Abstract
Bariatric surgery is an effective procedure to achieve weight loss in obese patients. However, homeostasis of essential metals may be disrupted as the main absorption site is bypassed. In this study, we determined Cu, Fe and Zn isotopic compositions in paired serum and whole blood samples of patients who underwent Roux-en-Y gastric bypass (RYGB) surgery for evaluation of longitudinal changes and their potential relation to mineral element concentrations and relevant clinical parameters used for monitoring the patient’s condition. Samples from eight patients were collected pre-surgery and at 3, 6 and 12 months post-surgery. Multi-collector inductively coupled plasma-mass spectrometry (MC-ICP-MS) was used for high-precision isotope ratio measurements. Alterations in metal homeostasis related to bariatric surgery were reflected in the serum and whole blood Cu, Fe and Zn isotopic compositions. The serum and whole blood Cu became isotopically lighter (lower δ65Cu values) after bariatric surgery, reaching statistical significance at 6 months post-surgery (p < 0.05). The difference between the serum and the whole blood Zn isotopic composition increased after surgery, reaching significance from 6 months post-surgery onwards (p < 0.05). Those changes in Cu, Fe and Zn isotopic compositions were not accompanied by similar changes in their respective concentrations, making isotopic analysis more sensitive to physiological changes than elemental content. Furthermore, the Zn isotopic composition correlates with blood glycaemic and lipid parameters, while the Fe isotopic composition correlates with glycaemic parameters.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity, defined as a body mass index (BMI) of 30 kg/m2 or more [1], is a risk factor contributing to numerous medical pathologies, including diabetes mellitus, cardiovascular disease, and cancer [2], and is associated with elevated mortality [1, 2]. Obesity influences metabolic homeostasis as a result of an enhanced occurrence of inflammation, insulin resistance, and oxidative stress [3]. While the prevalence of obesity is about 12% worldwide [4] and less than 30% in Europe [5], more than one third of the US population is obese [6]. A change of the individual’s lifestyle is usually the first option to reduce the BMI, as this can be effective with minimal health risk when done in a proper manner. However, this option is frequently ineffective at long term, as the desired BMI reduction is often not achieved and/or there is a lack of patient commitment.
Bariatric surgery is a common clinical practice for the weight management of obese patients, resulting in an effective and durable weight loss in patients with severe obesity. It is offered for adults with a BMI ≥ 40 kg/m2 without co-morbidities or a BMI ≥ 35 kg/m2 with co-morbidities, such as type 2 diabetes mellitus, hypertension, etc, and without excessive risk of post-operative morbidity [7]. Depending on the surgical procedure, bariatric surgery reduces food absorption by restricting the food intake, by inducing malabsorption or by a combination of both. A restrictive procedure reduces the stomach volume, e.g. by placing a band around the upper portion of the stomach, thus creating a small pouch, or by surgically removing part of the stomach. The procedure aiming at malabsorption limits digestion and absorption, e.g. by delivering chyme directly to the ileum in the case of biliopancreatic diversion (BPD) [8]. The most common surgical procedure is the Roux-en-Y gastric bypass (RYGB) [9], in which a small pouch in the stomach is connected to the last part of the jejunum, thereby bypassing most of the stomach, duodenum and upper jejunum [8]. The weight loss results from the reduced food intake accompanied by changes in metabolism and gut microbiota. Increased secretion of glucagon-like peptide-1 (GLP-1) is thought to be a major factor involved in the improvement of diabetes mellitus after bariatric surgery. This hormone induces insulin biosynthesis and secretion, along with β cell proliferation and differentiation [10]. Together with other so-called gut satiety hormones, which also include peptide YY (PYY), it may reduce the appetite via the gut–brain axis [3]. Furthermore, changes in gut microbiota which commonly occur after bariatric surgery may contribute to a reduced inflammation in the intestine [11].
As a consequence of a smaller absorption site and malabsorption, deficiency of essential nutrients is a common feature in bariatric surgery patients. Fe deficiency is reported in up to 50% of the patients, with menstruating women showing the highest risk [12]. Other micronutrients that are commonly depleted include water-soluble vitamins (thiamine, folic acid, vitamin B12), fat-soluble vitamins (vitamin A, vitamin D) and essential mineral elements (Ca, Zn, Cu) [8]. A nutritional deficiency can be developed not only in a relatively short post-surgery time, but also at long term. Vitamin B12 deficiency, for example, increases up to 62% 5 years after the bariatric surgery, while the prevalence at 12 months post-surgery is 4% only [8]. Therefore, long-term post-operative monitoring and patient follow-up are encouraged [7].
High-precision isotopic analysis of essential mineral elements is a powerful clinical tool complimentary to their quantitative determination, as small changes in the isotopic composition due to homeostatic alterations can be detected. Additionally, changes in isotope ratios accompanying diseases or variation in isotope ratios across body compartments indicate isotope fractionation accompanying biochemical processes and can provide an enhanced insight into the role of these elements in health and disease. The serum Fe isotopic composition for example has been shown to be heavier in chronic kidney disease (CKD) patients with iron-deficiency anaemia, but not in CKD patients with erythropoietin-related anaemia, compared to the control group [13]. Patients with end-stage liver disease show a lighter serum Cu isotopic composition than that of a healthy population [14, 15]. Normalised values after liver transplantation signal a restored liver function [16]. Recently, it has been shown that reduced Cu excretion through the bile, as in cholestatic liver disease, induced in mice via common bile duct ligation resulted in a light whole body Cu isotopic composition [17]. A lighter serum Cu isotopic composition was also observed in Wilson’s disease [18] and cancer patients [19, 20]. The whole blood Zn isotopic composition was shown to be affected by dietary habits [21]. Although tumour tissue was shown to be isotopically light, the serum/whole blood Zn isotopic composition was not significantly altered in the case of breast cancer [22].
The goal of the present study was to examine potential changes in the serum and whole blood Cu, Fe and Zn isotopic compositions as a result of bariatric surgery in a case series of eight patients. Paired serum and whole blood samples were collected from obese female patients before the gastric bypass and at 3, 6 and 12 months post-surgery. We investigated the association between the isotopic compositions, element concentrations and clinical parameters used for evaluation of the patient’s condition.
Materials and methods
Sampling
Whole blood and serum samples were withdrawn from eight patients who underwent laparoscopic gastric bypass at the Mayo Clinic (AZ, USA). All patients were female and their age ranged between 18 and 50 years at the time of surgery. The sampling was performed before the surgery (on the day of or 1 day before the surgery) and at 3, 6 and 12 months post-surgery. After the surgery, all patients received multivitamin supplements (also containing mineral elements) for preventing nutritional deficiencies. A wide set of clinical parameters were determined at the Mayo Clinic for monitoring the patient’s condition using standardised clinical protocols [23].
Ten serum samples from assumed healthy (non-obese) subjects collected at the Ghent University Hospital (UZ Gent, Belgium) and literature-reported data on serum Cu, Fe and Zn isotopic compositions were used for comparison purposes. These healthy subjects were all female with ages from 23 to 50 (average 42.5) years old (Electronic Supplementary Material (ESM), Table S1).
This study was approved by the Mayo Clinic Institutional Review Board (expedited review procedure 45 CFR 46.110, item 2). All patients provided written informed consent. Healthy volunteers provided informed consent at UZ Gent.
Sample preparation for isotopic analysis
Whole blood and serum samples were prepared for subsequent elemental and isotopic analysis in a class-10 clean lab (PicoTrace™, Germany), as described elsewhere [24]. The samples were digested with 14 M HNO3 (pro analysis, Chem-Lab, Belgium) and 9.8 M H2O2 (TraceSELECT®, Sigma-Aldrich, Belgium) at 110 °C and then evaporated to dryness. HNO3 was subjected to additional purification via sub-boiling distillation in a Savillex DST-4000 system (Savillex Corporation, MN, USA) prior to use. The samples were re-dissolved in 8 M Optima grade HCl (Fisher Chemical, UK) also containing ~ 0.001% H2O2. Cu, Fe and Zn were sequentially isolated from the sample digests by anion exchange chromatography using AG-MP1 resin (Bio-Rad Laboratories, CA, USA). A second chromatographic separation following the same protocol was applied to the serum Cu fractions to achieve negligible levels of Na [24], as the ratio Na/Cu in serum is about 2-fold higher than that in whole blood. Each purified metal fraction was re-dissolved in concentrated HNO3 and dried twice, and finally re-dissolved in 0.28 M HNO3 for further elemental and isotope ratio measurements. Ultrapure water used throughout was obtained from a Milli-Q Element water purification system (Merck Millipore, NE, USA).
Multi-collector and single-collector ICP-MS
The isotopic compositions of Cu, Fe and Zn were determined using a Thermo Scientific Neptune (Germany) multi-collector ICP-MS (MC-ICP-MS) unit, equipped with a high-transmission jet interface. The samples were measured in a sample-standard bracketing (SSB) approach using the isotopic reference materials Cu NIST SRM 976 (National Institute of Standards and Technology, NIST, MD, USA), Fe IRMM-014 (Institute for Reference Materials and Measurements, IRMM, Belgium) and Zn IRMM-3702 (IRMM, Belgium). The concentrations of the target element in the samples were matched within ± 5% to that in the isotopic standard. Ga, Ni or Cu (Inorganic Ventures, VA, USA) was always added as an internal standard for the Cu, Fe and Zn isotope ratio measurements, respectively. The isotope ratios were corrected for instrumental mass discrimination using the combination of internal correction by means of the revised Russell’s law and external correction (SSB) [25].
For QA/QC, single-element standard solutions of Cu, Fe and Zn (obtained by dilution of 1000 mg/L stock solutions from Inorganic Ventures, VA, USA) were measured every five samples in each measurement session. These standards were previously characterised isotopically (measurements spread over ~ 7 years [21]). The δ-values obtained (mean ± 2 times the standard deviation (SD)) along this work are 0.22 ± 0.04‰ (N = 30) for δ65Cu, 0.45 ± 0.06‰ (N = 29) for δ56Fe and − 7.04 ± 0.06‰ (N = 29) for δ66Zn; these values are in good agreement with earlier reported data [21, 24]. For method (including sample digestion and target element isolation) evaluation, the SeronormTM Trace Elements serum L-1 (lot number: 1801802, Sero, Norway) and whole blood L-1 (lot number: 1406263, Sero, Norway) reference materials were used. The results obtained for the SeronormTM serum L-1 (mean ± SD, N = 3) and for the SeronormTM whole blood L-1 (mean ± SD, N = 3) reference materials are − 0.22 ± 0.07 and 0.09 ± 0.06‰ for δ65Cu, − 1.52 ± 0.03 and − 2.35 ± 0.01‰ for δ56Fe and 0.01 ± 0.04 and − 0.02 ± 0.05‰ for δ66Zn, respectively; these values are in agreement with earlier reported data [21].
The elemental concentrations were determined using an Element XR (Thermo Scientific, Germany) single-collector sector-field ICP-MS instrument. External calibration, with Ga as internal standard (10 μg/L) to correct for matrix effects, signal drift and instrument instability, was applied for quantification.
Statistical analysis
Results are presented as mean ± SD, unless otherwise specified. Shapiro–Wilk’s test was used to determine the normality of the data set. Parametric tests were used for data with normal distribution; otherwise, equivalent non-parametric tests were used. One-way ANOVA (parametric) or Kruskal–Wallis (non-parametric) tests were used to compare the data between groups. Paired t test (parametric) or Wilcoxon signed rank test (non-parametric) was used to compare paired data from the same patient. The correlation between two variables was determined using Spearman’s rank-order correlation. A p-value ≤ 0.05 indicated significance. Statistical analysis was performed using SPSS Statistics 25 (IBM Analytics, Brussels, Belgium). SigmaPlot 13 (Systat Software Inc., CA, USA) was used for constructing graphs.
Results and discussion
Clinical parameters and mineral element concentrations
Table 1 compiles relevant clinical parameters used for the clinical follow-up of the bariatric surgery patients. In this cohort, the BMI was reduced by 27% at 12 months post-surgery on average. The levels of total protein, glycated haemoglobin HbA1c, cholesterol and low-density lipoproteins (LDL) had also decreased significantly at 3 and 6 months post-surgery (p < 0.05). The total protein, cholesterol and LDL values were within the normal range in all patients at all sampling points. One patient (#002) showed an abnormal HbA1c and an elevated glucose level pre- and post-surgery and two patients (#006 and #007) reached normal HbA1c and glucose levels 6 months post-surgery. The improvement of the glycaemic and lipid metabolism after bariatric surgery is consistent with other studies [26]. Protein deficiency (serum albumin < 3.5 mg/dL) is a severe complication associated with malabsorptive surgical procedures, e.g. RYGB and BPD, occurring during the first post-operative months, and is attributed to an acquired food intolerance towards protein-rich foods [27]. In spite of this, the individuals enrolled in this study showed normal serum albumin levels post-surgery. One patient (#007) developed iron-deficiency anaemia (reduced haemoglobin, ferritin and serum Fe levels; elevated total iron-binding capacity (TIBC)) after the bariatric surgery and remained so during the whole post-operative period of the study.
The intra-individual metal concentrations were compared pre- and post-surgery (one-tailed paired t test or Wilcoxon signed rank test). All individual data of Cu, Fe and Zn concentrations are provided in ESM Tables S1–S2. The intra-individual serum and whole blood Cu concentrations were significantly reduced post-surgery (p < 0.05) (Fig. 1). However, a significant decrease of the serum and whole blood Cu concentrations was not observed between groups (ANOVA or Kruskal–Wallis test) due to the large inter-individual variability. The serum and whole blood Fe and Zn concentrations did not reach a significant difference during the post-operative period compared to the pre-surgery values (Table 2).
Box plots for the Cu concentration in serum (a) and whole blood (b) for the bariatric surgery patients and assumed healthy subjects. Cu concentrations did not show a significant difference between groups (ANOVA or Kruskal–Wallis test). Paired comparisons reveal significant difference compared to pre-surgery values (one-tailed t test or Wilcoxon signed rank test). Data of healthy subjects (European non-obese females) were compiled from this study and literature [28]. Healthy subjects were not included in the statistical tests for data comparison. ap value from one-tailed t test; bp value from one-tailed Wilcoxon signed rank test
Mineral absorption mainly takes place in the duodenum and proximal jejunum, i.e. the portion of the intestine that is bypassed, resulting in a reduced gastric capacity and intestinal mineral absorption. Cu, Fe and/or Zn deficiency is frequently developed in RYGB patients, a condition that can remain for years [29,30,31,32] due to reduced intake, reduced absorption and/or increased intestinal losses, despite supplementation [29, 33, 34]. A prevalence of ~ 19% for Cu deficiency at 24 months post-surgery [29] and of ~ 24% for Fe deficiency anaemia after 18 months have been reported for RYGB patients [33]. Adequate multivitamin and mineral supplements may restore the Cu, Fe and Zn status at long term after bariatric surgery, but regular monitoring of the clinical parameters is still needed.
Serum and whole blood Cu, Fe and Zn isotopic compositions
Metal isotopic compositions were examined in the serum and whole blood pre- and post-surgery (Fig. 2). Individual data are provided in ESM Tables S3–S4. As can be seen in Fig. 2, the whole blood was enriched in the heavy 65Cu isotope (+ 0.3‰) and enriched in the light 54Fe isotope (− 0.2‰) compared to serum, whereas the Zn isotopic compositions of serum and whole blood were similar. This difference in isotopic compositions between serum and whole blood can be hypothetically attributed to the effect of redox processes and erythropoiesis and is in agreement with previous observations [28, 37].
a–f Box plots for the metal isotopic compositions (δ65Cu, δ56Fe and δ66Zn) in serum and whole blood for the bariatric surgery patients over time and for assumed healthy subjects (non-obese European females). The δ65Cu values among time points were significantly different in serum (ANOVA, p = 0.037) and whole blood (Kruskal–Wallis test, p = 0.038). Paired comparisons of δ65Cu show significant differences compared to pre-surgery values (two-tailed t test or Wilcoxon signed rank test). Data for healthy subjects were compiled from literature [28, 35, 36] and completed by additional analysis performed along this study, as summarised in ESM Table S5. The δ66Zn data from reference [28] were recalculated relative to the IRMM-3702 isotopic reference material [36]. Healthy subjects were not included in the statistical tests for data comparison. ap value from Tukey’s post hoc test of ANOVA; bp value from pairwise comparison of Kruskal–Wallis test, cp value from two-tailed t test; dp value from two-tailed Wilcoxon signed rank test
An age- and gender-matched European (French and Belgian) population consisting of assumed healthy (non-obese) individuals was included (ESM Table S5) to provide a general overview of variability in the isotopic compositions of these metals. For comparative purposes, age- and gender-matching is crucial, especially for δ56Fe, as the isotopic compositions differ significantly between male and female subjects and between pre-menopausal and post-menopausal female subjects [38]. To the best of our knowledge, there are no data available on metal isotopic compositions for either non-obese US individuals or obese Europeans to perform a strict comparison. Previous literature shows that for different European (French, Swedish and Swiss) cohorts of healthy individuals investigated, no significant differences in δ65Cu, δ56Fe and δ66Zn values were established [39]. The overall serum and whole blood Cu isotopic composition and serum Fe isotopic compositions of the obese patients were lighter than those of healthy (European) subjects, while whole blood δ56Fe and serum δ66Zn were heavier in obese patients. No difference was observed in the whole blood δ66Zn value between the healthy subjects and obese patients. A light Fe isotopic composition could be expected due to a low-grade inflammation typically accompanying obesity, a high Fe status and/or reduced intestinal Fe absorption [35, 40]. Furthermore, a high BMI is associated with reduced Fe absorption [41] and elevated serum hepcidin concentration [42]. Increased levels of the hormone hepcidin, as a result of increased cytokine production in obese patients [43], downregulate Fe release from enterocytes and thus intestinal uptake [42]. A low dietary intake of bioavailable heme-Fe can also lead to an iron-restricted erythropoiesis. However, as the healthy subjects are from different geographical origins, firm conclusions as to whether the difference is due to obesity and/or other factors cannot be derived. Populations from different geographical areas may have different metal isotopic compositions because of diet, metabolic activity, genetics, etc [44]. For example, healthy subjects from the circumpolar area of Russia showed significantly different δ65Cu and δ66Zn values compared to healthy subjects from Europe and Japan, while the latter also showed a δ56Fe value that was significantly different from that observed in healthy French subjects [39].
The use of healthy subjects from the same geographical origin receiving the same essential metal-containing multivitamin supplementation as the controls could be meaningful for comparison with the metal isotopic compositions of bariatric surgery patients. However, the absorption of micronutrients in healthy subjects and bariatric surgery patients is at different levels as the latter tend to absorb more nutrients from the multivitamin to address the deficiencies [45]. Moreover, as a common challenge in a nutritional study, the level of baseline nutrients (i.e. before intervention) also varies between healthy subjects, while a fraction of the healthy volunteers constituting a reference population often also show a sub-optimal level of micronutrients without any clinical symptom [46]. In this study, we preferred to use the pre-surgery value as control to evaluate whether Cu, Fe and Zn isotopic compositions are altered after bariatric surgery. By using the same individual as control, stronger conclusions can be achieved with a smaller number of participants [47]. All pre-surgery samples were acquired on the day of or 1 day before the surgery to reduce the variability due to different disease severity.
A significant difference was observed for serum and whole blood Cu isotopic compositions between the pre-surgery and 6 months (p = 0.011 and p = 0.037, respectively) and 12 months (p = 0.013 and p = 0.031, respectively) post-surgery samples (two-tailed paired t test or Wilcoxon signed rank test) (Fig. 2). The group-averaged Cu isotopic composition became gradually lighter over time, reaching significance at 12 months (p = 0.029 for serum and p = 0.016 for whole blood). This difference was not observed for the group-averaged Cu concentration (vide supra) in either serum or whole blood (Fig. 1), and thus, the Cu concentration was not conclusive. For this purpose, the small inter-individual variability in the Cu isotopic composition provides benefit compared to the Cu concentration.
The enrichment in the light 63Cu isotope in the serum/whole blood over time could be hypothetically associated with the reduced duodenal Cu absorption and mineral bioavailability as the amount of digestive juice in the stomach is limited after the gastric bypass. When the Cu level in the body is insufficient, the level of CTR1 (the major Cu importer) is up-regulated in the intestine [45, 48]. This process may affect the Cu isotope fractionation accompanying intestinal absorption. It has been shown in vitro that a high level of Ctr1 favours a lighter Cu isotopic composition in yeast [49]. Furthermore, changes in the composition and distribution of gut microbiota after bariatric surgery [11] can also contribute to an altered serum/whole blood Cu isotopic composition [50]. It has been shown that region-specific Cu isotopic changes in the intestine of antibiotics-treated mice, particularly in the duodenum and colon, are accompanied by an increased level of Cu–Zn superoxide dismutase (SOD1, an antioxidant enzyme) in the duodenum and a reduced level of the Cu transporters CTR1 and ATP7A in the colon [50].
Fe and Zn isotopic compositions did not show any systematic difference after surgery compared to the pre-surgery values in the intra-individual comparisons (two-tailed paired t test or Wilcoxon signed rank test) (Fig. 2). Among patients, those suffering from severe diabetes (#002) and Fe deficiency anaemia (#007) showed the heaviest whole blood Fe isotopic compositions (Fig. 3). The heavier Fe isotopic composition at pre-surgery in patient #007 might indicate that Fe deficiency had started before surgery, although the Fe concentration had not been altered yet. On the other hand, the Fe concentrations of those patients were within the range of the others, highlighting the superiority of isotopic composition over concentration. Thus, unlike the Fe concentration, which has high intra- and inter-individual variability and is barely changed, alteration in the Fe isotopic composition reflects a change in the Fe homeostasis.
Longitudinal change of the difference between the serum and whole blood Zn isotopic compositions
The serum Zn isotopic composition 6 months post-surgery was slightly heavier than that during pre-surgery, but the difference did not reach significance (Fig. 2e). This enrichment in the heavier Zn isotopes was not observed in the whole blood (Fig. 2f). However, the difference between the δ66Zn value in serum and that in whole blood (expressed as Δ66Zn) became gradually larger over the post-operative time (Fig. 4). The Δ66Zn values at 6 and 12 months post-surgery showed a significant difference compared to the pre-surgery value (Kruskal–Wallis test, p = 0.023 and 0.004, respectively). This effect was not observed for the Δ65Cu and Δ56Fe values (Kruskal–Wallis test, p = 0.197 and p = 0.343, respectively). The Δ66Zn seems to reflect the disrupted Zn homeostasis, impaired Zn status and/or reduced Zn absorption capacity in the bariatric surgery patients. Ruz et al. observed a reduced Zn absorption in bariatric surgery patients from 6 to 18 months post-surgery [34]. When the Zn supply is low, Zn is taken from the storage to fulfil Zn requirements. Intracellular Zn is bound to metallothionein via a Zn–S bond in cysteine clusters and can be released when the Zn level is low [51]. As the Zn–S bond favours the lighter Zn isotopes, the heavier isotopes would be preferentially released from intracellular metallothionein to serum in which it is transported to organs sensitive to Zn depletion. Additionally, changes in the Zn absorption involve many metal transport proteins, e.g. ZIP4 as a primary regulator of intestinal Zn uptake and ZnT1 for the Zn efflux from the enterocyte. The expression of Zn transporters is regulated by cytokines, hormones and the metal itself [52], and changes in their expressions can impact Zn homeostasis, as potentially reflected in the Δ66Zn value.
Correlations between isotopic compositions and clinical parameters
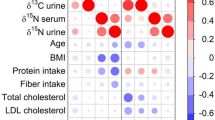
A schematic overview of the correlations between the Cu, Fe and Zn isotopic compositions and the clinical parameters used for the monitoring the patient’s condition is presented in Fig. 5. Serum and whole blood δ66Zn values showed significant correlations with glycaemic and lipid parameters, i.e. glucose, high-density lipoproteins (HDL) and LDL. The link between Zn and diabetes has been firmly established. Zn2+ and insulin are secreted from pancreatic β cells and both synergistically induce glucagon secretion from nearby α cells. In the target cells, Zn maintains an active state of the insulin receptor, thus prolonging the insulin activity [31]. Zn is also involved in lipogenesis and its deficiency disrupts lipid homeostasis [53, 54].
Schematic overview of the correlation between Cu (a), Fe (b) and Zn (c) isotopic compositions and clinical parameters. Only significant (p < 0.05) relationships are shown. Arrows with the same directions indicate positive correlation, while arrows with opposite directions indicate negative correlation. aCorrelation coefficient between 0.3 and 0.5. bCorrelation coefficient between 0.5 and 0.7. cCorrelation coefficient > 0.7. The individual Spearman’s correlation coefficients and the level of significance are provided in the Electronic supplementary information (ESM Table S6)
The serum δ56Fe correlated with the HbA1c and glucose levels in this population. HbA1c, which is the percentage of haemoglobin that binds glucose, is thought to be a more robust diabetic parameter as an erythrocyte is recycled every 120 days, thus carrying information of the blood glucose concentration over a long term [55]. However, the HbA1c value tends to be elevated when Fe is depleted because the erythrocyte cycle becomes longer [56]. Studies are needed to understand whether δ56Fe reflects blood glucose concentration or merely Fe deficiency.
Principal component analysis (PCA) was conducted using 35 variables (Fig. 6). Three principal components (PCs) explained 54% of the total variance. PC1 was mostly loaded by the number of white blood cells (WBC) and the levels of potassium, glucose, triglyceride, HbA1C, haemoglobin, platelets and alkaline phosphatase (ALP). PC2 was mostly loaded by the ferritin concentration, serum δ65Cu, whole blood δ65Cu and the levels of chloride, LDL and aspartate aminotransferase (AST). Meanwhile, PC3 was mostly loaded by the serum Cu concentration, whole blood Cu concentration, whole blood Zn concentration and whole blood Fe concentration. The PCA scores plot (PC1 vs. PC2) shows that data from patient #002 are distinct from those of the other patients (Fig. 6b). From the loading plot, it can be seen that the scores of these data are related to glycaemic parameters (HbA1c, glucose level, serum δ56Fe and blood δ56Fe). This patient has a severe case of diabetes and receives a high dose of insulin and oral diabetes medication—higher than that for two other patients receiving this type of medication in this cohort dose (data not shown). Thus, from the PCA, the heavy serum and whole blood δ56Fe of the patient can be hypothetically associated to severe diabetes.
Conclusions
The study of serum and whole blood Cu, Fe and Zn isotopic compositions showed to be useful for the follow-up of bariatric surgery patients. The Cu isotopic composition became gradually lighter over time, while the difference in Zn composition between whole blood and serum (Δ66Zn) was significantly increased 6 months post-bariatric surgery. Gastric bypass patients showed an improvement in metabolic parameters, reaching normal values post-surgery, but the mineral isotopic compositions were not fully restored 12 months post-surgery. The Fe isotopic compositions are altered in patients with disrupted metabolism. Those changes in Cu, Fe and Zn isotopic compositions are not accompanied by similar changes in their respective concentrations, making isotopic compositions more sensitive to physiological changes than elemental content, thus providing added value and advocating for further research in this context. Additionally, correlations between metal isotopic compositions and glycaemic and lipid parameters were established. However, whether these metal isotopic shifts are caused by metabolic changes or due to metal imbalance remains to be elucidated.
References
Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories. JAMA. 2013;309:71. https://doi.org/10.1001/jama.2012.113905.
Lenz M, Richter T, Mühlhauser I. The morbidity and mortality associated with overweight and obesity in adulthood. Dtsch Aerztebl Int. 2009;106:641–8. https://doi.org/10.3238/arztebl.2009.0641.
Sinclair P, Docherty N, Le Roux CW. Metabolic effects of bariatric surgery. Clin Chem. 2018;64:72–81. https://doi.org/10.1373/clinchem.2017.272336.
GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. https://doi.org/10.1056/NEJMoa1614362.
World Health Organization (WHO) (2017) Global Health Observatory (GHO) data—overweight and obesity. https://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/. Accessed 15 Oct 2019
Ogden CL, Carroll MD, Fryar CD, Flegal KM.Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015; 219; 1-8. https://doi.org/10.1017/S1368980017000088
Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21:1–27. https://doi.org/10.1002/oby.20461.
Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544–56. https://doi.org/10.1038/nrendo.2012.48.
Welbourn R, Pournaras DJ, Dixon J, Higa K, Kinsman R, Ottosson J, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the second IFSO Global Registry Report 2013–2015. Obes Surg. 2018;28:313–22. https://doi.org/10.1007/s11695-017-2845-9.
Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery: it’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care. 2013;36:S287–91. https://doi.org/10.2337/dcS13-2032.
Furet J-P, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. https://doi.org/10.2337/db10-0253.
Love AL, Billett HH. Obesity, bariatric surgery, and iron deficiency: true, true, true and related. Am J Hematol. 2008;83:403–9. https://doi.org/10.1002/ajh.21106.
Anoshkina Y, Costas-Rodríguez M, Speeckaert M, Van Biesen W, Delanghe J, Vanhaecke F. Iron isotopic composition of blood serum in anemia of chronic kidney disease. Metallomics. 2017;9:517–24. https://doi.org/10.1039/c7mt00021a.
Costas-Rodríguez M, Anoshkina Y, Lauwens S, Van Vlierberghe H, Delanghe J, Vanhaecke F. Isotopic analysis of Cu in blood serum by multi-collector ICP-mass spectrometry: a new approach for the diagnosis and prognosis of liver cirrhosis? Metallomics. 2015;7:491–8. https://doi.org/10.1039/C4MT00319E.
Lauwens S, Costas-Rodríguez M, Delanghe J, Van Vlierberghe H, Vanhaecke F. Quantification and isotopic analysis of bulk and of exchangeable and ultrafiltrable serum copper in healthy and alcoholic cirrhosis subjects. Talanta. 2018;189:332–8. https://doi.org/10.1016/j.talanta.2018.07.011.
Lauwens S, Costas-Rodríguez M, Van Vlierberghe H, Vanhaecke F. Cu isotopic signature in blood serum of liver transplant patients: a follow-up study. Sci Rep. 2016;6:30683. https://doi.org/10.1038/srep30683.
Costas-Rodríguez M, Van Campenhout S, Hastuti AAMB, Devisscher L, Van Vlierberghe H, Vanhaecke F. Body distribution of stable copper isotopes during the progression of cholestatic liver disease induced by common bile duct ligation in mice. Metallomics. 2019;1:3–5. https://doi.org/10.1039/c8mt00362a.
Aramendía M, Rello L, Resano M, Vanhaecke F. Isotopic analysis of Cu in serum samples for diagnosis of Wilson’s disease: a pilot study. J Anal At Spectrom. 2013;28:675. https://doi.org/10.1039/c3ja30349g.
Télouk P, Puisieux A, Fujii T, Balter V, Bondanese VP, Morel A-P, et al. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics. 2015;7:299–308. https://doi.org/10.1039/C4MT00269E.
Balter V, Nogueira da Costa A, Bondanese VP, Jaouen K, Lamboux A, Sangrajrang S, et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc Natl Acad Sci. 2015;112:982–5. https://doi.org/10.1073/pnas.1415151112.
Van Heghe L, Engström E, Rodushkin I, Cloquet C, Vanhaecke F. Isotopic analysis of the metabolically relevant transition metals Cu, Fe and Zn in human blood from vegetarians and omnivores using multi-collector ICP-mass spectrometry. J Anal At Spectrom. 2012;27:1327. https://doi.org/10.1039/c2ja30070b.
Larner F, Woodley LN, Shousha S, Moyes A, Humphreys-Williams E, Strekopytov S, et al. Zinc isotopic compositions of breast cancer tissue. Metallomics. 2015;7:107–12. https://doi.org/10.1039/c4mt00260a.
American College of Surgeons, American Society for Metabolic and Bariatric Surgery (2016) MBSAQIP Standards Manual v2.0—resources for optimal care of the metabolic and bariatric surgery patient 2016.
Lauwens S, Costas-Rodriguez M, Van Vlierberghe H, Vanhaecke F. High-precision isotopic analysis of Cu in blood serum via multi-collector ICP-mass spectrometry for clinical investigation: steps towards improved robustness and higher sample throughput. J Anal At Spectrom. 2017; 32: 597–608. https://doi.org/10.1039/C6JA00433D
Baxter DC, Rodushkin I, Engström E, Malinovsky D. Revised exponential model for mass bias correction using an internal standard for isotope abundance ratio measurements by multi-collector inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2006;21:427. https://doi.org/10.1039/b517457k.
Yan Y, Sha Y, Yao G, Wang S, Kong F, Liu H, et al. Roux-en-Y gastric bypass versus medical treatment for type 2 diabetes mellitus in obese patients. Medicine (Baltimore). 2016;95:e3462. https://doi.org/10.1097/MD.0000000000003462.
Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95:4823–43. https://doi.org/10.1210/jc.2009-2128.
Albarède F, Telouk P, Lamboux A, Jaouen K, Balter V. Isotopic evidence of unaccounted for Fe and Cu erythropoietic pathways. Metallomics. 2011;3:926–33. https://doi.org/10.1039/c1mt00025j.
Gletsu-Miller N, Broderius M, Frediani JK, Zhao VM, Griffith DP, Davis SS, et al. Incidence and prevalence of copper deficiency following Roux-en-Y gastric bypass surgery. Int J Obes. 2012;36:328–35. https://doi.org/10.1038/ijo.2011.159.
King JC, Shames DM, Woodhouse LR. Zinc and health: current status and future directions: homeostasis in humans. J Nutr. 2000;130:1360S–6S. https://doi.org/10.1093/jn/130.5.1437S.
Maret W. Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev Nutr Food Sci. 2017;22:1–8. https://doi.org/10.3746/pnf.2017.22.1.1.
Halfdanarson TR, Kumar N, Li CY, Phyliky RL, Hogan WJ. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol. 2008;80:523–31. https://doi.org/10.1111/j.1600-0609.2008.01050.x.
Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Rebolledo A, et al. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90:527–32. https://doi.org/10.3945/ajcn.2009.27699.
Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Basfi-fer K, et al. Zinc absorption and zinc status are reduced after Roux-en-Y gastric bypass: a randomized study using 2 supplements. Am J Clin Nutr. 2011;94:1004–11. https://doi.org/10.3945/ajcn.111.018143.
Van Heghe L, Delanghe J, Van Vlierberghe H, Vanhaecke F. The relationship between the iron isotopic composition of human whole blood and iron status parameters. Metallomics. 2013;5:1503–9. https://doi.org/10.1039/c3mt00054k.
Costas-Rodríguez M, Van Heghe L, Vanhaecke F. Evidence for a possible dietary effect on the isotopic composition of Zn in blood via isotopic analysis of food products by multi-collector ICP-mass spectrometry. Metallomics. 2014;6:139–46. https://doi.org/10.1039/c3mt00244f.
Von Blanckenburg F, Oelze M, Schmid DG, Van Zuilen K, Gschwind HP, Slade AJ, et al. An iron stable isotope comparison between human erythrocytes and plasma. Metallomics. 2014;6:2052–61. https://doi.org/10.1039/c4mt00124a.
Van Heghe L, Deltombe O, Delanghe J, Depypere H, Vanhaecke F. The influence of menstrual blood loss and age on the isotopic composition of Cu, Fe and Zn in human whole blood. J Anal At Spectrom. 2014;29:478–82. https://doi.org/10.1039/C3JA50269D.
Jaouen K, Gibert M, Lamboux A, Telouk P, Fourel F, Albarède F, et al. Is aging recorded in blood Cu and Zn isotope compositions? Metallomics. 2013;5:1016–24. https://doi.org/10.1039/C3MT00085K.
Cikomola JC, Flórez MR, Costas-Rodríguez M, Anoshkina Y, Vandepoele K, Katchunga PB, et al. Whole blood Fe isotopic signature in a sub-Saharan African population. Metallomics. 2017;9:1142–9. https://doi.org/10.1039/c7mt00170c.
Zimmermann MB, Zeder C, Muthayya S, Winichagoon P, Chaouki N, Aeberli I, et al. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int J Obes. 2008;32:1098–104. https://doi.org/10.1038/ijo.2008.43.
Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Holterman AXL, Galvani C, et al. Decreased serum hepcidin and improved functional iron status 6 months after restrictive bariatric surgery. Obesity. 2010;18:2010–6. https://doi.org/10.1038/oby.2009.490.
Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest. 2004;113:1251–3. https://doi.org/10.1172/JCI21441.
Costas-Rodríguez M, Delanghe J, Vanhaecke F. High-precision isotopic analysis of essential mineral elements in biomedicine: natural isotope ratio variations as potential diagnostic and/or prognostic markers. TrAC - Trends Anal Chem. 2016;76:182–93. https://doi.org/10.1016/j.trac.2015.10.008.
Turnlund J, Keyes W, Anderson H, Acord L. Copper absorption and retention in young men at three levels of dietary copper using the stable isotope, 65Cu. Am J Clin Nutr. 1989;49:870–8. https://doi.org/10.1093/ajcn/49.5.870.
Weaver CM, Miller JW. Challenges in conducting clinical nutrition research. Nutr Rev. 2017;75:491–9. https://doi.org/10.1093/nutrit/nux026.
Louis T, Lavori P, Bailar J, Polansky M. Statistics in Practice - Crossover and self-controlled designs in clinical research. N Engl J Med. 1984;310:24–31. https://doi.org/10.1186/1472-6904-6-1.
Kuo Y-M, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136:21–6. https://doi.org/10.1093/jn/136.1.21.
Cadiou J-L, Pichat S, Bondanese VP, Soulard A, Fujii T, Albarède F, et al. Copper transporters are responsible for copper isotopic fractionation in eukaryotic cells. Sci Rep. 2017;7:44533. https://doi.org/10.1038/srep44533.
Miller KA, Vicentini FA, Hirota SA, Sharkey KA, Wieser ME. Antibiotic treatment affects the expression levels of copper transporters and the isotopic composition of copper in the colon of mice. Proc Natl Acad Sci. 2019;116:5955–60. https://doi.org/10.1073/pnas.1814047116.
Maret W (2000) Zinc and health: current status and future directions a link between cellular zinc and redox state. 1455–1458
Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. https://doi.org/10.1074/jbc.r600011200.
Shen H, MacDonald R, Bruemmer D, Stromberg A, Daugherty A, Li X, et al. Zinc deficiency alters lipid metabolism in LDL receptor–deficient mice treated with rosiglitazone. J Nutr. 2007;137:2339–45. https://doi.org/10.1093/jn/137.11.2339.
Eder K, Kirchgessner M. Zinc deficiency and activities of lipogenic and glycolytic enzymes in liver of rats fed coconut oil or linseed oil. Lipids. 1995;30:63–9. https://doi.org/10.1007/BF02537043.
Little RR, Sacks DB. HbA1c: how do we measure it and what does it mean? Curr Opin Endocrinol Diabetes Obes. 2009;16:113–8. https://doi.org/10.1097/MED.0b013e328327728d.
English E, Idris I, Smith G, Dhatariya K, Kilpatrick ES, John WG. The effect of anaemia and abnormalities of erythrocyte indices on HbA1c analysis: a systematic review. Diabetologia. 2015;58:1409–21. https://doi.org/10.1007/s00125-015-3599-3.
Competing interests
The authors declare that they have no conflict of interest.
Funding
The Special Research Fund of Ghent University BOF-UGent is acknowledged for financial support. MC-R thanks FWO-Vlaanderen for her post-doctoral grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Mayo Clinic Institutional Review Board (IRB). All patients provided written informed consent at the Mayo Clinic. Healthy volunteers provided informed consent at UZ Gent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 291 kb)
Rights and permissions
About this article
Cite this article
Hastuti, A.A.M.B., Costas-Rodríguez, M., Anoshkina, Y. et al. High-precision isotopic analysis of serum and whole blood Cu, Fe and Zn to assess possible homeostasis alterations due to bariatric surgery. Anal Bioanal Chem 412, 727–738 (2020). https://doi.org/10.1007/s00216-019-02291-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02291-2