Abstract
Perilla oil is vulnerable to lipid oxidation owing to its high linolenic acid content. Microencapsulation using freeze- and spray-drying methods was applied to enhance the oxidative stability and change the physicochemical properties of perilla oil. Freeze-dried powder (FDP) possessed 11.77 to 38.48% oil content, whereas spray-dried powder (SDP) had 8.90–27.83% oil content. Encapsulation efficiency ranged from 51.22 to 85.71% by freeze-drying and from 77.38 to 90.74% by spray-drying. The oxidative stability of powders depends on the oil content and production methods. Generally, FDP had higher oxidative stability and water solubility, and lower moisture content and water activity than SDP. The particle size of FDP (154.00–192.00 μm) in volume-weight mean diameter was 2.56–24.49 times larger than that of SDP (7.84–72.03 μm). SDP had a lower volatile content at the initial time of storage than FDP, while more volatiles were observed in SDP as storage time increased. The microencapsulation method should be selected appropriately depending on the target property or usage in food applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perilla oil has a unique savory flavor and is used as a flavor enhancer and sauce in Korean cuisine (Lee et al., 2017). Perilla oil contains 54–64% linolenic acid and is one of the highest omega-3 rich vegetable oils known (Narisawa et al., 1994). Consumption of vegetable oils with a high linolenic acid content could be effective in preventing various diseases, including cardiovascular diseases, cancer, inflammatory arthritis, and rheumatoid arthritis (Asif et al., 2011). Despite its health-beneficial effects, perilla oil is vulnerable to lipid oxidation due to its high linolenic acid content, making it difficult to store and use.
Oxidation products from lipids possess a unique rancid odor that decreases consumer acceptance of lipid-rich foods. In addition, some chemicals generated by lipid oxidation, including formaldehyde, 2-hydroxyl nonenal, and 2-hydroxy hexanal, are toxic (Gutiérrez-del-Río et al., 2021). Therefore, protection of perilla oil or linolenic acid-rich oils from lipid oxidation could be a critical point during production, storage, and transport (Hasani et al., 2018).
Microencapsulation using various wall materials has been widely applied as an appropriate solution to enhance the oxidative stability of lipids during storage (Ahn et al., 2008). Preparation of an oil-in-water (O/W) emulsion matrix followed by drying techniques is a typical procedure for microencapsulating oil into a powder form. Maltodextrin, lactose, whey protein concentrate, and gum arabic are the most commonly used ingredients for the microencapsulation of oils (Carneiro et al., 2013; Himmetagaoglu et al., 2018; Jalali-Jivan et al., 2020). To increase the stability and applicability of bioactive compounds, spray-drying and/or freeze-drying that converts the liquid phase to a solid state has been performed.
Spray-drying is one of the most widely used drying methods in the food industry because of its low cost and flexibility (Fang and Bhandari, 2010). Spray-drying is well suited to the rapid production of micro-sized particles for encapsulation of flavor chemicals and oils (Nguyen et al., 2004). However, core materials are inevitably exposed to high temperatures, which may lead to significant oxidation of unsaturated fatty acids during spray-drying (Elik et al., 2021).
The freeze-drying method can be used for the microencapsulation of heat-labile bioactive compounds owing to its low temperature processes (Papoutsis et al., 2018). Although the operational cost is relatively high compared to spray-drying, freeze-drying can deliver heat-sensitive biomolecules with minimal structural damage caused by oxidation (Anwar et al., 2011; Naik et al., 2014).
Several oils, such as krill oil (Aziz et al., 2014; Sánchez et al., 2021) and fish oil (Kagami et al., 2003; Ramos et al., 2021), have been microencapsulated using various wall materials to enhance the oxidative stability and extend shelf life and applications in the food industry (Aziz et al., 2014; Sánchez et al., 2021). Although perilla oil requires protection against lipid oxidation due to high contents of linolenic acid, microencapsulation of perilla oil has not been adequately studied.
The objectives of this study were to determine the microencapsulation conditions of perilla oil in powder form and to evaluate the physicochemical properties and oxidative stability of microencapsulated perilla oil powders.
Materials and methods
Materials
Queensbucket ™ perilla oil was obtained from SeoulMills (Seoul, Korea). Whey protein concentrate 80 (WPC80) was purchased from Herbnare (Seoul, Korea). Maltodextrin, gum arabic from acacia trees, iron (II) sulfate heptahydrate, and ammonium thiocyanate were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1-Butanol and 2-propanol were purchased from Samchun (Seoul, Korea). Other chemicals, including n-hexane, ethanol, isooctane, methanol, cumene hydroperoxide, hydrochloric acid, and barium chloride, were purchased from Daejung Chemical Co. (Seoul, Korea).
Preparation of encapsulated perilla oil powder
The microencapsulation of perilla oil was performed using a freeze dryer and spray dryer as described by El-Messery et al. (2020) and Murali et al. (2015), respectively. A 10% (w/v) of maltodextrin and WPC80 solution was prepared at a ratio of 4:1 (v/v) and used as the continuous phase. Perilla oil (1, 2, 3, and 4%, w/w) was dispersed in the continuous phase and homogenized using an HB873AKR device (Tepal, Rumily, Haute-Savoie, France) for 5 min. The coarse emulsion was passed five times through a high-pressure homogenizer (APV 1000; SPX Flow, Charlotte, NC, USA) at 40 MPa. After homogenization, gum arabic solution (2.5 mL) was added to the O/W emulsion, and the mixture was homogenized again for 5 min. To obtain powdered perilla oil, the O/W emulsion samples were lyophilized using a model LP30 pilot scale freeze dryer (IlShinBioBase, Seoul, Korea) for 72 h or spray-dried using a model ADL 311/311SS spray dryer (Yamato Scientific Co., Ltd., Tokyo, Japan). For spray-drying, the rate of drying air flow was 0.42 m3/min and compressor air pressure was 0.2–0.25 MPa. The inlet and outlet air temperatures were 180 ± 5 °C and 90 ± 5 °C, respectively. After the drying process, the obtained powders were stored in moisture-impermeable plastic bags at − 20 °C for further characterization of their properties.
For the oxidative stability tests, 2 g of microencapsulated powder was placed in a 10 mL- vial under airtight conditions and stored at 60 °C in a dry oven (HYSCLAB, Seoul, Korea) to accelerate the oxidation process. Samples were analyzed for 1 week. All samples were prepared in triplicates.
Determination of encapsulation efficiency
Encapsulation efficiency was determined according to Bringas-Lantigua et al. (2011), with slight modification. Encapsulation efficiency can be defined as the ratio of the mass of the core material encapsulated in the wall material to the mass of the core material used in the formulation (Kaushik et al., 2015). The content of surface oil indicates that not all the perilla oil was encapsulated inside the wall materials and some oils remained on the surface of the powder. The surface of 1 g of microencapsulated powder was washed with 10 mL n-hexane and shaken for 10 min. The mixture was filtered through Whatman paper No. 1. After filtration, the remaining powder was washed three times with 10 mL of n-hexane. The solvent was evaporated at 60 °C in a dry oven for 24 h. Encapsulation efficiency (%) was calculated as:
where TO is the total oil content added to the powder sample and SO is the surface oil content remaining on the surface of the powder sample.
Particle size distribution
The size distribution of powder particles was determined using a Mastersizer3000 laser light diffraction instrument (Malvern Instruments, Malvern, England). The particle size distribution were expressed as the volume-weighted mean diameter D[4,3] and surface-weighted mean diameter D[3,2]. D[4,3] is volume or mass moment mean of spheres while D[3,2] is the diameter of a sphere that has the same volume/surface area ratio. Each powder sample was measured in triplicate.
Moisture content and water activity
The moisture content of the microencapsulated powders was determined using a model C20 coulometric Karl Fisher titrator (Mettler-Toledo Intl., Columbus, OH, USA). Approximately 10 mg of powder was mixed with 10 mL of methanol and titrated with Karl Fisher reagent (Fluka, Bushs, Switzerland) until the endpoint was reached. The water activity (Aw) of the powder samples was measured using a Series 4TE DUO Aqualab Water Activity Meter (Decagon Devices Inc, Pullman, WA, USA) at 25 °C.
Water solubility
The water solubility of the microencapsulated powders was determined according to the method proposed by Fernandes et al. (2013) with slight modifications. Powders (0.5 g) were dissolved in 25 mL distilled water by vortex mixing for 5 min. The solution was then centrifuged at 3000 g for 10 min using a table top centrifuge (VS-5500N, Vision Co., Seoul, Korea). Twenty milliliter aliquots of the supernatant were transferred to crystal dishes and dried overnight at 105 °C in a dry oven. Water solubility (%) was calculated as the percentage of dried supernatant relative to the amount of powder originally added (0.5 g).
Scanning electron microscopy (SEM)
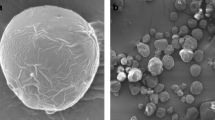
The external microstructures of the microencapsulated powders were observed by SEM using a model SNE-3000 M microscope (SEC Inc., Suwon, Korea). The samples were glued onto an adhesive tape mounted on the specimen stub, and the particles were covered with gold–palladium prior to analysis. Representative SEM images of freeze-dried and spray-dried samples are shown.
Headspace oxygen content analysis
The headspace oxygen content in airtight sample bottles of microencapsulated powders was analyzed according to the method of Kim et al. (2022). Briefly, 40 μL of headspace gas in the 10 mL vial was injected to a gas chromatograph (GC) with a thermal conductivity detector and peak area of oxygen molecules was calculated.
Lipid hydroperoxides analysis
Lipid hydroperoxides of the microencapsulated powders were determined as previously described (Yi et al., 2018). The samples were incubated for 20 min at room temperature and the absorbance at 510 nm was measured using a model UV-1650PC UV/VIS-spectrometer (Shimadzu, Kyoto, Japan). The concentration of lipid hydroperoxide was calculated using the cumene hydroperoxide standard curve.
Volatile analysis by solid phase microextraction-gas chromatography/mass spectrometry (SPME-GC/MS)
The headspace volatiles in encapsulated powders were determined using SPME-GC/MS (Agilent Technologies, Inc., Santa Clara, CA, USA) as previously described (Kim et al., 2016). Each powder sample (0.5 g) was put in a 10 mL vial and tightly air-sealed with a rubber septum and aluminum cap. All samples were prepared in triplicate.
Volatile identification was carried out by a combination of NIST Mass Spectra and retention index, which was calculated using standard hydrocarbon mixtures (C6–C17).
Statistical analysis
All data were statistically analyzed by analysis of variance (ANOVA) and Duncan’s multiple range test using the SPSS program version 19 (SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered statistically significant.
Results and discussion
Physicochemical properties
The physicochemical properties, including encapsulation efficiency, particle size, moisture, Aw, and water solubility of the freeze-dried powders (FDP) and spray-dried powders (SDP) are shown in Table 1. As the perilla oil content increased in the powders, the encapsulation efficiency of the powder samples decreased, regardless of the drying method. As the perilla oil content increased from 1 to 4%, the encapsulation efficiency decreased from 85.71 to 51.22% in FDP and from 90.74 to 77.38% in SDP. Spray-drying produced 1.04 to 1.51 times higher encapsulation efficiency values than freeze-drying. The finding implies that spray-drying is a more economical technique that decreases the loss of core materials during encapsulation. A certain amount of oil cannot be wrapped in the wall materials and remains on the external surfaces of the powders (Table 1), which could react with oxygen molecules and lower the oxidative stability of the perilla oil powder.
Generally, SDP had smaller particle sizes than FDP. The D[4,3] and D[3,2] values in the FDP increased from 154.0 to 192.0 μm and 52.17 to 73.07 μm, respectively as the amount of perilla oil increased from 1 to 4%. For SDP, the D[4,3] and D[3,2] values increased proportionally as the content of perilla oil increased from 1 to 3%, whereas SDP4 had a smaller particle size than the other samples (Table 1). Spray-drying transforms the O/W emulsion into powder through a complexation procedure, and the oil positioned on the surface of the powder may inhibit the complexation procedure, which could limit the growth of powder size when perilla oil was present at 4% in the O/W emulsion. This speculation is supported by observations of Guo et al. (2022) that when the oil-holding capacity of the wall materials was not sufficient to encapsulate all the amounts of oil, surplus oil on the surface of the droplets prevented aggregation during the spray-drying stage and resulted in a smaller particle size of the microcapsule.
The corresponding size distributions of the FDP and SDP powders are shown in Fig. 1. The particle size of FDP (154.00–192.00 μm) in volume-weight mean diameter was 2.56–24.49 times larger than that of SDP (7.84–72.03 μm) (Table 1). The diameter of most freeze-dried microparticles are approximately 100 μm, while the diameters of spray-dried particles are approximately 10 μm (Guo et al., 2020). Usually, freeze-drying tends to generate larger particles than spray-drying. The processing temperature is the major factor that determines the particle size. Relatively low processing temperatures can produce large particles, whereas high temperatures can produce small particles (Pellicer et al., 2019). Therefore, spray drying is recommended for the purpose of obtaining particles with a small size less than 100 μm.
Particle size of perilla oil powders with different oil contents using a freeze drier (A) and a spray drier (B). ‘FDP1’, ‘FDP2’, ‘FDP3’, and ‘FDP4’ are freeze-dried powders containing 1%, 2%, 3%, and 4% perilla oil in O/W emulsion before encapsulated, respectively. ‘SDP1’, ‘SDP2’, ‘SDP3’, and ‘SDP4’ are spray-dried powders with aformentioned oil content. Different letters on the graph were significantly different at 0.05
Moisture content of FDP powders ranged from 264.73 to 310.10 ppm, while those of SDP powders ranged from 551.50 to 711.90 ppm. The Aw of FDP and SDP powders ranged from 0.032 to 0.033, and 0.221 to 0.339, respectively. Moisture content and Aw are two essential parameters that affect the powder quality and shelf life (Papoutsis et al., 2018). The moisture content represents the water composition in a food system, whereas Aw represents the availability of free water in a food system that is responsible for biochemical reactions (Yamashita et al., 2017). The moisture content of the powders produced by spray-drying was significantly higher than that produced by freeze-drying (p < 0.05) (Table 1). The finding agrees with that of Ramírez et al. (2015), who reported that the microparticles produced by freeze-drying had a lower moisture content than those produced by spray-drying. The higher moisture content in SDP could be due to the relatively short treatment time compared to that of the freeze-drying procedure. Spray-drying takes approximately 5 h to produce SDP, while at least 48 h is required to produce FDP through freeze-drying. The relatively short treatment time of hot air may allow sufficient time for solidification through denaturation of whey proteins and gelatinization of maltodextrin, but is not a sufficient time for the complete removal of moisture from the wall materials.
The water solubility of FDP ranged from 67.50 to 76.50%, whereas that of SDP ranged from 65.00 to 75.50%. For both the FDP and SDP powders, the samples with lower moisture content showed higher water solubility. Tuyen et al. (2014) reported that lower moisture content leads to increased water solubility of the encapsulated powders. Water solubility describes the rate and extent to which the powder particles dissolve into water, and low water solubility can be attributed to the higher oil concentration, which results in a reduction in their affinity for water (Barroso et al., 2014). Even though the measured results of water solubility were similar for FDP and SDP, FDP was immediately dissolved in water at room temperature, whereas SDP required handshaking or vortex mixing to disperse the particles.
SEM images of the external microstructure of the FDP and SDP powders are shown in Fig. 2. The FDP particles had sharp edges and were amorphous and porous. These porous microstructures may be formed by freeze-drying using the principle of sublimation and dehydration (Silva et al., 2013). The SDP particles appeared spherical and homogenous without cavities or wrinkles on the surface of the samples. Similar morphological observations were reported by El-Messery et al. (2020) and Eratte et al. (2014). Pores of SDP powders than the FDP indicate lower permeability of external oxygen into the core and inhibit perilla oils as core materials from leaking out of powders. For this reason, SDP powders may have a higher encapsulation efficiency than FDP powders, as mentioned previously.
Oxidative stability
The changes in the headspace oxygen content of the FDP and SDP powders are shown in Fig. 3. The headspace oxygen content of FDP1, FDP2, FDP3, and FDP4 was15.45, 13.80, 5.98, and 4.96% on day 5, respectively, implying that FDP1 and FDP2 had higher oxidation stabilities than the others. However, FDP2 had a headspace oxygen content similar to that of FDP3 and 4 on day 7. Therefore, FDP1 exhibited the highest oxidative stability under these experimental conditions. Considering the encapsulation efficiency of FDP (Table 1), the oil remaining on the surface of the powders may play an important role in determining its oxidative stability.
Changes of headspace oxygen content of perilla oil powders with different oil contents using a freeze dryer (A) and a spray dryer (B) at 60 °C. Figure symbols are listed in the Fig. 1 legend. Different letters are significantly different in the same time at 0.05
The headspace oxygen content of SDP1 and SDP4 on day 5 was 11.34 and 11.01%, respectively, while those of all the SDP samples were approximately 5% on day 7, implying that SDP had lower oxidative stability than FDP. These results showed a similar trend in the results of Aw, and it could be inferred that samples with a lower Aw of approximately 0.221 and 0.231 compared to the SDP2 and 3 samples, which had a high headspace oxygen content on day 5.
Changes in the lipid hydroperoxides of FDP and SDP are shown in Fig. 4. The lipid hydroperoxides of FDP 1 and SDP1 increased to 55.96 and 114.66 mmol/kg oil, respectively, while other samples had lower lipid hydroperoxide values (Fig. 4). More than two lipid hydroperoxides have been identified in SDP1. Lipid hydroperoxides in both FDP1 and SDP1were the highest after 7 days of storage, which did not agree with the results of the headspace oxygen content (Fig. 3). SDP1 and FDP1, which have high encapsulation efficiency, should have high oxidative stability because of the small amount of oil remaining on the surface, which is not consistent with the results shown in Fig. 3. An assay using lipid hydroperoxides may not be appropriate for evaluating the oxidative stability of powders.
Changes of lipid hydroperoxides of perilla oil powders with different oil contents using a freeze dryer (A) and a spray dryer (B) at 60 °C. Figure symbols are listed in the Fig. 1 legend. Different letters are significantly different in the same time at 0.05
Volatile compounds
The volatile profile changes of FDP4 and SDP4 before and after 7 days of storage at 60 °C are shown in Table 2. RI can help to identify the peaks of volatiles in GC chromatogram. The amount of total volatile compounds of FDP increased from 4.25 to 212.45 (× 106) ion count after 7 days storage, while those of the SDP increased significantly from 4.17 to 154.56 (× 106) ion count. Although fewer than four volatiles were detected in both the FDP and SDP samples, the number of volatiles increased to 15 and 19 in FDP and SDP, respectively, after oxidation at 60 °C. Hexanal and 2-octenal, which are typical lipid oxidation products, increased more in FDP than in SDP. However, some volatiles, including 1-penten-3-ol, 1-octen-3-ol, (3E)-3-octen-2-one, and 3,5-octadien-2-one, were only detected in SDP, implying that a relatively high treatment temperature (inlet:180 °C and outlet:90 °C) during the drying process could accelerate volatile formation. In addition, straight-chain alcohols were detected only in SDP, and ketones were detected more frequently in SDP than in FDP. According to Curioni and Bosset (2002), ketones can be formed from related fatty acids via β-oxidation. These compounds can break down to the corresponding secondary alcohols. Spray-drying was adapted to a relatively high temperature for a short time, which could induce lipid oxidation, whey protein denaturation, and maltodextrin gelatinization. Although freeze-drying may not accelerate the above chemical reactions, lipid oxidation may take place quickly in FDP with relatively high surface areas.
In conclusion, FDP showed higher oxidative stability and water solubility than SDP, whereas SDP had a higher encapsulation efficiency and smaller particle size than FDP. Although perilla oils were successfully transformed into powder forms, the oxidative stability of the powders requires substantial improvement. Unencapsulated oils or oils on the surface may play a critical role in relatively low oxidative stability. Appropriate drying methods should be selected depending on the application of the powders in food matrices because encapsulated powders possess different water solubility and oxidative stability.
References
Ahn JH, Kim YP, Lee YM, Seo EM, Lee KW, Kim HS. Optimization of microencapsulation of seed oil by response surface methodology. Food Chemistry. 107:98-105 (2008)
Anwar SH, Kunz B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. Journal of Food Engineering. 105:367-378 (2011)
Asif M. Health effects of omega-3, 6, 9 fatty acids: Perilla frutescens is a good example of plant oils. Oriental Pharmacy and Experimental Medicine. 11:51-59 (2011)
Aziz S, Gill J, Dutilleul P, Neufeld R, Kermasha S. Microencapsulation of krill oil using complex coacervation. Journal of Microencapsulation. 31:774-784 (2014)
Barroso AKM, Pierucci APTR, Freitas SP, Torres AG, Rocha-Leão MHMD. Oxidative stability and sensory evaluation of microencapsulated flaxseed oil. Journal of Microencapsulation. 31:193-201 (2014)
Bringas-Lantigua M, Expósito-Molina I, Reineccius GA, López-Hernández O, Pino JA. Influence of spray-dryer air temperatures on encapsulated mandarin oil. Drying Technology. 29:520-526 (2011)
Carneiro HC, Tonon RV, Grosso CR, Hubinger MD. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. Journal of Food Engineering. 115:443-451 (2013)
Curioni PMG, Bosset JO. Key odorants in various cheese types as determined by gas chromatography-olfactometry. International Dairy Journal. 12:959-984 (2002)
Elik A, Yanık DK, Göğüş F. A comparative study of encapsulation of carotenoid enriched-flaxseed oil and flaxseed oil by spray freeze-drying and spray drying techniques. LWT. 143:111153 (2021)
El-Messery TM, Altuntas U, Altin G, Özçelik B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocolloids. 106:105890 (2020)
Eratte D, Wang B, Dowling K, Barrow CJ, Adhikari BP. Complex coacervation with whey protein isolate and gum arabic for the microencapsulation of omega-3 rich tuna oil. Food and Function. 5:2743-2750 (2014)
Fang Z, Bhandari B. Encapsulation of polyphenols–a review. Trends in Food Science and Technology. 21:510-523 (2010)
Fernandes RVDB, Borges SV, Botrel DA. Influence of spray drying operating conditions on microencapsulated rosemary essential oil properties. Food Science and Technology. 33:171-178 (2013)
Guo J, Li P, Kong L, Xu B. Microencapsulation of curcumin by spray drying and freeze drying. LWT. 132:109892 (2020)
Guo B, Zhu C, Huang Z, Yang R, Liu C. Microcapsules with slow-release characteristics prepared by soluble small molecular starch fractions through the spray drying method. International Journal of Biological Macromolecules. 200:34-41 (2022)
Gutiérrez-del-Río I, López-Ibáñez S, Magadán-Corpas P, Fernández-Calleja L, Pérez-Valero Á, Tuñón-Granda M, ..., Lombó F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants. 10:1264 (2021)
Hasani S, Ojagh SM, Ghorbani M. Nanoencapsulation of lemon essential oil in Chitosan-Hicap system. Part 1: Study on its physical and structural characteristics. International Journal of Biological Macromolecules. 115:143-151 (2018)
Himmetagaoglu AB, Erbay Z, Cam M. Production of microencapsulated cream: Impact of wall materials and their ratio. International Dairy Journal. 83:20-27 (2018)
Jalali-Jivan M, Garavand F, Jafari SM. Microemulsions as nano-reactors for the solubilization, separation, purification and encapsulation of bioactive compounds. Advances in Colloid and Interface Science. 283:102227 (2020)
Kagami Y, Sugimura S, Fujishima N, Matsuda K, Kometani T, Matsumura Y. Oxidative stability, structure, and physical characteristics of microcapsules formed by spray drying of fish oil with protein and dextrin wall materials. Journal of Food Science. 68:2248-2255 (2003).
Kaushik P, Dowling K, Barrow CJ, Adhikari B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. Journal of Functional Foods. 19:868-881 (2015)
Kim HR, Dhungana SK, Kim ID, Park IJ. Physicochemical and sensory characteristics of pepper oil sauce prepared from perilla oil. African Journal of Food Science. 10:352-358 (2016)
Kim YH, Lee YH, Lee JH. The oxidative stability and aldehyde formations in corn oils by infrared based and hot air-circulating cookers. Food Science and Biotechnology. 31:1433-1442 (2022)
Lee JH, Kim MJ, Jung MH. Seed oil (Sesame Seed, Perilla Seed). pp. 291-318. In: Korean Functional Foods: Composition, Processing and Health Benefits. Park KY, Kwon DY, Lee KW, Park S (eds), CRC press, Inc., Boca Raton, FL, USA (2017)
Murali S, Kar A, Mohapatra D, Kalia P. Encapsulation of black carrot juice using spray and freeze drying. Food Science and Technology International. 21:604-612 (2015)
Naik A, Meda V, Lele SS. Freeze drying for microencapsulation of α‐linolenic acid rich oil: A functional ingredient from Lepidium sativum seeds. European Journal of Lipid Science and Technology. 116:837-846 (2014)
Narisawa T, Fukaura Y, Yazawa K, Ishikawa C, Isoda Y, Nishizawa Y. Colon cancer prevention with a small amount of dietary perilla oil high in alpha‐linolenic acid in an animal model. Cancer. 73:2069-2075 (1994)
Nguyen XC, Herberger JD, Burke PA. Protein powders for encapsulation: a comparison of spray-freeze drying and spray drying of darbepoetin alfa. Pharmaceutical Research. 21:507-514 (2004)
Papoutsis K, Golding JB, Vuong Q, Pristijono P, Stathopoulos CE, Scarlett CJ, Bowyer M. Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-Carrageenan. Foods. 7:115 (2018)
Pellicer JA, Fortea MI, Trabal J, Rodríguez-López MI, Gabaldón JA, Núñez-Delicado E. Stability of microencapsulated strawberry flavour by spray drying, freeze drying and fluid bed. Powder Technology. 347:179-185 (2019)
Ramírez MJ, Giraldo GI, Orrego CE. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technology. 277:89-96 (2015)
Ramos FM, Junior VS, Prata AS. Impact of vacuum spray drying on encapsulation of fish oil: Oxidative stability and encapsulation efficiency. Food Research International. 143: 110283 (2021)
Sánchez CO, Zavaleta EB, García GU, Solano GL, Díaz MR. Krill oil microencapsulation: Antioxidant activity, astaxanthin retention, encapsulation efficiency, fatty acids profile, in vitro bioaccessibility and storage stability. LWT. 147:111476 (2021)
Silva KA, Coelho MAZ, Calado VM, Rocha-Leão MH. Olive oil and lemonsalad dressing microencapsulated by freeze-drying. LWT-Food Science and Technology. 50:569-574 (2013)
Tuyen CK, Nguyen MH, Roach PD, Stathopoulos CE. Microencapsulation of Gac oil: Optimisation of spray drying conditions using response surface methodology. Powder Technology. 264:298-309 (2014)
Yamashita C, Chung MMS, dos Santos C, Mayer CRM, Moraes ICF, Branco IG. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT. 84:256-262 (2017)
Yi B, Kim MJ, Lee JH. Oxidative stability of oil-in-water emulsions with α-tocopherol, charged emulsifier, and different oxidative stress. Food Science and Biotechnology. 27:1571-1578 (2018)
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2020R1A2C2006600), funded by the Ministry of Education, Science, and Technology and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (321023‐5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koo, H., Kim, S. & Lee, J. Comparison of physicochemical properties and oxidative stability of microencapsulated perilla oil powder prepared by freeze-drying and spray-drying. Food Sci Biotechnol 32, 1831–1839 (2023). https://doi.org/10.1007/s10068-023-01299-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-023-01299-w