive oxygen species in vitro. J Pineal Res 50:328–335. https://doi.org/10.1111/j.1600-079X.2010.00847.x
Chemical Book, Hesperidin | 520–26–3, (2019). https://www.chemicalbook.com/ChemicalProductProperty_EN_cb3234127.htm Accessed from 11 May 2020
Bousbia N, Vian MA, Ferhat MA, Meklati BY, Chemat F (2009) A new process for extraction of essential oil from Citrus peels: microwave hydrodiffusion and gravity. J Food Eng 90:409–413. https://doi.org/10.1016/j.jfoodeng.2008.06.034
Buonocore G, Perrone S, Tataranno ML (2010) Oxygen toxicity: chemistry and biology of reactive oxygen species. Semin Fetal Neonatal Med 15:186–190. https://doi.org/10.1016/j.siny.2010.04.003
Castro-Vargas HI, Baumann W, Ferreira SRS, Parada-Alfonso F (2019) Valorization of papaya (Carica papaya L.) agroindustrial waste through the recovery of phenolic antioxidants by supercritical fluid extraction. J Food Sci Technol 56:3055–3066. https://doi.org/10.1007/s13197-019-03795-6
Davies MJ (2003) Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun 305:761–770. https://doi.org/10.1016/S0006-291X(03)00817-9
Devasagayam TPA, Sundquist AR, Di Mascio P, Kaiser S, Sies H (1991) Activity of thiols as singlet molecular oxygen quenchers. J Photochem Photobiol B Biol 9:105–116. https://doi.org/10.1016/1011-1344(91)80008-6
Devi KP, Rajavel T, Nabavi SF, Setzer WN, Ahmadi A, Mansouri K, Nabavi SM (2015) Hesperidin: a promising anticancer agent from nature. Ind Crops Prod 76:582–589. https://doi.org/10.1016/j.indcrop.2015.07.051
Diaz-Uribe CE, Vallejo W, Castellar W, Trilleras J, Ortiz S, Rodriguez-Serrano A, Zarate X, Quiroga J (2015a) Novel (E)-1-(pyrrole-2-yl)-3-(aryl)-2-(propen-1-one) derivatives as efficient singlet oxygen quenchers: kinetics and quantum chemical calculations. RSC Adv 5:71565–71572. https://doi.org/10.1039/C5RA13203G
Diaz-Uribe CE, Vallejo W, Castellar W, Trilleras J, Ortiz S, Rodriguez-Serrano A, Zarate X, Quiroga J (2015b) Novel (E)-1-(pyrrole-2-yl)-3-(aryl)-2-(propen-1-one) derivatives as efficient singlet oxygen quenchers: Kinetics and quantum chemical calculations. RSC Adv. https://doi.org/10.1039/c5ra13203g
Diaz-Uribe CE, Oliveros G, Muñoz-Acevedo A, Vallejo Lozada WA (2016) Kinetic study of the quenching of singlet oxygen by naringin isolated from peels of the fruit of bitter orange (Citrus aurantium I.). Rev Cuba Plantas Med 21
Fatima K, Masood N, Luqman S (2016) Quenching of singlet oxygen by natural and synthetic antioxidants and assessment of electronic UV/Visible absorption spectra for alleviating or enhancing the efficacy of photodynamic therapy. Biomed Res Ther. https://doi.org/10.7603/s40730-016-0008-6
Fierascu RC, Fierascu I, Avramescu SM, Sieniawska E (2019) Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules. https://doi.org/10.3390/molecules24234212
Frisch DJ, Trucks MJ, Schlegel GW, Scuseria HB, Robb GE, Cheeseman MA, Scalmani JR, Barone G, Mennucci V, Petersson B, Nakatsuji GA, Caricato H, Li M, Hratchian X, Izmaylov HP, Bloino AF, Zheng J, Sonnenb G (2009) Gaussian 09, Revision E.01, in: Gaussian 09, Revision E.01. Gaussian, Inc., Wallingford CT. http://wild.life.nctu.edu.tw/~jsyu/compchem/g09/g09ur/m_citation.htm
Ghafoor K (2009) Optimization of ultrasound assisted extraction of phenolic compounds and antioxidants from grape peel through response surface methodology. J Korean Soc Appl Biol Chem 52:295–300. https://doi.org/10.3839/jksabc.2009.052
Goliomytis M, Orfanou H, Petrou E, Charismiadou MA, Simitzis PE, Deligeorgis SG (2014) Effect of hesperidin dietary supplementation on hen performance, egg quality and yolk oxidative stability. Br Poult Sci 55:98–104. https://doi.org/10.1080/00071668.2013.870328
Grimme S, Waletzke M (1999) A combination of Kohn-Sham density functional theory and multi-reference configuration interaction methods. J Chem Phys 111:5645–5655. https://doi.org/10.1063/1.479866
Hassan BA, Hamed FM, Alyaseen FF (2018) Phytochemical screened, characterization and antibacterial activity of hesperetin and hesperidin extracted and isolated from dried oranges peels. Int J Res Pharm Sci 9:1362–1367
Hocman G (1988) Chemoprevention of cancer: phenolic antioxidants (BHT, BHA). Int J Biochem 20:639–651. https://doi.org/10.1016/0020-711x(88)90158-9
Jayathilakan K, Sharma GK, Radhakrishna K, Bawa AS (2007) Antioxidant potential of synthetic and natural antioxidants and its effect on warmed-over-flavour in different species of meat. Food Chem 105:908–916. https://doi.org/10.1016/j.foodchem.2007.04.068
Klamt A, Schüürmann G (1993) COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc Perkin Trans 2:799–805. https://doi.org/10.1039/P29930000799
Larios-Cruz R, Buenrostro-Figueroa J, Prado-Barragán A, Rodríguez-Jasso RM, Rodríguez-Herrera R, Montañez JC, Aguilar CN (2019) Valorization of grapefruit by-products as solid support for solid-state fermentation to produce antioxidant bioactive extracts. Waste Biomass Valoriz 10:763–769. https://doi.org/10.1007/s12649-017-0156-y
Lee JH, Jung MY (2010) Direct spectroscopic observation of singlet oxygen quenching and kinetic studies of physical and chemical singlet oxygen quenching rate constants of synthetic antioxidants (BHA, BHT, and TBHQ) in methanol. J Food Sci 75:C506–C513. https://doi.org/10.1111/j.1750-3841.2010.01669.x
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter 37:785–789
Lemes AC, Álvares GT, Egea MB, Brandelli A, Kalil SJ (2016) Simultaneous production of proteases and antioxidant compounds from agro-industrial by-products. Bioresour Technol 222:210–216. https://doi.org/10.1016/j.biortech.2016.10.001
Lourenço SC, Moldão-Martins M, Alve VD (2019) Antioxidants of natural plant origins: from sources to food industry applications. Molecules. https://doi.org/10.3390/molecules24224132
Mannino G, Perrone A, Campobenedetto C, Schittone A, Margherita Bertea C, Gentile C (2020) Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: a new source of nutraceuticals. Food Chem 307:125515. https://doi.org/10.1016/j.foodchem.2019.125515
Marzo C, Díaz AB, Caro I, Blandino A (2019) Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manag Res 37:149–156. https://doi.org/10.1177/0734242X18798699
Matias I, Diniz LP, Buosi A, Neves G, Stipursky J, Gomes FCA (2017) Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-β1. Front Aging Neurosci 9:184. https://doi.org/10.3389/fnagi.2017.00184
Merkel PB, Kearns DR (1972) Radiationless decay of singlet molecular oxygen in solution an experimental and theoretical study of electronic-to-vibrational energy transfer. J Am Chem Soc 94:7244–7253. https://doi.org/10.1021/ja00776a003
Miyamoto S, Martinez GR, Medeiros MHG, Di Mascio P (2014) Singlet molecular oxygen generated by biological hydroperoxides. J Photochem Photobiol B Biol 139:24–33. https://doi.org/10.1016/j.jphotobiol.2014.03.028
Morales J, Günther G, Zanocco AL, Lemp E (2012) Singlet oxygen reactions with flavonoids a theoretical – experimental study. PLoS ONE 7:e40548. https://doi.org/10.1371/journal.pone.0040548
Nagai S, Ohara K, Mukai K (2005) Kinetic study of the quenching reaction of singlet oxygen by flavonoids in ethanol solution. J Phys Chem B 109:4234–4240. https://doi.org/10.1021/JP0451389
Nardello V, Marti M-J, Pierlot C, Aubry J-M (1999) Photochemistry without light: oxidation of rubrene in a microemulsion with a chemical source of singlet molecular oxygen (1O2, 1Dg). J Chem Educ 76:1285. https://doi.org/10.1021/ed076p1285
Neha K, Haider MR, Pathak A, Yar MS (2019) Medicinal prospects of antioxidants: a review. Eur J Med Chem 178:687–704. https://doi.org/10.1016/j.ejmech.2019.06.010
Nipornram S, Tochampa W, Rattanatraiwong P, Singanusong R (2018) Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem 241:338–345. https://doi.org/10.1016/j.foodchem.2017.08.114
O’Boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package-independent computational chemistry algorithms. J Comput Chem 29:839–845. https://doi.org/10.1002/jcc.20823
Ouchi A, Aizawa K, Iwasaki Y, Inakuma T, Terao J, Nagaoka SI, Mukai K (2010) Kinetic study of the quenching reaction of singlet oxygen by carotenoids and food extracts in solution. development of a singlet oxygen absorption capacity (SOAC) assay method. J Agric Food Chem 58:9967–9978. https://doi.org/10.1021/jf101947a
Papas AM (1999) Diet and antioxidant status. Food Chem Toxicol 37:999–1007. https://doi.org/10.1016/S0278-6915(99)00088-5
Pari L, Karthikeyan A, Karthika P, Rathinam A (2015) Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol Rep 2:46–55. https://doi.org/10.1016/j.toxrep.2014.11.003
Pereira GA, Arruda HS, de Morais DR, Eberlin MN, Pastore GM (2018) Carbohydrates, volatile and phenolic compounds composition, and antioxidant activity of calabura (Muntingia calabura L.) fruit. Food Res Int 108:264–273. https://doi.org/10.1016/j.foodres.2018.03.046
Racine P, Auffray B (2005) Quenching of singlet molecular oxygen by Commiphora myrrha extracts and menthofuran. Fitoterapia 76:316–323. https://doi.org/10.1016/j.fitote.2005.03.017
Ray RS, Mujtaba SF, Dwivedi A, Yadav N, Verma A, Kushwaha HN, Amar SK, Goel S, Chopra D (2013) Singlet oxygen mediated DNA damage induced phototoxicity by ketoprofen resulting in mitochondrial depolarization and lysosomal destabilization. Toxicology 314:229–237. https://doi.org/10.1016/J.TOX.2013.10.002
Reichardt C, Welton T (2010) Solvents and solvent effects in organic chemistry. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim, Germany
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1–15. https://doi.org/10.1186/s40643-017-0187-z
Schäfer A, Klamt A, Sattel D, Lohrenz JCW, Eckert F (2000) COSMO implementation in TURBOMOLE: extension of an efficient quantum chemical code towards liquid systems. Phys Chem Chem Phys 2:2187–2193. https://doi.org/10.1039/b000184h
Senthilkumar K, Naveen Kumar M, Chitra Devi V, Saravanan K, Easwaramoorthi S (2020) Agro-Industrial waste valorization to energy and value added products for environmental sustainability. Springer, Singapore, pp 1–9
Sindhi V, Gupta V, Sharma K, Bhatnagar S, Kumari R, Dhaka N (2013) Potential applications of antioxidants – A review. J Pharm Res 7:828–835. https://doi.org/10.1016/j.jopr.2013.10.001
Singanusong R, Nipornram S, Tochampa W, Rattanatraiwong P (2015) Low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) and lime (Citrus aurantifolia) peels and the antioxidant. Food Anal Methods 8:1112–1123. https://doi.org/10.1007/s12161-014-9992-6
Sjöberg B, Foley S, Staicu A, Pascu A, Pascu M, Enescu M (2016) Protein reactivity with singlet oxygen: influence of the solvent exposure of the reactive amino acid residues. J Photochem Photobiol B Biol 159:106–110. https://doi.org/10.1016/j.jphotobiol.2016.03.036
Toop TA, Ward S, Oldfield T, Hull M, Kirby ME, Theodorou MK (2017) AgroCycle - Developing a circular economy in agriculture. Energy Procedia. https://doi.org/10.1016/j.egypro.2017.07.269
Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang JJ, Bin Li H (2017) Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int J Mol Sci. https://doi.org/10.3390/ijms18010096
Acknowledgements
The authors would like to thank Universidad del Atlántico. Departamento del Atlántico (Sistema General de Regalías—Fondo de Ciencia, Tecnología e Innovación por apoyo financiero del Programa de Investigación “Implementación de proyectos de I + D (componentes Microalgas, Extractos de plantas y cadena productiva de la sábila, Producción 1,2 pro-panodiol y Plataforma informática) para promover el desarrollo y la transferencia tecnológica de cadenas productivas agroindustriales y la implementación de tecnologías de última generación para el procesamiento de biocombustibles en el departamento del Atlántico”. FONDECYT 1180565 and 1201880 and ANID -Millennium Science Initiative Program- NCN17_040. ANID/FONDAP/15110019.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diaz-Uribe, C., Vallejo, W., De la Hoz, T. et al. Theoretical and kinetic study of the singlet oxygen quenching reaction by hesperidin isolated from mandarin (Citrus reticulata) fruit peels. Chem. Pap. 76, 169–178 (2022). https://doi.org/10.1007/s11696-021-01825-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01825-2