Abstract
The aim of this study was to investigate the influence of spray drying on oxidative stability of dried microencapsulated fish oil (DMFO) coated with modified cellulose. DMFO samples were obtained by spray drying of prepared emulsions consisted of water solution of modified cellulose and fish oil. Appearance and size of particles were measured by electron microscopy and laser light microsizer. The oxidative stability of samples was evaluated by peroxide value measurements. Additionally the influence of different antioxidant substances on oxidative stability of the fish oil was investigated. It was observed that oxidation changes were much slower in bulk fish oil compared to DMFO. The most important factor determining shelf-life of the product was the access to air. It can be concluded, that the production of fish oil microcapsules by spray drying technique is possible, however its oxidative stability is not improved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish oil is the richest source of long-chain polyunsaturated fatty acids (PUFA) of omega-3 type. Beneficial health effects of increased intake of omega-3 PUFA – especially the long-chain eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – are well established [1]. Omega-3 EPA and DHA intake may prevent cardiovascular diseases, certain types of cancer, inflammations and allergies as well as improve proper development and function of central nervous system [2]. In several western countries average fish intake is far below the recommended minimum of two fish servings per week (i.e. approximately 200.0 mg EPA and DHA per day). Besides changing of eating and culinary habits, the increase of omega-3 PUFA level in a diet may be achieved by intake of fish oil supplements or food enriched with fish oil [3, 4]. Therefore, a number of functional foods enriched with omega-3 PUFA via fish oil addition have been developed. Dried microencapsulated fish oil (DMFO) is a powder, which is easily applied to instant powder products. Fish oil powder has been incorporated into many food products, e.g. bread, biscuits, fruit bars, low-fat cakes, diet powder, fruit juice, milk powder, instant soups, infant formula, etc. [1]. A direct incorporation of fish oil into food is possible, but there are some problems related to this process. Addition of fish oil in excessive amounts may decrease sensory acceptance while highly unsaturated omega-3 fatty acids are susceptible to oxidation, resulting in the formation of toxic hydroperoxides, off-flavors and a shorter shelf-life of the product [5]. These disadvantages may be avoided by using well-purified fish oil at moderate addition levels, especially when protected by the addition of antioxidants [6, 7].
The food industry applies microencapsulation for a number of reasons such as stabilization of the active substances, controlled release of the active substances, masking its unpleasant taste and smell. Today different techniques are employed for microencapsulation of food additives, including flavors, preservatives, leavening agents, vitamins and minerals [8]. It may also be useful for microencapsulation of omega-3 PUFA. Spray drying is a low-cost microencapsulation technology and the most commonly used in the food industry. This technique has been widely used for drying heat-sensitive foods, pharmaceuticals and other substances, because of the rapid evaporation of the applied solvent from the droplets.
Spray drying is used not only for drying various food products (e.g., dairy products such as milk) but also for specific microencapsulation of various food ingredients, such as flavourings, colouring substances, fats and oils. Spray drying is transformation of a feed from a liquid form (solution, dispersion or paste) into a powder by spraying the feed into a hot drying medium. It is a continuous operating process involving a combination of several stages, namely atomization, mixing of spray and hot air, evaporation and dry product separation [8, 9].
The advantages of this method are: its ability to handle heat-sensitive materials, availability of machinery and its variety, good keeping qualities of microcapsules, variety of particle sizes that can be produced and an excellent dispensability of particles in aqueous media [9, 10].
The disadvantage of this technology is the high temperature conditions necessary for drying and access to air. Moreover, parts of the product during drying may adhere to the surface of the capsules, which presents potential for oxidation and changes in the flavor balance of the finished food products [11].
There are several materials used for coat formation during microencapsulation by spray drying. The coatings are generally food-grade hydrocolloids such as gelatin, plant gums, modified starch, dextrin or non-gelling proteins [8, 11]. Modified celluloses as coating materials, especially methylcellulose (MC) and hydroxypropyl methylcellulose (HPMC), are already used for microencapsulation of drugs. In this application modified cellulose acts as a physical barrier protecting the stomach mucosa against irritation of enteral administrated drugs while creating a dense and stable wall [12, 13]. For this reason the use of modified cellulose as a microencapsulating agent may provide an alternative for materials already used in food industry. Moreover, Rampurna and Gullapalli [14] have indicated, that the addition of methylcellulose causes a significant reduction and narrowing of the particle size of all basic oil in water (o/w) emulsions [14]. According to special properties of modified cellulose it may also act as a good coating material for fish oil microen-capsulation.
Hence the aim of this study is to investigate the influence of spray drying on oxidative stability of dried microencapsulated fish oil, coated with modified cellulose.
Materials and methods
Fish oil used in this study (ROPUFA’30’ n-3 food oil) was produced by refination of crude fish oil obtained from different species of Atlantic fishes and contained approximately 300.0 g kg−1 of long-chain omega-3 PUFA (DSM Nutritional Products, Switzerland). According to the fish oil certificate of analysis the content of docosahexaenoci acid (DHA) was 174.0 g kg−1 and the sum of long-chain omega-3 PUFA, i.e. EPA plus DHA plus docosapentaenoic acid (DPA) was 318.0 g kg−1. The fish oil was a yellowish, oily liquid with a very faint, fishy-like odour, peroxide value of the oil was 1.05 meq O kg−1. Methylcellulose METHOCEL A15 (The Dow Chemical Company, USA) was used as coating material. Maltodextrin (Crystal Tex 626, National Starch & Chemical Ltd., USA) was used as an additional coating agent. Soy lecithin – 3-sn-phospatidylcholine (Fluka, Germany) was used as additional emulsifier to improve the homogenization process. Antioxidants: α-tocopherol (Fluka, Germany) and food grade lycopene Lyc-O-Malto 7% (LycoRed Ltd., Israel) were additionally used for higher protection against oxidation of fish oil.
Two types of samples were investigated: (a) fish oil with and without antioxidant addition, (b) dry microencapsulated fish oil powder of different fish oil content.
Fish oil samples were prepared from pure fish oil mixed with antioxidants. A total of 100 g samples were poured into glass vials and closed with aluminum caps. An aluminum foil cover was used to protect samples against light. High amounts of antioxidants were chosen according to literature suggestion that the addition of each gram of highly unsaturated EPA and DHA requires 5–6 mg of α-tocopherol to protect against oxidation [15].
Four fish oil samples were prepared and investigated during storage: (a) without addition of antioxidants, stored at room temperature, no exposure to light and air, (b) without addition of antioxidants, stored at low temperature (5±0.5 °C), (c) with 2.5 g kg−1 of α-tocopherol stored at room temperature, no exposure to light and air, (d) with 1.0 g kg−1 of lycopene preparation stored at room temperature, no exposure to light and air, (e) with 2.5 g kg−1 of α-tocopherol stored at low temperature (5±0.5 °C), no exposure to light and air. Fish oil samples were evaluated just after preparation, second evaluation was made after a week of storage, third one – after 5 days and next ones – every third day.
Formulations of the emulsions for spray drying were established after several preliminary tests with different materials and proportions of the components. Final formulations are presented in Table 1.
Fish oil was added to obtain powders with a predicted amount of fish oil of 200.0 and 400.0 g kg−1, respectively. Aqueous solutions of coatings were prepared by dissolving the wall materials in water and overnight storage at 5±0.5 °C. The maltodextrin was dissolved in cold water by gentle magnetic stirring, then MC-solution was added and mixed using a hand blender at speed 200 rpm (Braun, Germany). An aqueous suspension of lecithin was prepared using a heated magnetic stirrer. The materials were magnetically mixed for 30 min and chilled in an ice bath to 5±0.5 °C. Fish oil was poured into the prepared solution under stirring, after that the mixture was homogenized in an ice-water bath using laboratory homogenizer Ultraturax TK 25 (IKA-Labortechnik, Germany) at a speed of 10 000 rpm for 5 min. Each batch of prepared emulsion weighed approximately 2 kg. The appearance and stability of emulsions was evaluated in light microscopy under 100× magnifications.
Spray drying was employed using a pilot-scale spray dryer (Nubilo SA, Italy) with an atomizer nozzle of 500 μm diameter. The drying chamber diameter was 1.5 m, the inlet and outlet air temperatures were 160 and 65 °C respectively ±2 °C. The emulsion was fed into the dryer by a peristaltic pump at flow rate of 15.0 g min−1 and an inlet pressure of 350 kPa during spraying. The powders were collected in glass vessels.
Obtained DMFOs of fish oil content of 200.0 and 400.0 g kg−1 were placed in plastic bags and stored for 32 days under different conditions: (a) room temperature, no exposure to light, access to air – samples A-200, A-400; (b) room temperature, no exposure to light, vacuum – samples B-200, B-400; (c) low temperature (5±0.5 °C), no exposure to light, vacuum – samples C-200, C-400; (d) low temperature, no exposure to light, access to air – samples D-200, D-400.
Vacuum stored samples were closed using vacuum packer VAC-STAR 2000 (Vac Star AG, Switzerland), in the case of DMFOs stored with access to air; the bags were closed using an office-stapler. Fish oil samples and DMFO samples were evaluated every third day. After taking sample for measurements bags were closed again under vacuum and reopened after 3–6 days for the next measurement. The aim of this procedure was to investigate oxidation changes of polyunsaturated fatty acids in products being opened and closed during storage as happens under typical household conditions.
Soxhlet extraction was made to determinate the oil content in obtained DMFOs. The sample material was put into an extraction thimble which was closed with glass wool and then placed in a Soxhlet extractor. A condenser was installed on top of the Soxhlet extractor and fed with cooling water at a temperature of 2 °C. The prepared round evaporation flask was filled with 75.0 ml of petrol ether and connected to the Soxhlet extractor. The petrol ether was heated to boiling point and run for 4 h. Afterwards, the evaporation flask was put into an evaporator and the petrol ether was evaporated for 30 min at 50 °C. A water vacuum pump was used to accelerate the process. Then the evaporation flask was stored under an extraction hood for 15 min, then the content was weighed.
Peroxide value (PV) was measured to determinate the oxidation process during storage. The applied iodometric method is based on ISO 3960 – Animal and vegetable fats and oils – Determination of peroxide value (2001), with chloroform and glacial acetic acid as solvents. Samples representing 0.5 g of oil, i.e. 2.5 g DMFO sample of fish oil content of 200.0 g kg−1 or 1.25 g sample of 400.0 g kg−1 were placed in the Erlenmeyer flask and dissolved in 20 ml of chloroform. Then 30 ml of glacial acetic acid was added and the mixture was stirred for a few seconds to ensure the mixing. After that 0.5 ml of the potassium iodide (KJ) solution was added. After 1 min, 30 ml of deionized water was added and the titration started. When the dark-yellow color changed into a pale-yellow, 0.5 ml of starch-solution was added. Titration was finished when the color disappeared. The mixture was stirred magnetically during the procedure. Results were calculated as micro equivalents of active oxygen per 1 kg of fat sample (meq O kg−1).
Microscopic evaluation of samples was made using light and scanning electron microscopy (SEM). Obtained emulsions were examined by optical light microscopy applying 100× magnification by using light microscopy. The surface appearance and morphology of DMFOs were examined by SEM (XL 20 Philips, Netherlands). Samples were fixed onto double-sided adhesive carbon tabs mounted on SEM-tubs, coated with gold/palladium in a sutter coater, and examined by SEM operating at 5 kV.
Particle size analysis was carried out using the Sympatec laser light diffraction particle size analyzer (Sympatec, Germany).
Statistical analysis of variance (ANOVA) was applied to the data obtained from peroxide values measurements using the statistical software MS Excel.
Results
Modified cellulose showed very good emulsifying properties. However, mixing and homogenization of the solution provoked an air entrapment creating an excess of foam, which disappeared after 30 min. This disadvantage may lead to an excess of oxygen incorporation for a short time and should be avoided. It was also observed that a consequence of the homogenization step was that the temperature of the emulsion increased up to 25 °C, in spite of the fact that an ice-water bath was used.
The diameter of droplets in all prepared emulsions ranged from 10 to 40 μm. That was observed a tendency to aggregate smaller droplets resulted in an increase of average droplet size. However visual evaluation showed that obtained emulsions were stable for at least 3 h after homogenization process, which was sufficient for spray drying.
The structures of DMFOs observed by SEM are shown in Fig. 1a and b. In all samples, the average diameter of the microcapsules was 27 μm, there were few particles of lower or higher diameter (Fig. 2). In Fig. 2 sinusoid curve reflects statistical distribution of particle size, which the highest peak reflecting an average particle size (i.e. ca. 27 μm), S-shaped curve reflects percentage amount of particles of different sizes (e.g. 75% of all particles was between 10 and 50 μm, or 50% of all particles was smaller than 27 μm diameter). The sinusoid curve shows that particles of diameter between 10 and 20 μm were the most common occurring (30% of all particles). The diameter of the microcapsules depends probably on homogenization parameters, the materials used and conditions of the spray drying process. It was observed, that not all of the wall material was used for the formation of microcapsules. Some unbound cellulose was observed around the capsules. Not too many capsules were broken or damaged. The surface of samples was smooth and regular, however small holes occurred on surface of sample containing 200.0 g kg−1 of fish oil. It could be an effect of too high content of coating material in the formulation, which was not used for creation of microcapsules wall and occurred in the form of irregular small particles.
Measurements of fish oil content in the obtained powders showed high microencapsulation efficiency. In the case of the sample with estimated content of fish oil – 200.0 g kg−1 the real amount of oil was 173.0 g kg−1, representing an efficiency of 86.5%. In the case of sample of fish oil content 400.0 g kg−1, the real amount of oil was 339.5 g kg−1, representing an efficiency of 84.8%. Unbounded fish oil (i.e. 13.5 and 15.2% of oil used for sample preparation) probably remained at the inner wall of the spray dryer chamber as it is usually during spray drying of o/w emulsions.
Evaluation of oxidative stability of fish oil samples showed that addition of antioxidants (α-tocopherol, lycopene) did not improve the shelf-life of the samples. The peroxide values for these samples during storage increased and were significantly higher in comparison to pure fish oil (Fig. 3). Results of measurements showed that the sample without antioxidants addition gave a product of the highest stability. That effect could be provoked by too high level of antioxidant, which in a high amount may accelerate oxidation process. The highest peroxide value at the end of storage time showed fish oil sample with addition of lycopene.
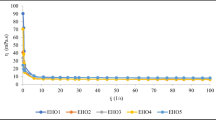
Initial peroxide value of pure fish oil was 1.05 meq O kg−1 and after microencapsulation process increased to approximately 4.06 and 2.10 meq O kg−1 for 200.0 and 400.0 g kg−1 DMFO samples, respectively. High temperature during spray drying resulted in acceleration of oxidation changes and higher peroxide values after the process. The formation of peroxide compounds was the highest in both DMFOs samples stored at room temperature with access to air, reaching at the end of the induction period values: 171.7 and 211.4 meq O kg−1 for 200.0 and 400.0 g kg−1 sample respectively, as shown in Fig. 4. The induction period was shorter for 200.0 g kg−1 sample than for 400.0 g kg−1 sample. Heinzelmann and Franke [16] considered the value of 20.0 meq O kg−1 as the upper limit for DMFO [16]. In the case of DMFO samples stored at low temperature peroxide values were much lower but with tendency to significant increase in time (Fig. 5).
Samples packed under vacuum were stable and mean peroxide values slightly increased during 30 days of storage. In the case of vacuum packed samples, the storage period was too short to observe the typical PV curve. After 30 days of storage peroxide value did not exceed 10.0 meq O kg−1. Moreover, there were no significant differences between vacuum packed samples stored in different temperatures in case 200.0 g kg−1 and 400.0 g kg−1 DMFOs. The most unexpected results were obtained in the case of vacuum stored DMFOs which were opened for a short time, closed and reopened again after 3–6 days for measurement. Peroxide values of these samples showed significant increase despite contact to air was very short and limited. Thus, access to oxygen, even for short time may have a strong effect on oxidative stability of DMFOs. Results of oxidation measurements of vacuum packed DMFOs are presented in Figs. 6 and 7.
Results of peroxides evaluation suggest that once initiated oxidative changes of fat runs more intensive in samples of higher fish oil content despite vacuum packaging. In the case of samples with full access to air, intensity of peroxides formation was higher in samples of lower fish oil content. This may suggest that the wall of DMFOs of fish oil content of 400.0 g kg−1 was more protective, i.e. more dense than in the case of 200.0 g kg−1 samples, which was also observed during SEM examination. This unexpected phenomenon could be explained by differences in proportion of components of both formulations. In the case of 400.0 g kg−1 DMFO utilization of used materials was probably more efficient and resulted in creation of more dense wall of particles than in the case of 200.0 g kg−1 sample, which contained significant amount of unbounded cellulose and more holes on the surface (Fig. 1).
Discussion
Heinzelmann and Franke [16] showed that contact between fish oil and oxygen during the emulsion production process may lead to acceleration of oxidation changes in emulsion before the drying process. It is impossible to prevent this contact completely. Moreover, a consequence of the homogenization process is that the temperature of emulsion increases. Maximal emulsion temperature during homogenization should not exceed 35 °C. In the present study it was observed that emulsion temperature increased during homogenization despite using ice-bath, though not exceeding 25 °C. An examination of oxidation deterioration in the obtained emulsions after homogenization showed no significant changes in peroxide value, which is in contradiction to results obtained by Heinzelmann and Franke [16] and Shahidi and Han [17].
In the present study it was observed that the drying procedure gave rise to oxidative degradation, which is in opposite to the results obtained by Heinzelmann et al. [17, 18]. The results obtained in this experiment confirmed previous conclusions that the main disadvantage of microencapsulation by spray drying technology is elevated temperature which may lead to an increased oxidation of PUFA [16, 17].
A number of studies have been reported on the oxidative stability of microencapsulated fish oil or fatty acids methyl esters, obtained either by spray drying or freeze drying techniques. Literature review on oxidative stability evaluations of different PUFA shows a wide variation of results that are difficult to interpret. Such inconsistent results may be due to changes in oxidation conditions and to the application of analytical methods that measure different endpoints of lipid oxidation. In many experiments on stability of fats and oils oxidation is accelerated under extreme conditions (e.g. elevated temperature of storage, forced air access) and many methods employed to determine the level of oxidation are not sufficiently sensitive to be relevant to flavor detection [19].
Explanation of poor shelf-life of fish oil sample with high addition of α-tocopherol may be its superior reactivity. Above a certain concentration, α-tocopherol loses efficiency in stabilizing PUFA [20]. Lampi et al. [20] indicated that the addition of α-tocopherol to vegetable oils does not generally improve their oxidative stability. It seems that this is because the natural α-tocopherol level in these oils is sufficient for protection against oxidation and its addition on higher level cannot elevate stability of the oil [20]. However, less is known about the reactions in more complex food systems, where it is possible that interactions with matrix and catalysts may cause different oxidation reactions. It has been observed that α-tocopherol may have a considerable pro-oxidant activity in aqueous systems in the absence of chelating agents and reducing compounds [20].
In the case of lycopene stabilized sample, the peroxide values were the highest in comparison to the others. The explanation of this results may be that too high doses of lycopene promote formation of peroxyl radicals acting as pro-oxidants and of the oil [21]. At the moment very little is known about the antioxidant activity of lycopene on polyunsaturated fatty acids present in fish oils. Further studies are necessary to evaluate possible stability effect of lycopene on fish oil.
Obtained results showed that fatty acids oxidation was much slower in the case of bulk fish oil samples in comparison to DMFO samples, which is similar to observations made by Marquez-Ruiz et al. [22]. The difference in oxidative stability is related to a high developed surface area of DMFOs in comparison to bulk fish oils [17, 22]. Therefore, difference in air access exerts an effect on the oxidative stability of examined samples. These was observed also during present investigation. Results of oxidation studies for vacuum-stored DMFOs are in agreement with other studies [18, 23, 24] which suggests that shelf-life of DMFOs may be improved when stored under nitrogen or vacuum. However, it is difficult to stop once oxidation process has started.
Conclusions
Results obtained during this study indicate that microencapsulation by spray drying technique did not significantly protect fish oil against oxidation in comparison to bulk fish oil. It was observed that oxidation changes were much slower in case of vacuum packed DMFO samples. However, vacuum packed samples opened during storage for a short time, closed under vacuum and reopened again after couple of days showed significantly higher oxidation changes in comparison to unopened ones. Results also showed that modified celluloses, especially methylcellulose, can be used as an alternative material for fish oil microencapsulation. Further studies are necessary to evaluate influence of addition of antioxidant as a factor increasing shelf-life of dried microencapsulated fish oils, as well as to optimize homogenization conditions, which may affect oxidative stability of the final food product.
References
Thautwein EA (2001) Eur J Lipid Sci Technol 103:45–55
Simopoulos AP, Leaf A, Salem N (1999) Ann Nutr Metab 43:127–130
Shahidi F, Wanasundra UN (1998) Trends Food Sci Technol 9:230–240
Wallace JMW, McCabe AJ, Robson PJ, Keogh MK, Murray CA, Kelly PM (2000) Ann Nutr Metab 44:157–162
Kolanowski W, Swiderski F, Berger S (1999) Int J Food Sci Nutr 50:39–49
Bimbo AP (1998) Informatics 5:180–188
Neil ME, Younger KM (1998) Microencapsulation of marine oils with a view to food fortification. In: Sadler MJ, Saltmarsh M (eds) Functional foods: the consumer, the product and the evidence. Springer, Berlin Heidelberg New York, pp 149–158
Ashady R (1993) J Microencaps 10:413–435
Re MI (1998) Drying Technol 16:1195–1236
Jackson LS, Lee K (1991) Lebensm-Wissens Technol 24:289–295
Kanawija SK, Pathania V, Singh S (1992) Indian Dairyman 44:6
Calderia AM, Goucha P, Almeida AJ (1998) Int J Pharm 164:147–154
Krawczyk T (2001) Informatics 12:1064–1080
Rampurna P, Gullapalli A (1997) Int J Pharm 151:249–253
Sanders TAB (1993) Proc Nutr Soc 52:457–461
Heinzelmann K, Franke K (1999) Colloids and Surf 12:223–229
Shahidi F, Han XQ (1993) Crit Rev Food Sci Nutr 33:501–547
Heinzelmann K, Franke K, Valesco J, Marquez-Ruiz G (2000) Eur Food Res Technol 211:234–239
Frankel EN, Satué-Gracia T, Meyer AS, German JB (2002) J Agric Food Chem 50:2094–2099
Lampi AM, Kataja L, Kamal-Eldin A, Piironen V (1999) JAOCS 76:749–755
Shi J, Le Maquer M (2000) Crit Rev Food Sci Nutr 40:1–42
Marquez-Ruiz G, Valesco J, Dobraganes C (1999) Eur Food Res Technol 221:13–18
Rabiskowa M, Song J, Opawale FO, Burgess DJ (1994) J Pharm Pharmacol 46:631–645
Young SL, Sarda X, Rosenberg M (1993) J Dairy Sci 76:2878–288
Acknowledgements
Authors express their thanks to Garman Academic Exchange Service – DAAD for partial support of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolanowski, W., Ziolkowski, M., Weißbrodt, J. et al. Microencapsulation of fish oil by spray drying--impact on oxidative stability. Part 1. Eur Food Res Technol 222, 336–342 (2006). https://doi.org/10.1007/s00217-005-0111-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-0111-1