Abstract
Relative quantities of d-amino acids, (%D) calculated from the sum of d- and l-amino acids were determined in bee honeys (n=6) by GC-SIM-MS. Amino acids were isolated by treatment with Dowex 50 W X8 cation exchanger and converted into N(O)-perfluoroacyl amino acid propyl esters. In all honeys d-Ala, ranging from 2.2–6.2% d-Ala, was detected. Other d-amino acids were also found, albeit not in all honeys and approached 5.9% d-Glx, 5.4% d-Lys, 3.0% d-Phe, 2.1% d-Orn, 1.7% d-Asx, 1.5% d-Ser, 0.1% d-Pro, and 0.4% d-Val in certain honeys. Quantities of d-amino acids increased very much on experimental heating of honeys in an oven and on a microwave treatment. Conventional heating of a forest honey (no. 1) at 65 °C for 450 h leads to an increase of d-Ala (2.2–12.5%), d-Pro (0.0–5.0%), d-Ser (1.5–9.1%), d-Asx (1.7–9.8%), d-Phe (0.4–5.0%) and d-Glx (1.5–5.8%); first numbers in parentheses refer to unheated honeys. Relative quantities of other d-amino acids also increased. Experimental heating of another forest honey (no. 2) in a microwave oven for 3 min at 180 W leads to an increase of d-Ala (3.7–11.0%), d-Glx (1.5–13.7%), d-Asx (0.7–10.2%), d-Phe (0.3–4.8%), d-Val (0–4.2%), and d-Pro (0.1–2.3%). Microwave treatment at 700 W for 1 min of a blossom honey (no. 3) leads to an increase of d-Ala (6.2–26.7%) and of d-Phe (3.0–10.9%). Microwave treatments were accompanied by intensive destruction of amino acids. Heating of a model mixture mimicking the major components of honey (d-glucose, d-fructose, and l-amino acids at 20% water content) at pH 2.6–9.0 and at 180 W for 1–3 min leads to the generation of d-amino acids and was also accompanied by intensive decay of amino acids. From the data it is concluded that d-amino acids are formed in honeys in the course of the Maillard reaction. A mechanism is presented based on amino acid racemization of reversibly formed Heyns and Amadori compounds (fructose-amino acids).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the definitions of the European Community [1] honey is a natural sweet substance produced by Apis mellifera L. from the nectar of plants or from secretions of living parts of plants or excretions of plant-sucking insects on the living parts of plants, which the bees collect, transform by combining with specific substance of their own, deposit, dehydrate, store, and leave in honeycombs to ripen and mature.
Honeys are classified according to the origin (blossom honey or nectar honey, and honeydew honey) and according to the mode of production and/or presentation. A product of lower quality is baker's honey, which is used for industrial uses or ingredient in foodstuffs, which are then processed. From a chemical point of view, honey is composed of d-fructose (about 38%) and d-glucose (about 31%) besides an abundance of minor mono- and oligosaccharides. The water content is about 20% and the pH of honeys ranges from 3.4 to 6.1. Honey contains about 100 mg amino acids in 100 g dry matter. The most abundant are Pro and Phe counting for about 70% and 15%, respectively, of all amino acids. Further, proteins including enzymes, flavor compounds and pigments are also present. Among various organic acids gluconic acid is the most abundant.
In order to control the quality, and authenticity, and the correct labeling of honey, analytical methods have been established such as determination of contents of sugars, moisture content, enzymic activity (diastase), pollen analysis, electrical conductivity, and the determination of quantities of 5-hydroxymethylfurfural (HMF) [2].
As the pattern and ratios of amino acids in certain honeys are characteristic and typical for regions and countries, these data have been used to identify the origin of honeys [3]. Accordingly, gas and liquid chromatographic methods for quantitative amino acid analysis have been described with the aim to use the amino acid profiles and ratios to discriminate between botanical sources or geographic regions of origin [4–6].
Few reports, however, are concerned with the chirality, i.e., the possible occurrence of enantiomeric l- and d-amino acids in honey. Occurrence of d-amino acids in a forest honey was first recognized in 1990 in our laboratories using capillary gas chromatography on Chirasil-l-Val and XE-60-l-Valine-(S)-α-phenylethylamide. Presence of 3.3% d-Asx (0.14 mg/100 g), 5.7% d-Glx (0.39 mg/100 g), 5.7% d-Ala; 5.7% d-Ala (0.11 mg/100 g), and 3.8% d-Phe (0.18 mg/100 g) were reported [7]. The gas chromatographic data were corroborated using quantification of l- and d-amino acids in a white fir honey by HPLC and pre-column derivatization with o-phthaldialdehyde (OPA) together with N-isobutyryl-l-cysteine (IBLC) or N-isobutyryl-d-cysteine (IBDC) and separation of the resulting diastereomeric amino acid derivatives by HPLC. Using OPA/IBLC or OPA/IBDC (in parantheses if determined) relative quantities of 5.2% (7.3%) d-Asp, 4.1% (4.2%) d-Glu, 1.8% (1.2%) d-Ser, 3.3% d-Ala, 1.8% d-Phe, and 6.9% (5.7%) d-Leu were determined in this honey [8].
Using pre-column derivatization of honey amino acids with FMOC-Cl or FMOC-Gly-Cl and separation of the resulting diastereomers by HPLC employing column switching and modified β-cyclodextrins as chiral stationary phases, relative quantities of 0.05–0.38% d-Pro, and 0.7–3.8% d-Phe and d-Leu could be detected in honeys and an increase of d-Pro and d-Leu on conventional and microwave heating was observed [9]. Owing to the method used only these d-amino acids could be analyzed.
From the data resulting from different chromatographic methods there was no doubt that certain d-amino acids occur naturally in honey. However, no convincing explanation on the mechanism of their formation could be presented. Bacteria, known to be good producers of d-amino acids in fermented or spoiled foodstuffs [10] do not grow in high quality honeys.
We had recently shown, however, that heating of l-amino acids together with reducing sugars leads to the formation of d-amino acids [11], a process that is usually referred to as racemization. The reaction of reducing sugars and amino acids is known as the Maillard reaction [12] or non-enzymic browning reaction. It was assumed that d-amino acids are generated from stable intermediates of this reaction, named Amadori compounds or fructose-amino acids.
In the following sections we present data on the contents of d-amino acids in bee honeys and their increase on heating, using either conventional heating or microwave heating. Further, we report on the generation of d-amino acids in a model mixture simulating bee honey. From the data a mechanism on the formation of d-amino acids via Amadori rearrangement products is proposed.
Experimental
Instruments
For enantioselective separations of derivatized amino acids a fused silica capillary column Chirasil-l-Val (N-propionyl-l-valine-tert-butylamide polysiloxane) of 25 m length × 0.25 mm ID and a film thickness 0.12 μm of the stationary phase (from Varian Inc., Darmstadt, Germany) was used together with a Model A17 gas chromatography coupled with a Model QP5000 mass spectrometer (Shimadzu, Kyoto, Japan).
The carrier gas was helium set at an inlet pressure of 5.0 kPa, a flow rate of 0.5 ml/min and a purge flow of 3 ml/min. Injector and interface temperatures were 250 °C and 0.5–1 μl aliquots of analytes were injected at a split ratio of 1:30. The temperature program was 70 °C for 1 min, increased at 2.5 °C/min to 100 °C, then 2 min isothermal, increased at 2.5 °C/min to 150 °C, increased at 5 °C/min to 150 °C, then at 20.0 °C/min to 190 °C, then for 8 min isothermal at 190 °C. The pressure program of the carrier gas was 5.0 kPa for 1 min, then 0.2 kPa/min to 7.0 kPa, 2 min isobaric; then 0.3 kPa/min to 10.8 kPa, then 1.4 kPa/min to 13.0 kPa, then 2.4 kPa/min to 15.0 kPa, then 5 min isobaric. For selected ion monitoring appropriate ion sets were selected and characteristic mass fragments (m/z) of the TFA/Prp or PFP/Prp esters of the amino acids were used: Ala (190,191); Val (218, 203); Thr (203, 202); Gly (176, 177); Pro (216); Leu (190, 232); Ser (188, 189); Asx = Asp + Asn (234, 216); Met (263, 203); Phe (91, 148); Glx = Glu + Gln (202, 203); Tyr (253, 266); Orn (216); Lys (230); GABA (232, 176). Fragments of TFA/Prp derivatives count 50 mass units less.
Solvents and chemicals
Methanol (MeOH), 1- and 2-propanol (1- and 2-PrOH), dichloromethane (DCM), acetyl chloride (AcCl), and trifluoroacetic acid anhydride (TFAA) were from Merck, Darmstadt, Germany; pentafluoropropionic anhydride (PFPAA) was from Pierce, Rockford, IL, USA; d-glucose, d-fructose, and antioxidant 3,5-di-tert-butyl-4-hydroxy toluene (BHT) were from Fluka, Buchs, Switzerland; aqueous ammonia (24%) and aqueous HCl (36%) of analytical grade were from Carl Roth, Karlsruhe, Germany; Dowex 50W X8 (200–400 mesh) was from Sigma, Deisenhofen, Germany. l- and d-amino acids were purchased from Sigma and Fluka and a standard mixture of dl-amino acids (ratio about 1:2) for calibrating the chiral column was prepared by mixing appropriate amounts followed by derivatization [13]. Doubly distilled water from a quartz distill was used exclusively.
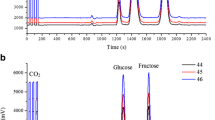
Chromatograms (GC-SIM-MS, TFA/1-Prp esters) of blossom honey no. 3. a Commercial product. b After heating at 65 °C for 450 h and c after microwave treatment for 1 min at 700 W; arrows indicate d-amino acids. For quantities of d-amino acids see Table 1. Note that varying baseline levels (offsets) are the results of varying amplifications of ion sets selected for SIM-MS. Peaks not assigned are unknown
Sources of honeys
Honeys were commercial products purchased from local retail outlets. Investigations were acrried out in forest honey no. 1 (Marlene, ‘Waldhonig’, Lidl, Germany); forest honey no. 2 (Biophar, ‘Wabenquell’, Braunschweig, Germany); blossom honey no. 3 (Maribel, ‘Sommerblütenhonig’, Lidl, Germany); pine honey no. 4 (‘Pinienhonig’ Immenhof, Germany); acacia honey no. 5 (‘Akazienhonig’ Immenhof, Germany); baker's honey no. 6, industrial product (provided by Landesbetrieb Hessisches Landeslabor, Giessen).
Treatment of honeys for analyses
To aliquots of honeys (about 1 g), water (10 ml) was added and pH 2.3 was adjusted by addition of 0.01 M HCl. Pasteur glass pipettes (0.5 cm ID × 23 cm total length) were sealed with glass wool plugs and the pipettes were filled with Dowex 50W X8 cation exchanger (6 cm high, corresponding to 1.5 ml bed volume). The analytes were passed through the ion exchangers at flow rates of about 1 ml/min. Then ion exchangers were washed with distilled water until the effluents were neutral and amino acids were eluted by 4 M aqueous ammonia. The effluents were evaporated to dryness using a rotary evaporator at 40 mbar and a bath temperature of 40 °C. To the remaining residues 0.1 M HCl (1 ml) was added, the solutions transferred to ReactiVials® (Wheaton, Millville, NJ, USA). Vials were tightly closed with Teflon® lined screw caps and the solvent removed in a stream of nitrogen. To the residues AcCl in 2-PrOH (1:4, v/v) (400 μl) and 1% BHT in 2-PrOH (10 μl) were added. The mixtures were heated to 100 °C for 1 h in a heating module equipped with a nitrogen flushing device (Pierce, Rockford, IL, USA). Reagents were removed in a stream of nitrogen, then DCM (200 μl) and TFAA or PFPAA (50 μl) were added and acylation was performed in closed vials at 110 °C for 20 min. Reagents were removed in a stream of nitrogen, DCM (200 μl) was added and aliquots of 0.5–1.0 μl were analyzed by GC-SIM-MS.
Conventional heating and microwave treatment of bee honeys
For conventional heating aliquots of the selected honeys (3 g) were kept in closed 5 ml ReactiVials® in an electrically heated oven at 65 °C for 44 h (honeys nos. 1 and 3) and 450 h (honeys nos. 4, 5 and 6), respectively.
Aliquots of forest honey no. 2 and blossom honey no. 3 (about 1 g) were put in open glass vessels (2.2 cm ID × 5.5 cm high) and heated in a domestic microwave oven with a rotating dish for 1, 1.5, 2.5, and 3 min at 180 W (no. 2) and for 1 min at 700 W (no. 3). Then water (10 ml) was added and analytes were treated as described above. Hydroxymethylfurfural (HMF) was determined colorimetrically in samples according to a standard protocol and using derivatization with barbituric acid and p-toluidine [14]. For data see Table 1.
Schema of the proposed generation of d-amino acids from fructose-l-amino acids (Amadori compounds) 1 serving as precursors via formation of 2,3-enols (2a) or 1,2-enols (2b), intermediate formation of carbanions (3a) and (3b), formation of (partly) racemized amino acid derivatives (4a) and (4b) on reprotonation, followed by release of a mixture of d- and l-amino acids (5) and 1- und 3-deoxyosones. R1 refers to amino acid side chain, R2 to –CH2OH of the sugar moiety, and asterisk indicates racemized amino acid
Preparation and microwave treatment of a model honey mixture
A model mixture simulating the composition of honey was prepared and treated as follows: To a mixture of d-glucose (40.0 g) and d-fructose (40.0 g) the l-enantiomers of Pro (304 mg), Ala (26 mg), Asp (37 mg), Val (26 mg), Glu (30 mg), Phe (29 mg), Lys (33 mg), Met (28 mg), Ser (32 mg) water (100 ml) was added. A pH 2.6 of the resulting solution was measured. The solution was divided into four 20 ml portions. One sample was left at pH 2.6 and the remaining three were adjusted by addition of 0.1 M NaOH to pH 5.1, pH 7.0, and pH 9.0. Weights of analytes were determined gravimetrically and solutions were concentrated to a final content of 20% water (w/w) using a rotary evaporator at 40 mbar and 40 °C bath temperature. Aliquots (3.5 g) of the syrupy residues were transferred to open vials (2.2 cm ID × 5.5 cm high) and treated in a microwave oven at 180 W for 2, 3, and 4 min. After immediate cooling in an ice bath, water (10 ml) was added. The resulting solutions were adjusted to pH 2.3 by addition of 0.1 M HCl and subjected to ion-exchange treatment, derivatization, and enantioselective analysis by GC-SIM-MS as described above. For data see Table 2. No d-amino acids were detected in the model mixture prior to microwave treatments.
Results
Analyses of native honeys nos. 1–6 and relative quantities of d-amino acids (%D=100D/(D+L)) detected therein are compiled in Table 1. As can be seen d-Ala, ranging from 2.2 to 6.2%, was detected in all honeys but varying kinds and quantities of other d-amino acids (ranging from about 1–3%) could also be detected. On conventional heating of honeys at 65 °C for 44 h and 450 h, respectively, quantities of d-amino acids increased and d-amino acids not detectable in unheated honeys were formed. As an example, in forest honey no. 1, quantities of 2.2% d-Ala increased, on heating at 65 °C for 44 and 450 h to 6.6% and 12.5%, respectively. No d-Pro was detected in this honey but 5.0% d-Pro was generated after 450 h heating. Microwave treatment for short periods of time increased relative quantities of d-amino acids very much. The absolute amounts of amino acids, however, decreased drastically as could be deduced qualitatively from the very much lowered signal intensities of the mass spectrometer. As examples, quantities of 3.7% d-Ala, 0.7% d-Asx and 1.5% d-Glx in untreated forestal honey no. 2 increased on heating for 3 min at 180 W to 11.0% d-Ala, 10.2% d-Asx and 13.7% d-Glx, respectively. Formation or increase of quantities of some other d-amino acids was also observed.
Microwave treatment of blossom honey no. 3 at 700 W for 1 min lead to an increase from 6.2 to 26.7% d-Ala and from 3.0 to 10.9% d-Phe.
d-amino acids in blossom honey no. 3 and change of relative quantities on conventional heating in comparison to microwave heating is demonstrated with chromatograms presented in Fig. 1. For relative quantities see Table 1.
Under the conditions of derivatization for GC the amino acid amides Asn and Gln are converted into Asp and Glu, therefore, Asx and Glx in Table 1 stand for the sum of Asp and Asn and Glu and Gln, respectively. Previous investigation of a honey by HPLC using derivatization with OPA/IBLC and OPA/IBDC, however, had shown that only the d-enantiomers of Asp and Glu were present in the fir honey analyzed [8]. Further, low quantities of the l-enantiomers but no d-enantiomers of His, Arg and Trp could be detected in this honey sample. The respective l-amino acids, however, are minor components in honeys.
Since HMF is an established indicator for heat treatment of honeys, the quantities were also determined in honeys before and after conventional heating and microwave treatment. Low amounts (7–27 mg HMF/kg) were detected in untreated honeys. Quantities drastically increase on conventional heating at 65 °C/450 min, amounting to 847–2369 mg HMF/kg. Microwave heating for 1 min at 700 W generated 4635 mg HMF/kg (see Table 1).
Relative quantities of d-amino acids resulting from microwave treatment of an artificial model honey at pH 2.6 to pH 9.0 are compiled in Table 2. As can be seen the time of microwave treatment at 180 W as well as pH control relative quantities of d-amino acids formed from l-amino acids and their decay. Notably, high relative amounts of d-Ala are formed approaching 39.0% and 41.3% at pH 7.0 and 9.0, respectively. Relative amounts of 21.3% d-Pro were generated at pH 2.6 and heating for 4 min at 180 W. The highest amounts of 24.6% d-Asx and 20.5% d-Glx were produced at pH 2.6 and microwave treatment at 180 W for 3 min but after 4 min d-Asx and d-Glx could not be detected anymore.
Representative chromatograms illustrating the data of tables are presented in Fig. 1.
Discussion
The data further confirm that d-amino acids occur in commercially available bee honeys. Relative quantities and kinds of d-amino acids detectable therein depend on analytes but both increase on conventional heating at 65 °C. Heating honey for such long periods of time is experimental but demonstrates increase and generation of additional d-amino acids under these conditions. Formation of high relative amounts of d-amino acids and destruction of d-and l-amino acids within short periods of time are observed on microwave heating of honeys. Such overheating might occur accidentally in the household but intensive conventional or microwave heating is performed under conditions of the industrial production of foodstuffs. Baker's honeys are defined as products of lower quality used in the food industry. Baker's honey no. 6, however, did not contain exceptionally high amounts of d-amino acids (see Table 1).
The data are in accordance with the recommendation that crystallized honey should be warmed gently in a water bath rather than putting it in a microwave owing to the risk of overheating usually accompanied by intensive browning reactions and formation of off-flavors.
The more or less intensive color of native honey is attributed, at least in part, to the Maillard reaction. Acceleration of the Maillard reaction in honeys on heating is indicated by intensive browning on heating and formation of HMF.
The occurrence and pattern of d-amino acids and in bee honey and the increase on conventional or microwave heating require an explanation. Bee honey is an excellent example for foodstuffs in which the Maillard reaction can proceed already at ambient temperature: it is rich in glucose and fructose and contains amino acids, the water activity is low (a w about 0.75) and the pH is weakly acidic to neutral. No inhibitors such as sulfites are added to honeys.
It was assumed that (partial) racemization of amino acids proceeds in the Amadori compounds (fructose-amino acids) resulting from the Amadori rearrangement [11, 15–18]. These compounds representing relatively stable intermediates of the Maillard reaction. The Amadori compounds (1-amino-1-deoxy-2-ketoses) easily undergo 1,2-enolisation under weakly acidic and 2,3-enolisation under neutral to weakly alkaline conditions. The enolisation favors the proton abstraction from the Cα-atom of the bonded amino acid with the formation of an intermediate sp3-hybridized carbanion. Enolization and proton abstraction with the formation of the carbanion might also be favored by the formation of intramolecular hydrogen bridges. Reprotonation of the carbanion can proceed from both sides of the more or less planar carbanion thus generating a (partly) racemized amino acid. Extend of the racemization depend on steric and electronic factors which are controlled by the amino acid side chains. Finally, decay of the Amadori compound with release of the racemized amino acid and 1- and 3-deoxyosones occurs. Notably, heating of synthetic fructose-l-phenylalanine or fructose-d-phenylalanine, serving as model compounds, leads to the release of a high percentage of the opposite amino acid enantiomer [16].
A tentative racemization mechanism based on a general schema on the reaction of carbohydrates with amino acids [19] is presented in Fig. 2 with the open-chain form of the Amadori compound.
The complex kinetics of the reaction are controlled by the general conditions governing the Maillard reaction and a strong temperature dependence is observed according to the Arrhenius equation [20, 21]. The amino acids released (both d- and l-enantiomers) can participate again in the Maillard reaction. At the end of the reaction amino acids are no longer detectable as they are irreversibly converted into Strecker aldehydes, heterocyclic flavor compounds or low and high molecular weight melanoidins [22]. Heyns compounds (2-amino-2-deoxyaldoses), resulting from the reaction of fructose and amino acids [23], are assumed to behave analogously to Amadori compounds. Amadori compounds have been detected in many dried or heated foodstuffs of animal or plant origin such as milk powder, plant and vegetable powders, dry fruits, tobacco, roasted cacao, and cacao products [24–28]. Consequently, occurrence of d-amino acids in some of these products has been attributed to release from Amadori compounds formed in the course of the Maillard reaction [15, 18, 29, 30].
References
Council Directive 2001/110/EC of 20 December 2001 relating to honey (L10/47 and Annex L10/50)
Anklam E (1998) Food Chemistry 63:549–562
White JW (1978) Adv Food Res 24:287–374
Davies AMC (1975) J Apicul Res 14:29–39
Gilbert J, Shepherd MJ, Wallwork MA, Harris RG (1981) J Apicul Res 20:125–135
Pirini A, Conte SL, Francioso O, Lercker G (1992) J High Res Chromatogr 15:165–170
Hausch M (1990) Dissertation, University of Hohenheim, Germany
Brückner H, Langer M, Lüpke M, Westhauser T, Godel H (1995) J Chromatogr A 697:229–245
Pawlowska M, Armstrong DW (1994) Chirality 6:270–276
Brückner H, Becker D, Lüpke M (1993) Chirality 5:385–392
Brückner H, Justus J, Kirschbaum J (2001) Amino Acids 21:429–433
Ledl F, Schleicher E (1990) Angew Chem Int Ed 29:565–594
Pätzold R, Brückner H (2005) In: Molnar-Perl I (ed) Quantitation of Amino Acids and Amines, Methods and Protocols. Journal of chromatography library, vol 70. Elsevier, Amsterdam, The Netherlands, pp 98–119
Winkler O (1955) Z Lebensm Unters Forsch 102:161–165
Brückner H, Pätzold R (2005) Amino Acids 29:61
Pätzold R, Brückner H (2005) In: Flegel M, Fridkin M, Gilon C, Slaninová J (eds) Proceedings of the 3rd international and 28th European peptide symposium, Kenes International, Geneva, Switzerland, pp 997–998
Bückner H, Kirschbaum J, Pätzold R (2002) In: Benedetti E, Pedone C (eds) Proceedings of the 27th European peptide symposium Edizioni Ziino, Napoli, Italy, pp 54–55
Ali H, Pätzold R, Brückner H (2005) Food Chem (in press), DOI 10.1016/j.foodchem.2005.08.056
Hofman T (1999) Eur Food Res Technol 209:113–121
van Boekel MAJS (2001) Nahrung/Food 65:150–159
Bell LN (1997) Food Chem 59:143–147
Hofman T, Heuberger S (1999) Z Lebensm Unters Forsch A 208:17–26
Heyns K, Paulsen H (1959) Liebigs Ann Chem 622:160–174
Anet EFLJ, Reynolds TM (1957) Aust J Chem 10:182–192
Noguchi M, Sato Y, Nishida K, Ando S, Tamaki E (1971) Agric Biol Chem 35:65–70.
Ciner-Doruk M, Eichner K (1979) Z Lebensm Unters Forsch A 168:9–20.
Cremer DR, Eichner K (2000) Eur Food Res Technol 211:247–251
Heinzler M, Eichner K (1991) Z Lebensm Unters Forsch 192:445–450
Pätzold R, Nieto-Rodgriguez A, Brückner H (2003) Chromatographia Suppl 57:S207–S211
Pätzold R, Brückner H (2005) J Agric Food Chem 53:9722–9729
Author information
Authors and Affiliations
Corresponding author
Additional information
Parts of the results have been presented at 9th International Congress on Amino Acids and Proteins, August 8–12, 2005, Vienna, Austria, and Euro Food Chem XIII, September 21–23, 2005, Hamburg, Germany.
Rights and permissions
About this article
Cite this article
Pätzold, R., Brückner, H. Gas chromatographic detection of d-amino acids in natural and thermally treated bee honeys and studies on the mechanism of their formation as result of the Maillard reaction. Eur Food Res Technol 223, 347–354 (2006). https://doi.org/10.1007/s00217-005-0211-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-0211-y