Abstract
The combined isothermal (10–60 °C) and isobaric (0.1–650 MPa) inactivation kinetics of lipoxygenase (LOX) extracted from tomatoes and reconstituted in a tomato purée were studied. Thermal inactivation of LOX at atmospheric pressure proceeded in the temperature range of 45–65 °C. LOX inactivation did not follow first order kinetics; the data could be fitted assuming that the two isoforms of LOX with different thermostability were present. Combined thermal and high pressure inactivation occurs at pressures in the range of 100–650 MPa combined with temperatures from 10–60 °C, and followed first-order kinetics. In the high-temperature/low-pressure range, (T≥50 °C and P≤300 MPa) an antagonistic effect is observed, therefore, the Arrhenius and Eyring equation cannot be used over the entire temperature and pressure range. Small temperature dependence is found in the low-temperature/high pressure range. A third degree polynomial model was successfully applied to describe the temperature–pressure dependence of the inactivation rate constants, which can be useful to predict inactivation rate constants of tomato LOX reconstituted in tomato purée in the temperature–pressure range studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipoxygenase (LOX, E.C. 1.3.11.12) catalyzes the hydroperoxydation of polyunsaturated fatty acids and esters by the use of molecular oxygen [1, 2]. Multiple forms or isozymes exist in many plant tissues showing differences in pH optimum, pI, and enzymic properties. Although the functional role of plant LOX is still largely unknown, metabolites of unsaturated fatty acids have been implicated in growth and development, plant senescence, and response to diseases and wounding [3]. Furthemore, in fruits and other plant food products, LOX plays a role in the formation of volatile flavor compounds [4, 5]. Tissue disruption results in the degradation of endogenous lipids to fatty acids, then they are oxidized by LOX to yield hydroperoxides, followed by selective lyase cleavage to form hexanal and hexenal from the 13-hydroperoxides of linoleic and linolenic acids, respectively. Conversion of these C6 aldehydes to the corresponding alcohols may occur through alcohol dehydrogenase [6]. The compounds formed are considered to be the major volatile compounds that contribute to the “fresh” flavor of tomatoes [7]. However, addition of these compounds above the recomended ranges results in the development of rancid off-flavours [1]. Therefore, it is interesting to investigate LOX stability in tomato based products during processing conditions.
Together with color and texture, flavor is one of the most important quality attributes of fresh tomato and tomato products (juices, purées or pastes) that can be affected after a conventional thermal treatment [8]. New nonthermal preservation technologies such as high hydrostatic pressure can be integrated in concepts of “minimal processing”, that is, the application of preservation technologies designed to retain the natural and as-fresh properties of foods [9]. In particular, the hurdle technology aims to this end by combining preservation treatments (e.g., high pressure and thermal treatment) at lower intensities that would have additive or synergistic effects while minimizing their impact on the sensory and nutritive properties of food [10]. High hydrostatic pressure is being applied on an industrial scale for some products commercialized in United States, Japan or Europe [11]. One of the main advantages of this technology is that, in contrast to high temperature processing, it shows a higher possibility to maintain the fresh quality attributes of foods because it slightly affects covalent bonds. Therefore, degradation processes such as Maillard reaction, off-flavor formation, and vitamin destruction occur at a slower rate during pressurization. Hence, there is an improved preservation of the nutritional and sensorial quality of processed products [8].
In the present work, a detailed kinetic study on the combined thermal and high pressure inactivation of tomato LOX reconstituted in tomato purée has been performed. Previous experimental results showed the instability of LOX in frozen tomatoes, so that, extraction of tomato LOX followed by its reconstitution in a tomato purée was necessary to accomplish the inactivation studies.
Materials and methods
Tomato LOX Extraction
Red tomatoes (Lycopersicom esculentum, cv Malpica), used in the tomato processing industry in Spain, were obtained from a wholesale market. Tomatoes were washed and cut and LOX was extracted as described by Bonnet and Crouzet [12] with slight modifications. Tomatoes were homogenized in 1:1 w/v with 0.5 mol/l Tris-HCl (pH 8.0) containing 1% (w/v) ascorbic acid, 1% (w/v) EDTA, and 0.001 mol/l phenylmethylsulfonyl fluoride (PMSF). The homogenate was filtered through a cheese cloth and centrifuged at 30,000×g for 10 min at 4 °C. The supernatant was brought to 0.020 mol/l CaCl2 by the addition of a 1 mol/l stock solution and after stirring for 2 h at 4 °C, the suspension was centrifuged at 30,000×g for 20 min at 4 °C. Further, protein precipitation was obtained by the addition of solid ammonium sulfate to 35% saturation, stirred for 1 h, and centrifuged at 12,000×g for 15 min at 4 °C. After the pellet was discarded, the (NH4)2SO4 concentration was increased to 60% saturation, stirred for 1 h again, and centrifuged at the same conditions. The pellet was resuspended in 0.01 mol/l MOPS-KOH at pH 6.8 and dialyzed overnight (MWCO 12–14 kDa, Medicell International Ltd. London, UK) against the same buffer. The dialyzed fraction was clarified by centrifuging at 10,000×g for 10 min at 4 °C. Crude fractions were frozen and kept at −40 °C until use.
Tomato purée preparation and reconstitution
Tomatoes were washed, cut, and homogenized in a blender. The resulting homogenate was filtered through a cheese cloth to remove peels and seeds and heat treated (90 °C, 10 min) to inactivate endogenous enzymes. The juice with an initial solid content of 4 Brix was concentrated at 70 °C by a rotary evaporator (Büchi RE111, Flawil, Switzerland) until 21.7 Brix. The purée was stored at −40 °C until use in reconstitution studies.
Tomato purée and LOX were thawed and mixed in a proportion to reach a final LOX activity of 0.35 units per 300 μl sample. Stability of the mixture was checked for 3 h.
Thermal treatments
Thermal inactivation experiments were performed in a thermostatic water bath at constant temperature. To ensure direct heating and cooling, reconstituted purée was filled manually with a syringe, in capillary tubes (Blaubrand-IntraMark, Brand GMBH+COKG, Germany; inner diameter=1.6 mm, length=14 cm). After filling, the tubes were placed in the water bath and the heating time was exactly measured. Immediately after withdrawal from the hot-water bath, the samples were placed in an ice-water bath and activity was measured within 1 h time. Treatment temperatures and times ranged from 45–65 °C and 0–30 min, respectively.
Combined thermal and high pressure treatments
Combined thermal and high pressure inactivation treatments were performed in a laboratory pilot scale, multivessel high-pressure equipment (HPIU-10000, 95/1994, Resato, Roden, The Netherlands). High pressure is generated using a pressure intensifier in the central pressure circuit. Eight individual vessels (volume=8 ml, diameter=10 mm, length=100 mm), each one surrounded by a thermostatic mantle linked to a cryostat, are connected to the central pressure circuit using T-joints and valves. The pressure transferring liquid is a glycol/oil mixture (TR15, Resato, Roden, The Netherlands).
This equipment allows performance of kinetic experiments because eight samples can be submitted simultaneously to treatments at the same pressure and temperature but for different preset treatment times. After the samples are enclosed in the thermostated vessels (filled in 0.4 ml flexible microcentrifuge tubes, BIOplastics, The Netherlands), only the central valve is closed and pressure is built up slowly (100 MPa/min) to control adiabatic heating, until the desired pressure level is reached. Once the pressure is reached, the individual vessels are isolated from the central pressure tubing and the central valve is opened. As a function of time, pressure can now be released in the individual vessels. To exclude the variable pressure–temperature conditions that arose from pressure building up and accompanying adiabatic heating, the blank was taken after a time where the pressure and temperature remained constant (2 min). Again, the samples were stored in an ice--water bath and activity was measured within 1 h time. Treatment pressures ranged between 100 and 650 MPa, treatment temperatures between 10–60 °C, and treatment times between 0 and 58 min.
Activity measurement
Following thermal and high pressure treatments, samples were prepared for LOX activity assay by mixing 300 μl of the treated sample with 120 μl of 0.5 mol/l sodium phosphate buffer at pH 6.5 and 0.5% Triton X-100 in a 1.5 ml microcentrifuge tube. Samples were centrifuged for 10 min at 10,000×g, 4 °C and the pellet was discarded [13].
The activity of LOX was measured by the formation of conjugated dienes from linoleic acid, using sodium linoleate as substrate [14]. The substrate was prepared as follows: 70 mg Tween 20 and 70 mg of linoleic acid were added to 4 ml of oxygen-free water. The solution was mixed with a Pasteur pipette and 0.6 ml of NaOH 1 mol/l was added to clear the solution. Finally, the solution was made up with demineralized water to a total volume of 25 ml, flushed with nitrogen and kept frozen (−80 °C) until use [15].
The activity measurement was carried out at 25 °C in a quartz cuvette. The assay mixture contained 2.7 ml of 0.2 mol/l sodium phosphate buffer at pH 6.5, 30 μl of the sodium linoleate substrate, and 100 μl of the enzyme extract. The absorption at 234 nm (Ultrospec 2100 pro, Amersham Biosciences, Uppsala, Sweden) was recorded as a function of time, for 4–5 min, and the activity was determined from the slope of the linear portion of the curve. One unit of enzyme is defined as the amount of enzyme producing one unit change in absorbance per minute at 25 °C.
Data analysis
The way in which the enzyme inactivation progresses as a function of time is expressed by the mathematical form of the kinetic model (for instance Eqs. (1)–(3)) and the rate of inactivation (k) is reflected by the numerical values of the kinetic parameter estimates [16].
Enzyme inactivation can often be described by a first-order reaction (Eq. (1)) [17].
where A is the enzyme activity at time t, A 0 is the initial enzyme activity and k is the inactivation rate constant.
A fractional-conversion model (Eq. (2)) is a special case of a first-order model that takes into account a constant non-zero activity (A ∞) after prolonged heating and/or pressurizing. The model applies when the enzyme sample contains a stable fraction that is not affected under the processing conditions studied
A biphasic kinetic model (Eq. (3)) represents the presence of two enzyme fractions, with different process stability, representing the labile (L) and the stable (S), one and both are inactivated according to first-order decay
The temperature dependence of the inactivation rate constant at constant pressure can be expressed in terms of the activation energy (E a) and estimated using the linearized Arrhenius equation (Eq. (4))
where E a is the activation energy (kJ/mol), k is the inactivation rate constant, k ref is the inactivation rate constant at a reference temperature, R T is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature (K), and T ref is the absolute reference temperature (K).
The pressure dependence of the enzyme inactivation rate constant at a constant temperature can be expressed in terms of the activation volume (V a), calculated using the linearized Eyring equation (Eq. (5))
where V a is the activation volume (cm3/mol), k is the inactivation rate constant, k ref is the inactivation rate constant at reference pressure, R p is the universal gas constant (8.314 J mol−1 K−1), P is the pressure (MPa), P ref is the reference pressure (MPa), T is the absolute temperature (K).
The estimation of the kinetic parameters can be done by a one-step (global) approach or by a two-step regression method. In a global approach, one-step method is considering the inactivation data obtained at different inactivation temperatures or pressures simultaneously. The Arrhenius or Eyring equations are incorporated in the inactivation rate equation and by a nonlinear regression analysis, the inactivation rate constant at reference conditions and temperature or pressure dependence coefficient are estimated. By the two-step regression method, first the inactivation rate constants are obtained from the experimental data by linear or nonlinear regression (temperature by temperature or pressure by pressure) and in a second step, the temperature or pressure coefficients are calculated from the regression analysis of the obtained inactivation rate constants as a function of temperature or pressure. Cohen et al. [18] and Haralampu et al. [19] suggested that the two-step regression approach gives the least accurate estimates probably because it estimates too many intermediate values and does not gain strength in the regression by considering the data set as a whole, but on the other hand, it will give the best fit at each temperature or pressure.
Results and discussion
Thermal inactivation of reconstituted LOX in tomato purée
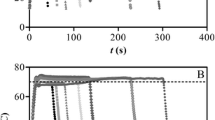
The data analysis of thermal inactivation of reconstituted LOX in tomato purée was done both by a global and two-step approach. As far as the global approach is concerned, experimental data for the temperature range of 45–60 °C were fitted to a fractional conversion model (Eq. (2)) showing the existence of a first-order inactivating thermolabile fraction and the occurrence of a thermostable fraction (Fig. 1). The parameter estimates indicate that at a reference temperature of 55 °C, LOX was inactivated at a rate of (0.4511±0.0203) min−1 and 14.33% of the LOX activity remained stable. The dependence of the rate constant with temperature (E a) was (189.90±5.11) kJ/mol.
As for the two-step approach, inactivation rate constants and A ∞ obtained by fitting experimental data of temperatures from 45 to 60 °C to a fractional conversion model (Eq. (2)) are shown in Table 1. For temperatures above 60 °C, the inactivation of reconstituted tomato LOX in tomato purée showed a biphasic behavior (Fig. 1), meaning that the enzyme fraction which was stable at lower temperatures (≈14%), is now being inactivated at a certain rate. Isothermal inactivation data for 62.5 and 65 °C were fitted by a nonlinear regression method to Eq. (3). Table 2 shows the kinetic parameters (k L and k S, A L and A S) for the different temperatures. As expected, for all temperatures studied, the inactivation rate constants increase with the treatment temperature. By plotting the logarithm of the rate constant versus the reciprocal of absolute temperature in the temperature range of 45–60 °C, a straight line with a good correlation coefficient (0.9952) was obtained. The temperature dependence of the inactivation rate constant, expressed in terms of activation energy (E a), was predicted using the Arrhenius Eq. (4) and yielded (191.44±6.64) kJ/mol. The value of E a obtained by the two-step approach ((191.44±6.64) kJ/mol), is in the range with the one obtained by the global approach ((189.90±5.11) kJ/mol), so that although the two-step method was supposed to give the least accurate estimates, no differences were found in the way the both methods describe the thermal dependence of the inactivation rate constants in the temperature range of 45–60 °C.
Likewise, thermal inactivation of LOX from many different sources such as green peas, green beans, potatoes, asparagus, wheat germ, germinated barley, broccoli, and tomato could be described either by a simple first-order model or by a two fraction first-order model (biphasic) [20–27]. A wide range of E a values can be found in the literature (65–655 kJ/mol) depending on the source of LOX and temperature range studied (40–100 °C) [28]. As far as tomatoes is concerned, Anthon et al. [13] studied LOX inactivation from tomato juice (without reconstitution). They described the inactivation kinetics by assuming three different isoforms that were inactivated following the firstorder kinetics. The rate constants obtained were more temperature dependent (308, 336 and 349 kJ/mol for the three different LOX fractions obtained) than the ones obtained in our study.
Combined thermal and high pressure treatment of reconstituted LOX in tomato purée
Combined thermal and high pressure inactivation of reconstituted tomato LOX in tomato purée was conducted for different combinations of moderate temperatures (10–60 °C) and pressures (100–650 MPa). First-order kinetics was assumed to analyze inactivation data under these conditions (Fig. 2). Experimental data were fitted to Eq. (1) via linear regression yielding regression coefficients between 0.95 and 0.99. Kinetic constants obtained are shown in Table 3. From the inactivation rate constants, an antagonistic effect at combinations of high temperature (T≥50 °C) and low pressure (P<300 MPa) can be observed. In this region, a pressure increase at constant temperature causes the inactivation rate constant to decrease. An antagonistic effect of pressure and temperature is frequently encountered for enzyme denaturation. This effect is mostly limited to pressures lower than 300 MPa [29–35]. Opposite effects of pressure and temperature with respect to hydrophobic interactions and hydrogen bonds could be the cause of this effect. It is generally accepted that hydrogen bonds are destabilized by elevated temperatures while pressure often stabilizes them [30]. On the other hand, endothermic hydrophobic interactions are enhanced at elevated temperatures whereas pressure weakens them [29].
Combined thermal-high pressure inactivation of tomato LOX reconstituted in tomato purée at different temperatures and pressures 300 (o), 350 (◆), 400 (□), 450 (▲), 500 (×), 550 (▪), 600 (Δ), and 650 MPa (•) fitted to first-order model (Eq. (1))
Low inactivation rate constants are observed in the temperature range of 20–30 °C combined with high pressures showing LOX to exhibit maximal pressure stability at these treatment conditions. Ludikhuyze et al. [28] also found this behavior by treating soybean LOX at low temperatures combined with high pressure.
Pressure dependence of inactivation rate constants at elevated temperatures (T≥50 °C) did not obey the Eyring equation (Eq. (5)) due to the above mentioned antagonistic effect. However, when considering the pressure range 300–650 MPa, the pressure dependence of the inactivation rate constant is described accurately by the Eyring equation and activation volumes are reported in Table 4. In this pressure range, at all temperatures studied the activation volume is negative, hence as stated by Le Chatelier principle, LOX inactivation is favored by an increase in pressure. Furthermore, activation volumes seem rather constant up to temperatures of 50 °C, but they decrease slightly above 50 °C, meaning that the inactivation rate constants are less sensitive to pressure at high temperatures. Similar results have been reported by other authors with soybean LOX [28].
Modeling of combined heat and high-pressure dependence of inactivation rate constants
Mathematical models based on different equations, Arrhenius [32, 33] or Eyring [36], have been formulated to describe the pressure–temperature dependence of inactivation rate constants for enzymes.
However, in the present study an empirical third-degree polynomial equation, based on a thermodynamic kinetic model governing the behavior of the system during pressure and temperature change [35], was applied
Experimental rate constants were fitted to Eq. (6). Significance of the different terms was analyzed (p≤0.05) by multilinear analysis (Proc REG, SAS). Parameter values were estimated using 40 °C and 500 MPa as reference temperature and pressure (Table 5), respectively. Dependence of inactivation rate constant with pressure and temperature is described with Eq. (7)
By using Eq. (7), pressure–temperature combinations resulting in specific preset inactivation rate constants for reconstituted tomato LOX in tomato purée were simulated and depicted in isorate contour plots (Fig. 3). The antagonistic effect of pressure and temperature, previously discussed can be observed in the domain of temperatures higher than 45 °C and pressures lower than 300 MPa. The low temperature dependency of the rate constants at high pressures can also be confirmed.
Correlation between the natural logarithm of the experimental k values and the ones predicted according to the model (Eq. (7)) for pressure–temperature inactivation of tomato LOX reconstituted in a tomato purée
To assess predictions made by the developed secondary model the Accuracy factor (Af) [37] was compared
where n is the number of observations. The predicted/observed ratio refers to the relationship between the natural logarithm of the rate constant predicted by the model in certain process conditions and the one observed experimentally. It yielded a value of 1.088, indicating an error of 8.8% for the predictions. No autocorrelation was found by plotting the residuals versus temperature, pressure, experimental k or predicted k values (data not shown). Neither were the observed deviations from the bisector, when plotting the predicted k values versus the experimental ones (Fig. 4), indicated that no heteroscedasticity problems were detected. Table 6 shows correlation between the model parameters, there being only one combination whose absolute value is higher than 0.7.
Thermal inactivation data of reconstituted tomato LOX in tomato purée at atmospheric pressure could be fitted assuming that the two isoforms of LOX with different thermostability were present. A thermolabile fraction in a proportion of around 86% that begins to inactivate at 45 °C and is completely inactivated after 30 min at 52.5 °C, and a thermostable fraction that begins to inactivate at temperatures above 60 °C. Estimates of E a obtained by two different regression approaches (global and two step) are in the same range. Combined thermal and high pressure treatments follow first-order kinetics. An antagonistic effect of temperature and pressure, at pressures lower than 300 MPa and temperatures higher than 50 °C was observed. A mathematical model was suggested to describe the combined heat and high pressure dependence of inactivation rate constants of reconstituted tomato LOX in tomato purée. The model can be useful in identifying processing conditions to inactivate LOX in tomato-based products in the temperature–pressure range studied.
References
Eskin MNA, Grossman S, Pinsky A (1977) Crit Rev Food Sci Nutr 4:1–40
Galliard T, Chan HWS (1980) In: Stumpf PK (ed) The biochemistry of plants, lipids: structure and function, Vol 4. Academic Press, New York, pp 131–161
Yilmaz E (2001) Turk J Agric For 25:291–296
Galliard T, Matthew JA (1977b) Phytochemistry 16:339–343
Hatanaka A, Kajiwara T, Matsui K (1995) Z Naturforsch 50c:467–475
Galliard T, Matthew JA, Wright AJ, Fishwick MJ (1977a) J Sci Food Agric 28:863–868
Kazeniac SJ, Hall RM (1970) J Food Sci 35:519–530
Krebbers B, Matser AM, Hoogerwerf SW, Moezelaar R, Tomassen MMM, van den Berg RW (2003) Innov Food Sci Emerg Tech 4:377–385
Leistner L (2000) Int J Food Micro 55:181–186
Manvell C (1997) Food Sci Technol Today 11:107–111
Heremans K (2000) In: Hayashi R (ed) Trend in high pressure bioscience and biotechnology. Elsevier Science, Amsterdam pp 1–6.
Bonnet JL, Crouzet J (1977) J Food Sci 42:625–628
Anthon GE, Barret DM (2003) Food Chem 81:275–279
Axelrod B, Cheesebrough TM, Laakso S (1981) Methods Enzym 71:441–451
Koch E, Meier BM, Eiben HG, Slusarenko A (1992) Plant Physiol 99:571–576
Van Loey A, Indrawati, Smout C, Hendrickx M (2003) In: Whitaker JR, Voragen GJ, Wong DWS (eds), Handbook of enzymology. Marcel Dekker, New York, pp 49–59.
Whitaker JR (1972) Principles of enzymology for the food sciences. In: Principles of enzymology for the food sciences. Marcel Dekker, New York, pp 159–163.
Cohen E, Saguy I (1985) J Food Proc Preserv 9:273–290
Haralampu SG, Saguy I, Karel M (1985) J Food Proc Preserv 9:129–143
Svensson SG, Eriksson CE (1972) Lebensm Wiss Technol 5:118–123
Park KH, Kim YM, Lee CW (1988) J Agric Food Chem 36:1012–1015
Ganthavorn C, Nagel CW, Powers JR (1991) J Food Sci 56:47–49
Zhang Q, Cavalieri RP, Powers JR, Wu J (1991) J Food Sci 56:719–721
Bhirud PR, Sosulski FW (1993) J Food Sci 58:1095–1098
Günes B, Bayindirli A (1993) Lebensm-Wiss Technol 26:406–410
Hugues M, Bovin P, Gauillard F, Nicolas J, Thiry JM, Richard-Forget F (1994) J Food Sci 59:885–889
Morales-Blancas EF, Chandia VE, Cisneros-Zevallos L (2002) J Food Sci 67:146–154
Ludikhuyze L, Indrawati, Van den Broeck I, Weemaes C, Hendrickx M (1998a) J Agric Food Chem 46:4074–4080
Balny C, Masson P (1993) Food Rev Int 9:611–628
Heremans K (1993) The behaviour of proteins under pressure In: Winter R (ed) High-pressure chemistry, biochemistry and materials science, Jonas J, Dordrecht , pp 443–469
Mozhaev VV, Lange R, Kudryashova EV, Balny C (1996) Biotechnol Bioeng 52:320–331
Weemaes CA, Ludikhuyze LR, Van den Broeck I, Hendrickx ME (1998) Biotechnol Bioeng 60:292–300
Van den Broeck I, Ludikhuyze LR, Van Loey AM, Hendrickx ME (2000) J Agric Food Chem 48:1960–1970
Indrawati, Van Loey AM, Ludikhuyze LR, Hendrickx ME (2000) Biotechnol Prog 16:109–115
Ly-Nguyen B, Van Loey AM, Smout C, Eren Özcan S, Fachin D, Verlent I,Vu Truong S, Duvetter T, Hendrickx ME (2003) J Food Sci 68:1377–1383
Ludikhuyze L, Indrawati, Van den Broeck I, Weemaes C, Hendrickx M (1998b) J Agric Food Chem 46:4081–4086
Ross T (1996) J Appl Bacteriol 81:501–508
ACKNOWLEDGEMENTS
This research was supported by the Research Council, KULeuven. D. Rodrigo holds a postdoctoral fellowship from Spanish Ministry of Science and Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigo, D., Jolie, R., Van Loey, A. et al. Combined thermal and high pressure inactivation kinetics of tomato lipoxygenase. Eur Food Res Technol 222, 636–642 (2006). https://doi.org/10.1007/s00217-005-0128-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-0128-5