Abstract
Continuous-flow microwave pasteurization provides important advantages over conventional heat exchangers such as fast volumetric heating, lower tube surface temperature, and possible non-thermal effects that enhance enzymatic and bacterial inactivation. Conventional and microwave-assisted inactivation of pectin methylesterase (PME), polyphenol oxidase (PPO), and peroxidase (POD) in cloudy apple juice were investigated to evaluate non-thermal effects. Experiments were conducted to provide uniform heating with accurate temperature acquisition and similar temperature profiles for conventional and microwave treatments. A two-fraction first-order kinetic model was successfully fitted to the data in a procedure that took into account the whole time-temperature profile instead of assuming isothermal conditions. Predicted inactivation curves for pasteurization at 70 and 80 °C of the cloudy apple juice showed that PME has the highest thermal resistance (residual activity of 30% after 250 s at 80 °C) and that there was no evidence of non-thermal microwave effects on the inactivation of these enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food pasteurization is aimed to provide rapid and uniform heating ensuring safety and quality of the processed product (Hebbar and Rastogi 2012). However, high temperatures can induce undesired chemical and physical changes that impair fresh-like characteristics demanded by consumers (Rawson et al. 2011). From these present and growing demands, food companies are increasingly interested in implementing technologies alternative to conventional thermal processes. In the case of fruit juices, the high-pressure technology (HPP), for example, is already in use to deliver products with higher quality than those processed using heat exchangers (Huang et al. 2017). However, continuous-flow microwave pasteurization still holds potential in high-temperature short-time processing of fluid foods, since the fast volumetric heating and lower tube surface temperature can reduce the loss of product quality during the heating step (Tang 2015). Moreover, specific non-thermal effects of microwave are reported to enhance the inactivation rate of microorganisms and enzymes (Guo et al. 2017).
The ability of microwave radiation to induce various types of molecular alterations, such as denaturation/refolding of proteins, and also changes in cell morphology, is a current subject of study (Shazman et al. 2007; Shamis et al. 2012; Lopes et al. 2015). Tajchakavit and Ramaswamy (1997), for example, reported that inactivation of pectin methylesterase in orange juice subjected to microwave heating was significantly more effective when compared to conventional thermal heating. Another study reported that the apple juice inoculated with Lactobacillus plantarum and Saccharomyces cerevisiae under microwave heating resulted in a lower D-value when compared to conventional heating (Tajchakavit et al. 1998). Matsui et al. (2008) showed that enzymes of green coconut water under microwave heating presented lower thermal resistance than under conventional heating. Rougier et al. (2014) presented evidences that microwave radiation can induce non-thermal alterations on cell membrane integrity of Escherichia coli. Siguemoto et al. (2018) presented results of inactivation of Escherichia coli O157:H7 and Listeria monocytogenes in apple juice under microwave heating that were mostly more efficient than predicted for conventional heating under the same conditions.
However, other studies did not detect the occurrence of enhanced effects of microwaves, e.g., polyphenol oxidase and peroxidase inactivation of red beet samples under microwave treatment at 250 W (Latorre et al. 2012), microbial stability changes during the storage of tomato juice pasteurized by continuous-flow microwave system (Stratakos et al. 2016), lipase and lipoxygenase inactivation of wheat germ by single mode focused microwave reactor (Xu et al. 2016), or peroxidase inactivation of tomato puree by a semi-industrial continuous microwave heating under low power/long time (390 to 770 W, 460 to 848 s) (Arjmandi et al. 2017).
In order to evaluate the microwave effects on biological matrices, the authors emphasize the importance of using a microwave system that provides accurate and reproducible time-temperature profiles that are similar or equivalent to those obtained with conventional heating (Hamoud-Agha et al. 2013). However, difficulties in obtaining accurate time-temperature profiles using a domestic microwave oven were reported by Tajchakavit et al. (1998) and Benlloch-Tinoco et al. (2014) considering the inhomogeneous microwave field distribution in the cavity.
Based on these considerations, the purposes of the present study were (1) to study the inactivation kinetics of pectin methylesterase (PME), peroxidase (POD), and polyphenol oxidase (PPO) in cloudy apple juice using conventional and microwave heating with accurate acquisition of the time-temperature history and (2) to compare the effectiveness of microwave and conventional heating on inactivation of these enzymes in cloudy apple juice in search for evidence of microwave enhanced effects.
Material and Methods
Sample Preparation
Apples (Malus domestica Borkh, cv Fuji Suprema) were obtained from an orchard in the state of Santa Catarina, Brazil. The selected fruits were cut in halves and pressed with a household juice extractor SS-1080 (Fun Kitchen, USA). An ascorbic acid solution (0.2 g/L) was mixed to retard browning. The time between extraction and thermal treatment was less than 30 min.
Physicochemical characterization of the juice was carried out. The results are means of three replicates ± standard deviation: pH = 3.86 ± 0.01 using a pH-Stat PHM-290 (Radiometer, Copenhagen, Denmark), soluble solids content = 10.9 ± 0.1 °Brix at 20 °C (711,849 refractometer, Carl Zeiss Jena, Jena, Germany), and titratable acidity = 0.27 ± 0.01 mg malic acid/100 mL (AOAC 2010).
Enzymatic Activity Assessment
Pectin Methylesterase Activity
The pectin methylesterase (PME) activity was determined titrimetrically according to Rouse and Atkins (1953) by the amount of NaOH solution (Synth, Diadema, Brazil) required to maintain pH = 7.5 at 30 °C for 15 min using a pH-stat PHM290 (Radiometer, Copenhagen, Denmark) with an automatic titration ABU901 (Radiometer, Copenhagen, Denmark). A 1% apple pectin solution (70–75% degree of methylation, Sigma Aldrich, Gallen, Switzerland) was prepared daily and adjusted to pH 7.5 with NaOH solution at 30 °C. A 2.0 mL sample of cloudy apple juice was added to 25 mL of the 1% apple pectin solution containing 0.15 M NaCl (Synth, Diadema, Brazil). The mixture was titrated with NaOH solution to reach pH = 7.5, and the consumption of NaOH solution was obtained after 15 min. The PME activity was analyzed in duplicate for each processing condition. One unit of PME activity was expressed as pectin methylesterase unit (PEU) according to Eq. (1), where V was the consumed volume of NaOH (mL), N was the NaOH solution concentration (mol/L), V′ was the volume of the sample (2.0 mL), and t r was the reaction time (15 min):
Polyphenol Oxidase Activity
The protocol for determining the polyphenol oxidase (PPO) activity was carried out according to Siguemoto and Gut (2017) by measuring the increase in absorbance at 420 nm of the reaction with pyrocatechin (Sigma Aldrich, St Louis, USA). The apple juice was centrifuged at 2000 rpm for 15 min at room temperature (Tominaga Works, Tokyo, Japan), and the supernatants were analyzed immediately. Volumes of 100 μL of 50 mM phosphate buffer pH 6.5 (35 mM KH2PO4 and 15 mM Na2HPO4·7H2O) and 33 μL of the apple juice supernatant were pipetted and added to a microplate well (Greiner Bio-one, Frickenhausen, Germany). The mixture was then incubated at 25 °C for 1 min. A freshly prepared solution of pyrocatechin (50 mM, 67 μL) was added, and the absorbance was monitored every 10 s using a SpectraMax plus 384 spectrophotometer (Molecular Devices, Sunnyvale, USA) for 3 min. The reference value (0.000 absorbance) was determined using a blank solution containing phosphate buffer and pyrocatechin. The reaction curves obtained (absorbance versus time) were linear, and the slope was taken as the PPO activity. One unit of enzymatic activity (U) was defined as absorbance increase of 0.001 per minute under the assay conditions. The PPO activity was analyzed in quadruplicate for each processing condition.
Peroxidase Activity
The peroxidase (POD) activity was determined by a colorimetric method based on the change of absorbance at 405 nm as a result of ABTS oxidation (Siguemoto and Gut 2017). The apple juice was centrifuged at 2000 rpm for 15 min at room temperature (Tominaga Works, Tokyo, Japan) and the supernatants were analyzed immediately. In a microplate well (Greiner Bio-one, Frickenhausen, Germany), 125 μL of 67 mM phosphate buffer pH 6.0 (59 mM KH2PO4 and 8 mM Na2HPO4·7H2O) and 42 μL of apple juice supernatant were added. The mixture was incubated at 25 °C for 1 min and then 17 μL of 1.7 mM ABTS solution (Sigma Aldrich, St Louis, USA) and 17 μL of 0.8 mM hydrogen peroxide solution (Lafan, Várzea Paulista, Brazil) were added. Absorbance at 405 nm was monitored every 20 s for 5 min using a SpectraMax plus 384 spectrophotometer (Molecular Devices, Sunnyvale, USA). The reference value for absorbance was set using the phosphate buffer as the sample for the assay (blank). The increase in absorbance at 405 nm during the reaction time showed a linear trend under the assay conditions. One unit of enzymatic activity (U) was defined as an absorbance increase of 0.001 per minute. The POD activity was analyzed in quadruplicate for each processing condition.
Conventional and Microwave Treatments
Samples of the cloudy apple juice were treated using conventional heating (hot water bath) and microwave heating (focused microwave reactor) for comparison on enzymatic inactivation. In order to make a fair comparison between treatments, some care had to be taken:
-
1.
The time-temperature histories of the samples should be similar between treatments. This was accomplished by adjusting the microwave power incidence during heating and holding steps and using the same procedure for cooling.
-
2.
The temperature in the sample must be uniform to provide a homogeneous treatment. This was accomplished using small volumes under stirring or agitation.
-
3.
The whole recorded time-temperature history of the samples should be taken into account instead of assuming isothermal conditions. This was accomplished by including the lethality calculation in the parameter estimation problem.
-
4.
The temperature acquisition must be made using a small time step and a sensor with fast response and negligible heat capacity in order to get reliable temperature data. This was accomplished by using a thin fiber-optic probe inserted in the sample.
Conventional Treatment
For the conventional treatment of the cloudy apple juice, 3 mL samples were placed in polyethylene pouch (35 mm width, 150 mm height, and 1.2 mm thickness). Because of the low thickness of the material, it offers reduced thermal resistance to heat transfer (Aguiar et al. 2012). The pouch also flattens the sample, shortening the path from surface to center. To improve temperature uniformity, pouches were agitated during immersion in hot water bath.
A Fluoroptic STF-1M temperature probe (LumaSense Technologies, Santa Clara, USA) was placed with the tip at the center of the sample. This fiber-optic sensor has a response time of only 0.25 s, which is important to study rapid temperature changes. The conventional heat treatment was accomplished by immersion and agitation of the pouch in a thermostatic water bath MA-184 (Marconi, Piracicaba, Brazil), followed by immersion in an ice water bath, until the temperature reached 10 °C, as proposed by Aguiar et al. (2012). The temperature data was recorded every 0.5 s using a fiber-optic thermometer Luxtron 812 (LumaSense Technologies, Santa Clara, USA). Different combinations of bath temperatures (50, 60, 70, 80, and 90 °C) and immersion times (20, 40, 60, 90, 120, 210, and 270 s) were tested to obtain residual activities between 95 and 5%. Treated samples were stored in a plasma freezer 349FV (Fanem, São Paulo, Brazil) at − 30 °C and thawed for enzymatic activity assessment.
Microwave Treatment
Microwave heating was conducted using a Discover Reflux microwave reactor (CEM Corporation, Charlotte, USA). This reactor has a cylindrical cavity where a cylindrical flask containing the sample is placed at its center for heating at 2450 MHz with maximum power output of 300 W. Microwave heating can be conducted with a fixed power output or with controlled temperature. In this case, an infrared sensor reads the temperature at the bottom of the flask and the controller manipulates the power incidence on the sample to reach the temperature set-point. A solenoid valve automatically releases air jets over the surface of the flask to assist on temperature control. Magnetic stirring helps to provide a uniform temperature distribution in the liquid.
The juice sample (7 mL) was placed in a borosilicate glass test tube (16 mm inner diameter and 200 mm length). Since glass is transparent to microwaves, the sample directly absorbs the transmitted radiation. Firstly, microwave heating started with a fixed power setting to obtain a temperature increase rate similar to the one obtained with conventional heating. When the temperature of the sample was close to the desired value, the reactor program was changed from “fixed power” to “controlled temperature,” in which power incidence only compensates heat losses to keep the holding temperature constant.
After holding time was complete, the sample was quickly moved to an ice water bath until a temperature of 10 °C was reached. The time-temperature profile was recorded with the same probe and thermometer used in conventional processing. A glass stick kept the probe tip at the center of the sample while magnet stirring provided mixing.
The treated apple juice samples were immediately stored in a plasma freezer 349FV (Fanem, São Paulo, Brazil) at − 30 °C and thawed for enzymatic activities analysis. The same combinations of holding temperatures (50, 60, 70, 80, and 90 °C) and exposure times (20, 40, 60, 90, 120, 210, and 270 s) used in conventional treatment were tested.
Inactivation Kinetic Model
For the mathematical modeling of the inactivation, the enzyme activity was normalized as in Eq. (2), where AR is the residual activity, A is the activity after, and A0 is the activity before treatment.
The enzymatic inactivation was assumed to follow first-order kinetics with the possible presence of two enzymatic fractions (isoenzymes) with different heat resistances (Matsui et al. 2008; Aguiar et al. 2012; Falguera et al. 2013). This model (Eqs. 3, 4a, and 4b) has five parameters, where α represents the initial activity contribution from the thermolabile fraction while (1 − α) represents the contribution from the thermostable fraction, D1, Tref and D2, Tref are the D-values of the isoenzymes at the reference temperature (time for decimal reduction on AR), and z1 and z2 are the corresponding z-values (temperature increase for a decimal reduction on the D-values). In these equations, F T is the duration of the thermal treatment at a constant and uniform temperature T and alog() is the antilogarithmic function.
For a non-isothermal treatment with a temperature history T(t), the equivalent processing time at the reference temperature (T ref ) or integrated lethality (F) can be calculated from Eq.(5a) for the thermolabile fraction and from Eq.(5b) for the thermostable fraction.
By combining Eqs. (3), (4a), (4b), (5a), and (5b), the model for first-order inactivation with two isoenzymes and variable temperature can be expressed as
where the model parameters are α, D1, Tref, z1, D2, Tref, and z2. For the case of a single enzymatic fraction, parameter α is set to 1.0 and the model parameters are reduced to D1, Tref and z1.
Model Fitting and Statistical Analyses
The two-fraction first-order inactivation model in Eq.(6) was fitted to the experimental results of residual enzymatic activity. The two integrals in Eq.(6) were numerically solved by the trapezoidal method using the recorded temperature history of each experiment. The sum of squared errors for the prediction of residual activity in Eq.(7) was minimized using the nonlinear generalized reduced gradient algorithm in Excel 2016 Solver (Microsoft, Redmond, USA) (Aguiar et al. 2012), where n is the total number of tests:
Various initial guesses of the model parameters were tested to search for local optima in this nonlinear optimization problem. Initially, the problem was solved considering a single enzymatic fraction (α = 1.0), then all five parameters were optimized to verify if a significant improvement was obtained in the model prediction.
The results were evaluated by the minimized SSE, the coefficient of determination R2, the number of parameters (2 or 5), and the error dispersion in a parity chart of predicted versus experimental values of AR.
Results and Discussion
Time-Temperature Profiles and Residual Activities
Figure 1 presents time-temperature profiles recorded during conventional and microwave treatments of cloudy apple juice at 70 °C. Similar profiles were obtained for treatments at 50, 60, 80, and 90 °C. This figure allows verifying that similar rates of heating and cooling could be achieved. Nevertheless, the whole recorded temperature profile T(t) was used in the calculations through Eq.(6) instead of assuming isothermal conditions.
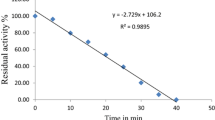
Figure 2 presents the residual enzymatic activity of the three enzymes as a function of the exposure time (hot water bath or microwave heating) to illustrate the inactivation trends. The 90 °C curves for PPO and POD were omitted because residual activity was under the detection limits.
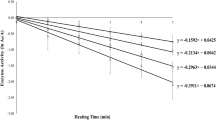
Clearly, PME was more thermal-resistant when compared to PPO and POD in the cloudy apple juice. PME was not fully inactivated after 270 s at 90 °C. The minimum residual activity reached was 18% with conventional heating and 12% with microwave heating (270 s, 90 °C).
Denès et al. (2000) reported 10% residual activity in PME extracted from Golden Delicious after treatment for 12 s at 65 °C. The medium was a buffer solution with sodium sulfite and sodium chloride. In this work, the residual PME activity in the juice for similar conditions would be around 90% according to Fig. 2a, b. The large difference in thermal resistance can be attributed to the medium where the enzyme was present. Juice components, such as sugars and salts, can stabilize the enzymatic structure minimizing the effect of the exposure to heat (Wilińska et al. 2008).
As verified in Fig. 2c, d that the PPO activity dropped rapidly for temperatures between 50 and 70 °C and then stabilized around 20%, which indicates the presence of isoenzymes with significantly different thermal resistances. According to some authors, short exposures to temperatures between 70 and 80 °C are sufficient for PPO inactivation in fruit juices. Krapfenbauer et al. (2006), for example, evaluated the effect of temperature on PPO activity of juices from eight apple varieties, and no residual activity was observed after 20 s at 80 °C.
POD in the cloudy apple juice showed thermostability at 50 °C (Fig. 2e, f). Similar heat stability was reported by Dubey et al. (2007) in the range of 50 to 60 °C for four apple varieties. Moreover, an initial over-activation of POD was observed for both thermal treatments (Fig. 2e, f). Matsui (2006) also reported over-activation of POD from horseradish after microwave heating at 46 °C. The over- or re-activation of POD in thermally processed fruits and vegetables varies with the heating conditions, pH, food matrix, and enzyme molecular weight (Rodrigo et al. 1996; Thongsook and Barrett 2005). The inactivation of POD occurs when the distance between the heme group and tryptophan residue increases or when the calcium atom is lost (Veitch 2004). Re-activation of POD after thermal treatment could be explained by a conformational change from a reversible denatured form to native form, due to refolding capacity and consequent approximation of heme group with tryptophan (Carvalho et al. 2003).
Inactivation Kinetics by Conventional and Microwave Treatments
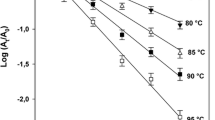
The first-order inactivation model with two isoenzymes and variable temperature in Eq.(6) was fitted to the data of PME, PPO, and POD inactivation by conventional and microwave heating. For the three enzymes, the first-order inactivation model with two fractions provided a superior fit than the model with a single fraction. The adjusted parameters are shown in Table 1 for T ref = 80 °C, along with the corresponding coefficients of determination. Figures 3, 4, and 5 brings the predicted inactivation curves by the adjusted models for conventional and microwave heating. These figures also present the parity charts of the predicted versus experimental values of A/A0. Note that the distributions of points in the parity charts show a scattering around the 45° line and there are no large deviations (less than 0.1). These results indicate the suitability of the kinetic model in Eq.(6) for PME, PPO, and POD inactivation in cloudy apple juice under these processing conditions.
Tajchakavit et al. (1998) studied the heat resistance of Lactobacillus plantarum and Saccharomyces cerevisiae in apple juice under continuous-flow microwave and conventional heating. For both thermal treatments, the D-values for L. plantarum (D80 ° C= 1.2 s for conventional treatment and D80 ° C= 0.00013 s for microwave treatment) and S. cerevisiae (D80 ° C= 0.32 s for conventional treatment and D80 ° C= 0.00051 s for microwave treatment) are lower than the D-values obtained in this study (Table 1) indicating that these microorganisms have a lower thermal resistance than the enzymes. Similarly, Siguemoto et al. (2018) found lower thermal resistance of inoculated Escherichia coli O157:H7 and Listeria monocytogenes in apple juice submitted to microwave and conventional heating when compared to enzymes of cloudy apple juice for a reference temperature of 80 °C.
Similarly to cloudy apple juice, the PME from orange juice also showed to be more heat-resistant than lactic acid bacteria and yeasts present in the juice (Tajchakavit and Ramaswamy 1995; Tajchakavit and Ramaswamy 1997; Tribess and Tadini 2006; Cinquanta et al. 2010). From a technological point of view, the thermo-resistant PME can be used as an indicator or target for the pasteurization of cloudy apple juice.
Comparison between Conventional and Microwave Treatments
To compare the inactivation of enzymes PME, POD, and PPO of cloudy apple juice under conventional and microwave heating conditions, inactivation curves for pasteurization temperatures of 70 and 80 °C (Tajchakavit et al. 1998; Sinha 2006) were built from the adjusted models and are presented in Fig. 6.
Figure 6 shows that the inactivation curves are very close for conventional and microwave heating. Small differences between curves can be attributed to experimental errors on the determination of residual enzymatic activities, uncertainty of the adjusted kinetic parameters, and adequacy of the model to the inactivation behavior.
For comparing between conventional and microwave curves in Fig. 6, it must be taken into account that the average standard deviation for the experimental determination of A/A0 was 0.09 for PME, 0.02 for PPO, and 0.03 for POD, and the largest standard deviation for determining A/A0 was 0.19 for PME, 0.05 for PPO, and 0.10 for POD. Moreover, the average absolute difference for the prediction of A/A0 by the adjusted model was 0.04 for the three enzymes and the maximum absolute difference for the prediction of A/A0 was 0.14 for PME, 0.15 for PPO, and 0.12 for POD.
For the pasteurization temperature of 70 °C (Fig. 6a, c, e), the absolute difference between conventional and microwave curves was under 0.06, with a mean of 0.02 for PME, 0.03 for PPO, and 0.02 for POD. For the pasteurization temperature of 80 °C (Fig. 6b, d, f), the average absolute difference between curves was 0.03 for PME, 0.03 for PPO, and 0.09 for POD. Differences higher than 0.10 were observed for PPO for times between 8 and 40 s and for POD for times between 22 and 130 s.
Considering the uncertainty in the determination of the curves in Fig. 6, it cannot be concluded that there is significant difference in the inactivation of PME and PPO at 70 and 80 °C using conventional or microwave heating. In the case of POD, Fig. 6f shows that at 80 °C, the thermolabile fraction is quickly inactivated (D1 under 5 s in Table 1) and then the thermostable fraction is inactivated at a slower rate. The visible separation between curves with a maximum difference of 0.14 at 61 s suggests that the thermostable fraction of POD would be more efficiently inactivated by microwave heating than by conventional heating. Nevertheless, before concluding that non-thermal effects of microwares were observed in these conditions, it is important to take into account the prediction uncertainties and also that POD is an enzyme with a complex behavior regarding thermal inactivation, often showing over-activation (Matsui 2006), re-activation (Gibriel et al. 1978; Thongsook and Barrett 2005), or inactivation rate that depends on heating rate (Aguiar et al. 2012).
Studies evaluating the non-thermal effects of microwave heating on microorganisms and enzymes provide contradictory conclusions: (1) inactivation was solely a result of heating (Latorre et al. 2012; Stratakos et al. 2016; Arjmandi et al. 2017) or (2) enhanced inactivation was observed and attributed to instantaneous temperature effects due to microwaves (Tajchakavit and Ramaswamy 1997; Tajchakavit et al. 1998; Matsui et al. 2008; Shamis et al. 2012). However, various studies have used microwave systems with inadequate temperature control or non-homogeneous heating, which can compromise the evaluation of non-thermal effects, as discussed by Shazman et al. (2007), Herrero et al. (2008), and Hamoud-Agha et al. (2014).
Conclusion
The inactivation of pectin methylesterase (PME), polyphenol oxidase (PPO), and peroxidase (POD) in cloudy apple juice was studied under conventional and microwave heating, and it was possible to adjust a kinetic model using the whole time-temperature profile of the samples instead of assuming isothermal conditions. It was also possible to provide similar heating and cooling curves for both treatments, for a fair comparison. The two-fraction first-order inactivation model provided a good fit and was used to build inactivation curves for pasteurization at 70 and 80 °C. Results showed that PME has the highest heat resistance and, therefore, is suitable as a process indicator or target. The comparison between conventional and microwave heating did not show significant differences in enzyme inactivation rate; therefore, no evidence of non-thermal or enhanced microwave effects was observed in the inactivation of PME, PPO, and POD in cloudy apple juice.
References
Aguiar, H. F., Yamashita, A. S., & Gut, J. A. W. (2012). Development of enzymic time-temperature integrators with rapid detection for evaluation of continuous HTST pasteurization processes. LWT - Food Science and Technology, 47(1), 110–116.
AOAC. (2010). Official methods of analysis of AOAC international (18th ed.). Washington, DC: AOAC, Association of official analytical chemists.
Arjmandi, M., Otón, M., Artés, F., Artés-Hernández, F., Gómez, P. A., & Aguayo, E. (2017). Microwave flow and conventional heating effects on the physicochemical properties, bioactive compounds and enzymatic activity of tomato puree. Journal of the Science of Food and Agriculture, 97(3), 984–990.
Benlloch-Tinoco, M., Martínez-Navarrete, N., & Rodrigo, D. (2014). Impact of temperature on lethality of kiwifruit puree pasteurization by thermal and microwave processing. Food Control, 35(1), 22–25.
Carvalho, A. S. L., Melo, E. P., Ferreira, B. S., Neves-Petersen, M. T., Petersen, S. B., & Aires-Barros, M. R. (2003). Heme and pH-dependent stability of an anionic horseradish peroxidase. Archives of Biochemistry and Biophysics, 415(2), 257–267.
Cinquanta, L., Albanese, D., Cuccurullo, G., & Di Matteo, M. (2010). Effect on orange juice of batch pasteurization in an improved pilot-scale microwave oven. Journal of Food Science, 75(1), E46–E50.
Denès, J. M., Baron, A., & Drilleau, J. F. (2000). Purification, properties and heat inactivation of pectin methylesterase from apple (cv Golden Delicious). Journal of the Science of Food and Agriculture, 80(10), 1503–1509.
Dubey, A., Diwakar, S. K., Rawat, S. K., Kumar, P., Batra, N., Joshi, A., & Singh, J. (2007). Characterization of ionically bound peroxidases from apple (Mallus pumilus) fruits. Preparative Biochemistry & Biotechnology, 37(1), 47–58.
Falguera, V., Gatius, F., Ibarz, A., & Barbosa-Cánovas, G. V. (2013). Kinetic and multivariate analysis of polyphenol oxidase inactivation by high pressure and temperature processing in apple juices made from six different varieties. Food and Bioprocess Technology, 6(9), 2342–2352.
Gibriel, A. Y., El-Sahrigi, A. F., Kandil, S. H., El-Mansy, A., & H. (1978). Effect of pH, sodium chloride and sucrose on heat-inactivation and reactivation of peroxidases in certain foods. Journal of the Science of Food and Agriculture, 29(3), 261–266.
Guo, Q., Sun, D. W., Cheng, J. H., & Han, Z. (2017). Microwave processing techniques and their recent applications in the food industry. Trends in Food Science & Technology, 67, 236–247.
Hamoud-Agha, M. M., Curet, S., Simonin, H., & Boillereaux, L. (2013). Microwave inactivation of Escherichia coli K12 CIP 54.117 in a gel medium: experimental and numerical study. Journal of Food Engineering, 116(2), 315–323.
Hamoud-Agha, M. M., Curet, S., Simonin, H., & Boillereaux, L. (2014). Holding time effect on microwave inactivation of Escherichia coli K12: experimental and numerical investigations. Journal of Food Engineering, 143, 102–113.
Hebbar, H. U., & Rastogi, N. K. (2012). Microwave heating of fluid foods. In P. J. Cullen, B. K. Tiwari, & V. P. Valdramidis (Eds.), Novel thermal and non-thermal technologies for fluid foods (pp. 369–404). San Diego: Academic press.
Herrero, M. A., Kremsner, J. M., & Kappe, C. O. (2008). Nonthermal microwave effects revisited: on the importance of internal temperature monitoring and agitation in microwave chemistry. The Journal of Organic Chemistry, 73(1), 36–47.
Huang, H. W., Wu, S. J., Lu, J. K., Shyu, Y. T., & Wang, C. Y. (2017). Current status and future trends of high-pressure processing in food industry. Food Control, 72, 1–8.
Krapfenbauer, G., Kinner, M., Gössinger, M., Schönlechner, R., & Berghofer, E. (2006). Effect of thermal treatment on the quality of cloudy apple juice. Journal of Agricultural and Food Chemistry, 54(15), 5453–5460.
Latorre, M. E., Bonelli, P. R., Rojas, A. M., & Gerschenson, L. N. (2012). Microwave inactivation of red beet (Beta vulgaris L. var. conditiva) peroxidase and polyphenoloxidase and the effect of radiation on vegetable tissue quality. Journal of Food Engineering, 109(4), 676–684.
Lopes, L. C., Barreto, M. T., Goncalves, K. M., Alvarez, H. M., Heredia, M. F., de Souza, R. O. M., Cordeiro, Y., Dariva, C., & Fricks, A. T. (2015). Stability and structural changes of horseradish peroxidase: microwave versus conventional heating treatment. Enzyme and Microbial Technology, 69, 10–18.
Matsui, K. N. (2006). Inativação das enzimas presentes na água de coco verde (Cocos nucifera L.) por processo térmico através de micro-ondas (Doctoral dissertation). Retrieved from Teses USP Database. https://doi.org/10.11606/T.3.2006-180716.
Matsui, K. N., Gut, J. A. W., De Oliveira, P. V., & Tadini, C. C. (2008). Inactivation kinetics of polyphenol oxidase and peroxidase in green coconut water by microwave processing. Journal of Food Engineering, 88(2), 169–176.
Rawson, A., Patras, A., Tiwari, B. K., Noci, F., Koutchma, T., & Brunton, N. (2011). Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: review of recent advances. Food Research International, 44(7), 1875–1887.
Rodrigo, C., Rodrigo, M., Alvarruiz, A., & Frigola, A. (1996). Thermal inactivation at high temperatures and regeneration of green asparagus peroxidase. Journal of Food Protection, 59(10), 1065–1071.
Rougier, C., Prorot, A., Chazal, P., Leveque, P., & Lepratb, P. (2014). Thermal and nonthermal effects of discontinuous microwave exposure (2.45 gigahertz) on the cell membrane of Escherichia coli. Applied and Environmental Microbiology, 80(16), 4832–4841.
Rouse, A. H., & Atkins, C. D. (1953). Further results from a study on heat inactivation of pectinesterase in citrus juices. Food Technology, 7(6), 221–223.
Shamis, Y., Croft, R., Taube, A., Crawford, R. J., & Ivanova, E. P. (2012). Review of the specific effects of microwave radiation on bacterial cells. Applied Microbiology and Biotechnology, 96(2), 319–325.
Shazman, A., Mizrahi, S., Cogan, U., & Shimoni, E. (2007). Examining for possible non-thermal effects during heating in a microwave oven. Food Chemistry, 103(2), 444–453.
Siguemoto, É. S., & Gut, J. A. W. (2017). Validation of spectrophotometric microplate methods for polyphenol oxidase and peroxidase activities analysis in fruits and vegetables. Food Science and Technology, 37(s1), 148–153.
Siguemoto, É. S., Gut, J. A. W., Martinez, A., & Rodrigo, D. (2018). Inactivation kinetics of Escherichia coli O157:H7 and Listeria monocytogenes in apple juice by microwave and conventional thermal processing. Innovative Food Science & Emerging Technologies, 45, 84–91.
Sinha, N. K. (2006). Apples. In Y. H. Hui (Ed.), Handbook of fruits and fruit processing (pp. 265–278). Hoboken: Blackwell Publishing.
Stratakos, A. C., Delgado-Pando, G., Linton, M., Patterson, M. F., & Koidis, A. (2016). Industrial scale microwave processing of tomato juice using a novel continuous microwave system. Food Chemistry, 190, 622–628.
Tajchakavit, S., & Ramaswamy, H. S. (1995). Continuous-flow microwave heating of orange juice: evidence of nonthermal effects. Journal of Microwave Power and Electromagnetic Energy, 30(3), 141–148.
Tajchakavit, S., & Ramaswamy, H. S. (1997). Thermal vs. microwave inactivation kinetics of pectin methylesterase in orange juice under batch mode heating conditions. LWT - Food Science and Technology, 30(1), 85–93.
Tajchakavit, S., Ramaswamy, H. S., & Fustier, P. (1998). Enhanced destruction of spoilage microorganisms in apple juice during continuous flow microwave heating. Food Research International, 31(10), 713–722.
Tang, J. (2015). Unlocking potentials of microwaves for food safety and quality. Journal of Food Science, 80(8), E1776–E1793.
Thongsook, T., & Barrett, D. M. (2005). Heat inactivation and reactivation of broccoli peroxidase. Journal of Agricultural and Food Chemistry, 53(8), 3215–3222.
Tribess, T. B., & Tadini, C. C. (2006). Inactivation kinetics of pectin methylesterase in orange juice as a function of pH and temperature/time process conditions. Journal of the Science of Food and Agriculture, 86(9), 1328–1335.
Veitch, N. C. (2004). Horseradish peroxidase: a modern view of a classic enzyme. Phytochemistry, 65(3), 249–259.
Wilińska, A., Rodrigues, A. S. F., Bryjak, J., & Polakovič, M. (2008). Thermal inactivation of exogenous pectin methylesterase in apple and cloudberry juices. Journal of Food Engineering, 85(3), 459–465.
Xu, B., Wang, L. K., Miao, W. J., Wu, Q. F., Liu, Y. X., Sun, Y., & Gao, C. (2016). Thermal versus microwave inactivation kinetics of lipase and lipoxygenase from wheat germ. Journal of Food Process Engineering, 39(3), 247–255.
Funding
The authors acknowledge financial support from the São Paulo Research Foundation—FAPESP (grants 2013/07914-8, 2014/06026-4, and 2014/17534-0) and from the National Council for Scientific and Technological Development—CNPq (grant 459177/2014-1) and also Prof. Adalberto Pessoa Junior from the Faculty of Pharmaceutical Sciences at the University of São Paulo for access to their facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siguemoto, É.S., Pereira, L.J. & Gut, J.A.W. Inactivation Kinetics of Pectin Methylesterase, Polyphenol Oxidase, and Peroxidase in Cloudy Apple Juice under Microwave and Conventional Heating to Evaluate Non-Thermal Microwave Effects. Food Bioprocess Technol 11, 1359–1369 (2018). https://doi.org/10.1007/s11947-018-2109-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-018-2109-2