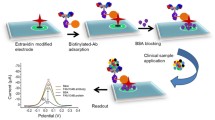
Abstract
Proteases are involved in cancer‚ taking part in immune (dis)regulation, malignant progression and tumour growth. Recently, it has been found that expression levels of one of the members of the serine protease family, trypsin, is upregulated in human cancer cells of several organs, being considered as a specific cancer biomarker. Considering the great attention that electrochemical peptide sensors have nowadays, in this work, we propose a novel electroanalytical strategy for the determination of this important biomolecule. It implies the immobilization of a short synthetic peptide sequence, dually labelled with fluorescein isothiocyanate (FITC) and biotin, onto neutravidin-modified magnetic beads (MBs), followed by the peptide digestion with trypsin. Upon peptide disruption, the modified MBs were incubated with a specific fluorescein Fab fragment antibody labelled with horseradish peroxidase (HRP-antiFITC) and magnetically captured on the surface of a screen-printed carbon electrode (SPCE), where amperometric detection was performed using the hydroquinone (HQ)/HRP/H2O2 system. The biosensor exhibited a good reproducibility of the measurements (RSD 3.4%, n = 10), and specificity against other proteins and proteases commonly found in biological samples. This work reports the first quantitative data so far on trypsin expression in human cell lysates. The developed bioplatform was used for the direct determination of this protease in lysates from pancreatic cancer, cervix carcinoma and kidney cells in only 3 h and 30 min using low amounts (~ 0.1 μg) of raw extracts.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteases are enzymes whose activity is expressed in proteolysis processes hydrolyzing peptide bonds by attacking the carbonyl groups of peptides [1, 2]. Although they are essential in fundamental biological processes in normal cells regulating cellular processes as gene expression, differentiation, and cell death, proteases are also involved in tumour growth and progression, both at primary and metastatic sites. Around one-third of all proteases are classified as serine (Ser) proteases containing a nucleophilic Ser residue at their active site. These proteases play a very important role in a variety of pathological processes like inflammation, atherosclerosis and cancer. Through a catalytic mechanism, the hydroxyl group of the active site Ser residue shows nucleophilic activity against the peptide bond resulting in the dissolution of some proteins implicated in vital activities [3, 4]. Trypsinogens and their corresponding active forms, trypsins from various sources, are Ser proteases that not only are involved in the digestion of dietary proteins but also induce proteolytic cascades by activating other proteases, such as metalloproteinases, and degrade many extracellular matrix components, thus playing a significant role in tumour progression [3, 5, 6]. Trypsin, produced in the pancreas by trypsinogen, is a well-characterized serine digestive enzyme that catalyses the selective hydrolysis of polypeptide chains of arginine or lysine amino acids in the C-terminal and has an important role in the regulation of pancreatic exocrine function [2, 7]. Owing to its anti-inflammatory and anti-swelling properties, it is used for surgical inflammation, ulcers and traumatic injuries [8, 9]. However, changes in trypsin levels are related to pathological processes as pancreatitis, vesicular or cystic fibrosis and cancer progression metastasis [3, 4, 6, 7, 9,10,11,12,13,14,15]. From an estimated and 18 million cancer cases around the world in 2018, 2.7% corresponded to pancreas cancer with a somewhat higher incidence in men (243,033 new cases) than in women (215,885 new cases) [16]. This cancer, almost always fatal, causes around 4% of all cancer deaths. In particular, trypsin participates in the invasion and metastasis of pancreatic cancer through promotion of the extracellular matrix degradation and may activate protease-activated receptor 2 (PAR-2) to stimulate pancreatic cancer cell proliferation and adhesion [5, 11]. Moreover, trypsin expression has been associated with colorectal cancers with a poor prognosis and shorter disease-free survival [10]. Consequently, trypsin is quantified in serum and urine as a reliable noninvasive biomarker and/or prognosticator for acute pancreatitis, cystic fibrosis, cancer and other pathological diseases screening [4, 17]. Clinically relevant ranges for trypsin in serum samples have been set at 5–15 nM for healthy individuals and 34–85 nM for pancreatic conditions patients [14, 18, 19]. Trypsin overexpressed levels of 26–281 nM have also been reported for cystic fibrosis, pancreatic carcinoma, and meconium ileus patients [20]. Detection of trypsin in pancreatic cell lysates has been reported as an important means for the diagnosis of chronic pancreatic diseases [21].
Therefore, the quantification of trypsin levels has attracted wide attention in recent years. However, there are still many challenges to overcome in this field and the development of sensitive, selective and noninvasive methods for the efficient and cost-effective determination of trypsin for monitoring of high-risk individuals and for clinical diagnoses and therapies is highly demanded nowadays [2, 9, 11, 13, 15, 20]. In this context, different techniques have been used for the detection of trypsin including (in alphabetical order): capacitance [12], chronocoulometry [22], colorimetry [2, 4], cyclic voltammetry [11], differential pulse voltammetry (DPV) [15], impedimetric measurements [23], fluorescence [2, 9, 17, 20, 24], phosphorescence [13, 21], photoelectrochemistry [7], photoluminescence [14], piezoelectric measurements [8], potentiometry [25] and square-wave voltammetry (SWV) [19, 26].
Seeking for a methodology for routine trypsin determination in an affordable way, implying simple and fast protocols, compatible with decentralized environments, this work reports the first peptide-modified MB-assisted electrochemical biosensing approach for such purpose. Biomedical peptide-based biosensing is a booming research field due to inherent peptide properties such as the possibility to be used as probes taking advantage of their capability to form tertiary structures and recognize a diversity of target analytes. In addition, the easy conjugation with signal and/or immobilizations tags results in versatile sensors applicable in many fields [27]. Electrochemical peptide-based biosensors have been proposed for the determination of trypsin [15, 19, 26]. In fact, the use of electrochemical detection and peptides as bioreceptors make these biotools competitive compared to the conventional ELISA long adopted in centralized laboratories for the analysis of protein antigens (such as proteases). ELISA requires relatively expensive (plate readers), hardly portable and miniaturizable instrumentation as well as the use of antibodies, bioreceptors less favourable than peptides in terms of size, production, versatility of functionalization and stability [27, 28]. However, the peptide-based electrochemical biosensors reported so far for trypsin determination involve quite complex or time-consuming protocols for the modification of the conventional electrode surfaces and only one of them showed applicability for the analysis of spiked human serum samples [15].
Unlike the reported electrochemical biosensors, the strategy described in this paper involves a simpler and shorter protocol implemented on the surface of MBs and amperometric detection at unmodified SPCEs. The strategy is based on the immobilization of a dually FITC and biotin-modified peptide probe on the surface of magnetic microbeads (MBs), its further cleavage by the target enzyme and enzymatic labelling with Fab fragments from anti-fluorescein antibody conjugated with horseradish peroxidase (HRP-anti-FITC Fab fragments). Therefore, the method is an “on-off” approach because of the lower number of HRP-anti-FITC Fab fragments attached upon the loss of the peptide fragment bearing the FITC moiety from the MBs surface due to the trypsin enzymatic cleavage. The modified MBs were magnetically captured on the surface of the working SPCE and the activity of the HRP attached to the MBs was amperometrically measured at a potential of − 0.20 V vs. the Ag pseudoreference electrode, in the presence of the enzymatic substrate (H2O2) and hydroquinone (HQ) as redox mediator.
Materials and methods
Apparatus and electrodes
A CHI1140A (CH Instruments, Inc.) potentiostat controlled by the software CHI1140A, and a Magellan V 7.1 (TECAN) ELISA plate reader were used to make the amperometric and spectrophotometric measurements, respectively. Screen-printed carbon electrodes (SPCEs) (DRP-110) and the specific cable connector (DRP-CAC) were purchased from Metrohm DropSens, S.L. All measurements were carried out at room temperature. Other instruments used were as follows: Vortex (Velp Scientifica), BioSan TS-100 constant temperature incubator shaker (Thermo), magnetic separator DynaMag®2 (Invitrogen – ThermoFisher Scientific), magnetic stirrer (Inbea S.L.) and Basic pH-meter (Basic 20+, Crison). The capture of the modified MBs onto the SPCE surface was controlled by a neodymium magnet (AIMAN GZ) embedded in a home-made polymethacrylate casing.
Reagents and solutions
Neutravidin-modified magnetic microbeads (neutravidin-MBs, Ø = 1 μm, 10 mg mL−1) were purchased from SpeedBeads™ (GE Healthcare, ref. 78,152,104,010,350). The dually labelled peptide (FITC-[AEEA]FRR[AEEA]-Btn, sequence adapted from [19, 26]) was synthesized and provided by Pepscan. The peptide consisted of three amino acids (two l-arginine (R) and one l-phenylalanine (F)) with spacers on each side ((2-[2-(Fmoc-amino)ethoxy]ethoxy)acetic acid (AEEA)) and labelled with biotin (Btn) on C-terminus and fluorescein isothiocyanate (FITC) at the other end. A small ethylenediamine spacer was used in front of the biotin functionalization on the C-terminus as well as in front of the FITC to prevent cleavage of the FITC from a so-called Edman degradation reaction. Blocker casein solution (BB solution: phosphate-buffered saline (PBS) containing 1% w/v casein, pH 7.4) and trypsin-EDTA (0.05%, ref. 25,300,054) were purchased from Thermo Fisher Scientific, and sodium hydroxide (NaOH) from Labkem. Sodium chloride (NaCl), potassium chloride (KCl), sodium di-hydrogen phosphate dihydrate (NaH2PO4 × 2H2O) and anhydrous di-sodium hydrogen phosphate (Na2HPO4) were purchased from Scharlab. Calcium nitrate tetrahydrate (Ca(NO3)2 × 4H2O) was acquired in Panreac (ref. 141,231.1211). Casein, hydroquinone (HQ), hydrogen peroxide (H2O2) (30%, w/v), trypsin (ref. T0303) and α-chymotrypsin from porcine pancreas (ref. C3142), lysozyme human (ref. L1667), pepsin from porcine gastric mucosa (ref. P6887), proteinase K from Tritirachium album (ref. P2308), human haemoglobin (ref. H7379) and IgG from human serum (ref. I2511) were purchased from Sigma-Aldrich. Fab fragments from anti-fluorescein antibody conjugated with horseradish peroxidase (HRP-anti-FITC Fab fragments) were purchased from Roche Diagnostics GmbH (ref. 11,426,346,910). Bovine serum albumin (BSA from GERBU Biotechnik GmbH, ref. 1507.0025), recombinant full length human p53 protein from EMD Millipore Corporation (ref. P6249) and recombinant human TNFα protein from BD Pharmigen (ref. 55,618) were also used. All reagents were of the highest available grade.
The following buffer solutions, prepared with deionized water from a Milli-pore Milli-Q purification system (18.2 MΩ cm), were employed: 0.05 M phosphate buffer, pH 6.0; and phosphate-buffered saline (PBS) consisting of 0.01 M phosphate buffer solution containing 137 mM NaCl and 2.7 mM KCl, pH 7.5. In both cases, pH was adjusted with 2 M NaOH.
Other solutions used were as follows: 0.01 μM peptide solution in PBS (pH 7.5); 1/1000 HRP-anti-fluorescein diluted in 1:1 mixture of BB and PBS (1:1 PBS/BB); trypsin and trypsin mixed with potential interferences solutions in PBS (pH 7.5); and freshly prepared 0.1 M HQ and 0.1 M H2O2 solutions in 0.05 M phosphate buffer (pH 6.0).
Peptide-based biosensing
A 2-μL aliquot of neutravidin-MBs was transferred into a 1.5-mL Eppendorf tube and washed twice with 50 μL of PBS solution, pH 7.5. MBs were placed in the magnetic separator and concentrated for 3 min before removing the supernatant. Thereafter, neutravidin-MBs were functionalized by incubation with 25 μL of 0.01 μM peptide solution under continuous stirring for 15 min (37 °C, 950 rpm). Subsequently, MBs were washed twice with 50 μL of PBS and incubated for 3 h (37 °C, 950 rpm) in 25 μL of a solution containing variable concentrations of the target protease (or the sample to be analysed) to allow the peptide digestion process. After two further washings, with 50 μL of a 1:1 PBS/BB mixture solution, the peptide-MBs conjugates were labelled by incubation in 25 μL of a 1/1000 HRP-anti-FITC Fab fragments solution for 15 min (37 °C, 950 rpm). The resulting MBs were washed twice with a 1:1 PBS/BB mixture solution and re-suspended in 50 μL of 0.05 M phosphate buffer (pH 6.0) to perform the amperometric measurements. Amperometric detection was carried out as described previously [29].
Analysis of a commercial trypsin standard and cell lysates
The developed peptide-based biosensing method was used for the analysis of a trypsin-EDTA standard with a known concentration (0.05% w/v) of the enzyme. The trypsin standard was composed by trypsin, Phenol Red, EDTA, d-glucose and some inorganic salts (KCl, KH2PO4, NaHCO3, NaCl and Na2HPO4 × 7H2O), and it is commonly used for cell and tissue dissociation and routine cell culture passage. The determination of trypsin was carried out by applying the standard additions method in the presence of a 1/200 diluted reference pattern in PBS by spiking it with increasing concentrations (25–100 nM) of trypsin standard solutions.
In addition, the developed peptide-based biosensor was used to analyse lysates from human embryonic kidney cell line, which expresses a mutant version of the SV40 large T antigen (HEK293T), as well as lysates from cervical (HeLa) and pancreatic (BxPC3 and PANC-1) cancer cells. All these cells, obtained from the ATCC cell line repository, were cultured and lysed as described previously [30, 31] and stored at − 80 °C till used. The quantification of trypsin in the cell lysates was performed by applying the standard additions method by adding increasing concentrations of trypsin standard solutions (25–100 nM) in the presence of 0.1 μg of each cell lysate.
Results and discussion
Figure 1 shows a scheme of the on-off peptide-based biosensing platform developed for the determination of trypsin. The preparation protocol involved a first step where a FITC and biotin double-labelled peptide was immobilized through the biotinylated end on the surface of neutravidin-MBs. This type of MBs were chosen as solid supports because they possess advantages in terms of lower non-specific adsorptions and higher affinity toward biotin over the most commonly used Streptavidin-MBs [32, 33]. After incubation in the presence of the target trypsin, which selectively cleaves the peptide on the C-terminal end of arginine, the peptide portion containing the FITC moiety was released and washed away from the modified MBs. Subsequently, the modified MBs were incubated in the presence of HRP-anti-FITC Fab fragments, and the on-off electrochemical biosensing procedure was implemented through magnetic capture of the MBs on the SPCE surface where amperometric transduction was carried out using the hydroquinone (HQ)/HRP/H2O2 system at a detection potential of − 0.20 V (vs the Ag pseudoreference electrode). In the absence of trypsin, the peptide probe is intact and a high number of HRP-anti-FITC Fab fragments were attached to the MBs providing a large amperometric response (amperogram b) in Fig. 1). However, the higher the trypsin concentration in the sample, the higher number of peptide molecules are cleaved and a lower amount of HRP-anti-FITC Fab fragments were attached to the MBs resulting in smaller amperometric responses (amperogram a) in Fig. 1).
Optimization of the experimental variables
The working variables involved in the preparation and behaviour of the peptide-based biosensing platform, together with the tested ranges and the selected values, are summarized in Table 1 and the corresponding experimental results shown in Fig. 2. The respective variable value was selected according to the ratio obtained at − 0.20 V (vs. the Ag pseudoreference electrode) between the amperometric signals measured for 0.0 (blank, B) and 250 (signal, S) nM trypsin (blank-to-signal, B/S, ratio). The detection potential and the HQ/H2O2 concentrations were the same that those previously optimized [34, 35].
Optimization of the experimental variables involved in the construction of the peptide-based biosensing platform developed for the determination of trypsin. MBs volume (a); peptide concentration (b); peptide incubation time (c); trypsin incubation time (d); HRP-anti-FITC Fab fragments dilution factor (e); HRP-anti-FITC Fab fragments incubation time (f). Amperometric responses measured in the absence (white bars) or in the presence (grey bars) of 250 nM trypsin standard solutions, and the resulting blank-to-signal ratios (B/S, red lines). Error bars estimated at triple of the standard deviation (n = 3)
A higher reaction efficiency was observed when the peptide-modified MBs/trypsin mixture was incubated at 950 rpm and 37 °C, close to the physiological temperature. This is in agreement with previous reports that showed an increase of the trypsin digestive activity at 37 °C [13, 15, 20]. As it can be seen in Fig. 2a, a volume of 2 μL MBs provided larger B/S ratios. Larger volumes provoked a decrease in such ratio due to an increase of the blank signals, as expected for a higher amount of immobilized peptide, but also because of an increase in the amperometric signals measured after incubation with trypsin, which was probably due to steric hindrance hampering trypsin digestive action in the presence of large amounts of immobilized peptide. In fact, the same effect was observed for increased concentrations of the peptide at a fixed MB volume (Fig. 2b). Moreover, the amperometric signals were stabilized from a peptide immobilization time of 30 min (Fig. 2c). Figure 2d shows a larger B/S ratio was obtained when a digestion time of 3 h was used. It is assumed that trypsin cannot act with the same efficacy for shorter times, leaving more undigested peptide and, therefore, more FITC to bind the signal tags (HRP-anti-FITC Fab fragments), thus providing larger amperometric signals. Moreover, if longer trypsin incubation times were employed, a progressive increase in the target signal was observed which again could be due to steric hindrance, preventing proteolysis of some peptide bonds by trypsin.
The influence of the HRP-anti-FITC Fab fragments concentration on the amperometric signals through dilution of the commercial solution with a 1/1 PBS:BB solution in the range between 1/5000 and 1/250 is shown in Fig. 2e. Although both the blank and the current measured in the presence of trypsin increased progressively with the concentration of HRP-anti-FITC Fab fragments, the values of the B/S ratio did not change significantly. It was decided to select a 1/1000 HRP-anti-FITC Fab fragments dilution because this value allowed precise measurements in the presence of trypsin and provided convenient B/S ratios. The influence of the HRP-anti-FITC Fab fragments incubation time on the amperometric responses was evaluated in the range between 15 and 60 min using a 1/1000 HRP-anti-FITC Fab fragments dilution. As can be seen in Fig. 2f, the B/S ratio decreased as this incubation time increased which may be due to an increase of nonspecific adsorptions with long HRP-anti-FITC Fab fragments incubation times. Therefore, 15 min HRP-anti-FITC Fab fragments incubation was selected.
In stepwise procedures, it is important to reduce the assay time by lowering the number of the involved steps. Thus, two procedures involving a different number of incubation steps were tested. A three-step protocol with successive incubations for the immobilization of the peptide on MBs (15 min), followed by trypsin digestion (3 h) and subsequent labelling by incubation of the modified MBs for 15 min in the HRP-anti-FITC Fab fragments solution. A two-step procedure where, after the 15 min peptide immobilization on the MBs, the modified MBs were incubated for 3 h in a mixture solution of trypsin and the HRP-anti-FITC Fab fragments. A significant increase in the B/S ratio was observed when changing from the two to the three steps protocol (not shown). This fact was attributed to the steric hindrance produced when the HRP-anti-FITC Fab fragments and trypsin were incubated together in the same solution most likely because the proteolytic efficiency of the target protein is lower for HRP-anti-FITC Fab fragments-tagged peptides than for the free peptide.
Calibration graph, analytical characteristics, stability and selectivity
Under the optimized experimental variables listed in Table 1, the developed amperometric peptide-based biosensing platform provided a linear calibration plot over the 23 to 250 nM trypsin concentration range (Fig. 3a), with a slope value of (−14.7 ± 0.5) nA nM−1, an intercept of (7.58 ± 0.07) μA and a correlation coefficient (R2) of 0.9996. As can be observed, the decreasing signal tended to level off without reaching zero which has been attributed to some redox-tagged peptides were not accessible to trypsin cleavage [26]. Limits of detection (LOD) of 7 nM (calculated as 3 × sb/slope) and quantification (LQ) of 23 nM (10 × sb/slope) were obtained. These values are lower than the established clinical threshold trypsin level in serum of patients with pancreatic cancer [19], which advanced the possible usefulness of this bioplatform for the analysis of clinical samples. In addition, a relative standard deviation value of 3.4 % was calculated for ten assays, thus showing the good reproducibility of the developed methodology allowing the determination of trypsin in about 3 h. Moreover, the bioconjugates formed by the double-labelled peptide-modified MBs could be stored in 10 mM filtered PBS (pH 7.5) at 4 °C for 7 days without a significant loss of sensitivity in the further measurements.
Calibration graphs and amperometric traces recorded with different trypsin concentrations (a). Peptide-based biosensor selectivity toward trypsin (b); current values measured for 0.0 (white bars) and 100 (grey bars) nM trypsin in the absence (buffer) and in the presence of: 1.0 mg mL−1 IgG, 5.0 mg mL−1 BSA, 5.0 mg mL−1 haemoglobin, 100 nM casein, 1 mM Ca2+, 10 ng mL−1 TNFα, 200 ng mL−1 p53, and 0.4 μM pepsin, proteinase K, α-chymotrypsin and lysozyme. Supporting electrolyte, 0.05 M sodium phosphate solution, pH 6.0; Eapp = −0.20 V vs. the Ag pseudoreference electrode. Other conditions are as described in Table 1 (selected values column). B/S ratios (red line) are those obtained for each experimental point. Error bars estimated as triple that of the standard deviation (n = 3)
Table 2 shows a comparison of the fundamentals and main analytical characteristics of the developed method with those reported in the last 5 years for the determination of trypsin. As it can be seen, most of the recently reported methodologies have been tested in spiked serum and urine samples from healthy individuals. Only Wu et al. [21] assayed their phosphorescence-based sensor in cell lysates but, although their method could differentiate between pancreatic and non-pancreatic cells, no quantitative data of trypsin concentration were given. Furthermore, most of the reported methodologies need long and laborious synthesis and fabrication procedures to prepare the sensing systems, either optical or electrochemical, while the approach described in this work only needs 15 min to prepare the peptide-MBs. Moreover, although several methods based on proteins hydrolysis by trypsin have been described, only two groups have reported peptide-based electrochemical sensors [15, 19, 26]. Although they obtained lower LODs than that achieved in this work, none of such sensors used disposable electrodes, and demanded relatively complex or time-consuming protocols (~ 13 h) for the modification of the conventional electrode surfaces. In addition, these methods were not tested in real samples [19, 26] or were applied to spiked human serum samples [15]. Moreover, as it is shown below, the sensitivity achieved with the peptide-based biosensor reported here, which compares favourably with that of most of the reported methods, is enough for the direct determination of the target enzyme in a low amount of cell lysates.
The biosensor selectivity was evaluated by measuring the B/S ratios for 0 and 100 nM trypsin standards prepared in the absence and in the presence of potential interferences produced by other proteins (IgG, BSA, haemoglobin, casein), ions (Ca2+), cancer biomarkers (TNFα, p53) or proteases (pepsin, proteinase K, α-chymotrypsin and lysozyme) that coexist with trypsin in biological fluids. Figure 3b shows that the calculated B/S ratios at the high non-target concentration levels considered (1.0 mg mL−1 IgG, 5.0 mg mL−1 BSA, 5.0 mg mL−1 haemoglobin, 100 nM casein, 1 mM Ca2+, 10 ng mL−1 TNFα, 200 ng mL−1 p53, and 0.4 μM pepsin, proteinase K, α-chymotrypsin and lysozyme) were always within the experimental error range. Therefore, we can conclude that the developed biosensing strategy is highly selective to the target protease which can be attributed to the specific trypsin-triggered peptide proteolysis.
Determination of trypsin in a commercial standard and cell lysates
Firstly, the developed peptide-based biosensor was validated by determining the trypsin content in a commercial standard of known concentration (0.05 %) commonly used in cell and primary tissue dissociation. Three replicates were analysed after a simple 1/200 dilution in PBS (pH 7.5) by using the standard additions method. A trypsin concentration of (0.049 ± 0.002) % (n = 3, α = 0.05) was obtained indicating the great precision and accuracy of the measurements carried out with the bioplatform.
Furthermore, the peptide-based biosensor was used to analyse lysates from pancreatic cancer (BxPC3 and PANC-1), cervix carcinoma (HeLa) and kidney (HEK293T) cells. The determination of trypsin in this type of complex biological samples was carried out by the standard additions method, using only 0.1 μg of sample in each case. As can be seen in Fig. 4, a remarkably larger trypsin concentration was found in pancreatic cancer cell lysates (1850 ± 60 ng μg−1 for BxPC3 cells, and 1200 ± 100 ng μg−1 for PANC-1 cells) in comparison with kidney (410 ± 20 ng μg−1 for HEK293T) and cervical (490 ± 50) ng μg−1 for HeLa) cell lysates. The results obtained in the analysis of PANC-1, HEK293T and HeLa cells were in agreement with those found by Wu et al. using a label-free phosphorescent sensing method involving cytochrome C-capped Mn:ZnS quantum dots [21]. These findings show the ability of the developed biosensor to discriminate between pancreatic and non-pancreatic cancer cells providing as far as we know the first quantitative data of the target protease in this type of sample. However, it is important to mention that future efforts should focus on a more exhaustive validation by analysing more samples, by different users at different environments and using different methodologies.
Determination of trypsin determination in cancer cell lysates (0.1 μg) with the developed peptide-based biosensing platform (inset: representative examples of amperometric responses obtained for pancreatic cancer (BxPC3) and kidney (HEK293T) cell lysates). Error bars estimated as triple that of the standard deviation (n = 3)
Conclusions
This work describes the first peptide-MBs based biosensing method for the on-off amperometric determination of trypsin. This selective, precise, accurate and low-cost method has competitive advantages compared to other previously reported peptide-based electrochemical methodologies to be adapted for routine trypsin determination at the point-of-care due to the use of simpler, shorter and requiring fewer reagent protocols.
The high sensitivity of this bioplatform allows the determination of trypsin in real clinical samples and the quantification of the trypsin content in cell lysates with the ability to discriminate between pancreatic and non-pancreatic cancer cells. This research opens the way to future development of multiplexed systems allowing the simultaneous determination of various proteases in a single sample and integration of this kind of bioplatforms in automatic point-of-care systems. These advances would result in great advantages for diagnosis and routine clinical follow-up of prevalent diseases. It is important to note also that the developed method can easily be transferred to the determination of other biomarkers such as hydrolytic abzymes of great clinical relevance in neurodegenerative diseases such as multiple sclerosis.
References
Eatemadi A, Aiyelabegan HT, Negahdari B, Mazlomi MA, Daraee H, Daraee N, et al. Role of protease and protease inhibitors in cancer pathogenesis and treatment. Biomed Pharmacother. 2017;86:221–31.
Zhou Z, Liu W, Wang Y, Ding F, Liu X, Zhao Q, et al. A fluorometric and colorimetric method for determination of trypsin by exploiting the gold nanocluster-induced aggregation of hemoglobin-coated gold nanoparticles. Microchim Acta. 2019;186:272. https://doi.org/10.1007/s00604-019-3380-2.
Nyberg P, Ylipalosaari M, Sorsa T, Salo T. Trypsins and their role in carcinoma growth. Exp Cell Res. 2006;312:1219–28.
Lin X, Zhu Z, Zhao C, Li S, Liu Q, Liu A, et al. Robust oxidase mimicking activity of protamine-stabilized platinum nanoparticles units and applied for colorimetric sensor of trypsin and inhibitor. Sensors Actuators B Chem. 2019;284:346–53.
Nakanuma S-I, Tajima H, Okamoto K, Hayashi H, Nakagawara H, Onishi I, et al. Int J Oncol. 2010;36:793–800.
Peregrina-Sandoval J, del Toro-Arreola S, Oceguera-Villanueva A, Cerda-Camacho F, del Toro-Arreola A, Gonzalez-Ramella O, et al. Trypsin proteolytic activity in cervical cancer and precursor lesions. Int J Clin Exp Pathol. 2017;10(5):5587–93.
Kong W, Li Q, Xia L, Li X, Sun H, Kong R-M, et al. Photoelectrochemical determination of trypsin by using an indium tin oxide electrode modified with a composite prepared from MoS2 nanosheets and TiO2 nanorods. Microchim Acta. 2019;186:490. https://doi.org/10.1007/s00604-019-3589-0.
Karasevaa NA, Pluhara B, Beliaevab EA, Ermolaevab TN, Mizaikoff B. Synthesis and application of molecularly imprinted polymers for trypsin piezoelectric sensors. Sensors Actuators B Chem. 2019;280:272–9.
Liu Y, Zhang F, Heb X, Ma P, Huang Y, Tao S, et al. A novel and simple fluorescent sensor based on AgInZnS QDs for the detection of protamine and trypsin and imaging of cells. Sensors Actuators B Chem. 2019;294:263–9.
Soreide K, Janssen EA, Körner H, Baak JPA. Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–56.
Yi Q, Liu Q, Gao F, Chen Q, Wang G. Application of an electrochemical immunosensor with a MWCNT/PDAA modified electrode for detection of serum trypsin. Sensors. 2014;14:10203–12 https://www.mdpi.com/1424-8220/14/6/10203. Accessed 27 Nov 2019.
Ertürk G, Hedström M, Mattiasson B. A sensitive and real-time assay of trypsin by using molecular imprinting-based capacitive biosensor. Biosens Bioelectron. 2016;86:557–65.
Liu W, Li H, Wei Y, Dong C. A label-free phosphorescence sensing platform for trypsin based on Mn-ZnS QDs. RSC Adv. 2017;7:26930–4.
Xia T, Ma Q, Hu T, Su X. A novel magnetic/photoluminescence bifunctional nanohybrid for the determination of trypsin. Talanta. 2017;170:286–90.
Lin Y, Shen R, Liu N, Yi H, Dai H, Lin J. A highly sensitive peptide-based biosensor using NiCo2O4 nanosheets and g-C3N4 nanocomposite to construct amplified strategy for trypsin detection. Anal Chim Acta. 2018;1035:175–83.
Worldwide cancer data: Global cancer statistics for the most common cancers. CUP Continuous Update Project: Analysing research on cancer prevention and survival. World Cancer Research Fund International; American Institute for Cancer Research. 2018. https://www.wcrf.org/dietandcancer/cancer-trends/worldwide-cancer-data Accessed 27 Nov 2019.
Poon C-Y, Li Q, Zhang J, Li Z, Dong C, Lee AW-M, et al. FRET-based modified graphene quantum dots for direct trypsin quantification in urine. Anal Chim Acta. 2016;917:64–70.
Artigas JMG, Faure MRA, Garcia ME, Gimeno AMB. Serum trypsin levels in acute pancreatic and non-pancreatic abdominal conditions. Postgrad Med J. 1981;57:219–22.
González-Fernández E, Avlonitis N, Murray AF, Mount AR, Bradley M. Methylene blue not ferrocene: optimal reporters for electrochemical detection of protease activity. Biosens Bioelectron. 2016;84:82–8.
Li H, Yang M, Kong D, Jin R, Zhao X, Liu F, et al. Sensitive fluorescence sensor for point-of-care detection of trypsin using glutathione-stabilized gold nanoclusters. Sensors Actuators B Chem. 2019;282:366–72.
Wu P, Zhao T, Zhang J, Wu L, Hou X. Analyte-activable probe for protease based on cytochrome C-capped Mn:ZnS quantum dots. Anal Chem. 2014;86:10078–83.
Park S, Kim G, Seo J, Yang H. Ultrasensitive protease sensors using selective affinity binding, selective proteolytic reaction, and proximity-dependent electrochemical reaction. Anal Chem. 2016;88:11995–2000.
Banis G, Beardslee LA, Ghodssi R. Gelatin-enabled microsensor for pancreatic trypsin sensing. Appl Sci. 2018;8:208 https://www.mdpi.com/2076-3417/8/2/208. Accessed 27 Nov 2019.
Zhou G, Jiang H, Zhou Y, Liu P, Jia Y, Ye C. Peptide-coated palladium nanoparticle for highly sensitive bioanalysis of trypsin in human urine samples. Nanomater Nanotechnol. 2018;8:1–8.
Liang R, Ding J, Gao S, Qin W. Mussel-inspired surface-imprinted sensors for potentiometric label-free detection of biological species. Angew Chem Int Ed. 2017;56:6833–7.
González-Fernández E, Staderinia M, Avlonitis N, Murray AF, Mount AR, Bradley M. Effect of spacer length on the performance of peptide-based electrochemical biosensors for protease detection. Sensors Actuators B Chem. 2018;255:3040–6.
Karimzadeh A, Hasanzadeh M, Shadjou N, De la Guardia M. Peptide based biosensors. Trends Anal Chem. 2018;107:1–20.
Liu Q, Wang J, Boyd BJ. Peptide-based biosensors. Talanta. 2015;136:114–27.
Muñoz-San Martín C, Pedrero M, Manuel de Villena FJ, Garranzo-Asensio M, Rodríguez N, Domínguez G, et al. Disposable amperometric immunosensor for the determination of the E-cadherin tumor suppressorprotein in cancer cells and human tissues. Electroanalysis. 2019;31:309–17.
Barderas R, Desmet J, Timmerman P, Meloen R, Casal JI. Affinity maturation of antibodies assisted by in silico modeling. Proc Natl Acad Sci U S A. 2008;105(26):9029–34.
Barderas R, Babel I, Díaz-Uriarte R, Moreno V, Suárez A, Bonilla F, et al. An optimized predictor panel for colorectal cancer diagnosis based on the combination of tumor-associated antigens obtained from protein and phage microarrays. J Proteome. 2012;75(15):4647–55.
Zhao S, Walker DS, Reichert WM. Cooperativity in the binding of avidin to biotin-lipid-doped Langmuir-Blodgett films. Langmuir. 1993;9:3166–73.
Nguyen TT, Sly KL, Conboy JC. Comparison of the energetics of avidin, streptavidin, neutravidin, and anti-biotin antibody binding to biotinylated lipid bilayer examined by second-harmonic generation. Anal Chem. 2012;84:201–8.
Eguílaz M, Moreno-Guzmán M, Campuzano S, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM. An electrochemical immunosensor for testosterone using functionalized magnetic beads and screen-printed carbon electrodes. Biosens Bioelectron. 2010;26:517–22.
Gamella M, Campuzano S, Conzuelo F, Reviejo AJ, Pingarrón JM. Amperometric magnetoimmunosensor for direct determination of D-dimer in human serum. Electroanalysis. 2012;24:2235–43.
Funding
The financial support of the CTQ2015-64402-C2-1-R (Spanish Ministerio de Economía y Competitividad) Research Project and the TRANSNANOAVANSENS-CM Program from the Comunidad de Madrid (Grant S2018/NMT-4349) and predoctoral contract from Universidad Complutense de Madrid (C.M.-S.M.) are gratefully acknowledged. R.B. acknowledges the financial support of the PI17CIII/00045 Grant from the AES-ISCIII program. A.M-C. is a recipient of a FPU fellowship from the Ministerio de Educación, Cultura y Deporte.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Female Role Models in Analytical Chemistry.
Rights and permissions
About this article
Cite this article
Muñoz-San Martín, C., Pedrero, M., Gamella, M. et al. A novel peptide-based electrochemical biosensor for the determination of a metastasis-linked protease in pancreatic cancer cells. Anal Bioanal Chem 412, 6177–6188 (2020). https://doi.org/10.1007/s00216-020-02418-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02418-w