Abstract
Methylmalonic acid (MMA), a functional indicator of vitamin B12 insufficiency, was measured in the US population in the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2004 using a GC/MS procedure that required 275 μL of sample and had a low throughput (36 samples/run). Our objective was to introduce a more efficient yet highly accurate LC-MS/MS method for NHANES 2011–2014. We adapted the sample preparation with some modifications from a published isotope-dilution LC-MS/MS procedure. The procedure utilized liquid–liquid extraction and generation of MMA dibutyl ester. Reversed-phase chromatography with isocratic elution allowed baseline resolution of MMA from its naturally occurring structural isomer succinic acid within 4.5 min. Our new method afforded an increased throughput (≤160 samples/run) and measured serum MMA with high sensitivity (LOD = 22.1 nmol/L) in only 75 μL of sample. Mean (±SD) recovery of MMA spiked into serum (2 d, 4 levels, 2 replicates each) was 94 % ± 5.5 %. Total imprecision (41 d, 2 replicates each) for three serum quality control pools was 4.9 %–7.9 % (97.1–548 nmol/L). The LC-MS/MS method showed excellent correlation (n = 326, r = 0.99) and no bias (Deming regression, Bland-Altman analysis) compared to the previous GC/MS method. Both methods produced virtually identical mean (±SD) MMA concentrations [LC-MS/MS: 18.47 ± 0.71 ng/mL (n = 17), GC/MS: 18.18 ± 0.67 ng/mL (n = 11)] on a future plasma reference material compared with a GC/MS method procedure from the National Institute of Standards and Technology [18.41 ± 0.70 ng/mL (n = 15)]. No adjustment will be necessary to compare previous (1999–2004) to future (2011–2014) NHANES MMA data.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cobalamin (vitamin B12) is an essential cofactor for two enzymes involved in one-carbon metabolism: methylmalonyl CoA mutase and methionine synthetase [1]. Reduced function of the former enzyme results in increased serum methylmalonic acid (MMA) concentrations. Untreated vitamin B12 deficiency leads to megaloblastic anemia and/or irreversible central nervous system degeneration [2]. Serum or plasma vitamin B12 concentrations, particularly in the low-normal range, may not adequately reflect tissue vitamin B12 status [3]. Increased MMA concentrations are specific for and believed to be the first indication of vitamin B12 deficiency [4]. MMA in serum or plasma was therefore found to be a useful indicator of vitamin B12 deficiency, especially in patients with few or no hematological abnormalities, normal results for the Schilling test, or normal or only slightly depressed serum vitamin B12 concentrations [5].
Various analytical methods for the measurement of MMA in biological fluids have been reported, mainly since the 1980s. Different approaches were taken for sample preparation (e.g., ultrafiltration, solid-phase or liquid-liquid extraction) and analytical measurement (e.g., GC/MS, electrophoresis, and, more recently, LC-MS/MS) [6–19]. The analysis of serum MMA presents several challenges: this dicarboxylic acid has a small molecular weight, is hydrophilic, nonvolatile, and its concentration in serum is relatively low, especially in the generally healthy population with a low prevalence of vitamin B12 deficiency. This makes direct analysis by GC/MS challenging, and various types of derivatization (silylation, cyclohexanol, chloroformate, and butanol) have been reported to improve detection [6–9]. LC-MS/MS methods [10–18] yield shorter run times and better sensitivity compared with GC/MS procedures. These two factors are critical when a method is used for population monitoring because they translate to higher throughput and lower patient sample volume.
The NHANES has measured plasma MMA concentrations in the US population from 1999 to 2004 using a GC/MS procedure that required 275 μL of sample and had a low throughput (36 samples/run) [19]. No MMA data are available for 2005–2010. A 2010 expert roundtable discussing “NHANES Monitoring of Biomarkers of Folate and Vitamin B-12 Status” recommended that serum MMA measurements be reinstated in future NHANES in conjunction with serum vitamin B12 measurements [20]. Our primary objective was to introduce a more efficient yet highly accurate LC-MS/MS method for NHANES 2011–2014. Our secondary objective was to compare this new method to the previously used GC/MS procedure in order to provide continuity in interpreting NHANES data. Lastly, we aimed to assess how these two procedures compare with the GC/MS measurement procedure used by the National Institute of Standards and Technology (NIST).
Materials and methods
Chemicals, reagents, and samples
MMA, succinic acid, tert-butyl methyl ether (MTBE), and 3 M hydrochloric acid in n-butanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deuterium-labeled MMA (d3-MMA) was purchased from CDN Isotopes (Quebec, Canada). ACS grade methanol, phosphoric acid (H3PO4), glacial acetic acid, and acetonitrile were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Purified water (18 MΩ) was obtained from an Aqua Solutions water purification system (Aqua Solutions, Inc., Falmouth, ME, USA) and was used to prepare all samples, calibrators, and reagents. Quality control (QC) pools (low, medium, and high) were prepared in-house from pooled human serum purchased from a US blood bank. All units were screened for MMA and spiked with MMA standard solution as needed to achieve desired concentrations. All specimens were stored at –70 °C when not in use.
Sample preparation and analysis by LC-MS/MS
Samples were prepared by a modified procedure reported by Pedersen et al. [16]. Master stock solutions of MMA and d3-MMA were prepared in water (50 μmol/L). Six individual MMA working calibrators were prepared from the stock solution in water (25–2500 nmol/L) and stored (as 400 μL aliquots) at –70 °C until analysis. We prepared a fresh working internal standard (ISTD) solution (1 μmol/L) from the d3-MMA stock solution on the day of analysis in 50 % methanol/water. The sample preparation steps involved liquid–liquid extraction and generation of MMA dibutyl ester (Fig. 1). MMA was separated from succinic acid and from other compounds on an Accela UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) by isocratic elution (0.1 % acetic acid:methanol 40:60; 0.4 mL/min) within 4.5 min on a Hypersil Gold C18 analytical column (2.1 mm × 50 mm, 1.9 μm particle size) (Thermo Fisher Scientific) held at 35 °C. The total run time was 6 min. Samples were maintained at 7 °C within the instrument during the run. Injection volume was 3 μL in a partial loop mode. Mass detection was carried out on a TSQ Vantage (Thermo Fisher Scientific) under multiple-reaction monitoring (MRM) conditions in positive electrospray ionization (ESI) mode. The mass spectrometer parameters were as follows: spray voltage 4500 V, vaporizer temperature 350 °C, nitrogen sheath gas pressure 30 arbitrary units, auxiliary gas flow 5 arbitrary units, ion transfer capillary temperature 290 °C, argon collision gas pressure 1.2 mTorr, scan time 200 ms, scan width 0.1 Da, mass resolution in Q1 and Q3 0.7 full width at half maximum. We monitored two transitions each for MMA (m/z 231 → 119 and 175.1) and for d3-MMA (m/z 234.1 → 122.1 and 178.1) and used the sum of the two signals for quantitation, MRM mode. We also used the two individual transitions in selected reaction monitoring (SRM) mode to calculate confirmation ion ratios. In this calculation, the more abundant transition (m/z 231 → 119) was the quantitation ion (QISRM), whereas the less abundant transition (m/z 231 → 175.1) was the confirmation ion (CISRM). We established confirmation ion ratio (CISRM/QISRM) limits (mean ± 2 SD) for MMA from repeated analysis of the calibrators (n = 20). Quantitation was based on peak area ratios [analyte/internal standard (ISTD)] interpolated against a six-point aqueous calibration curve (1/×weighting), using XCalibur software (Thermo Fisher Scientific). We reinjected the calibrators at the end of each run to assess drifting. Each run consisted of a reagent blank (no ISTD), a blank (with ISTD), a set of calibrators, three serum QC samples prepared in duplicate (bracketing the unknown samples), and one blind QC sample for every 20 unknown samples (see Electronic Supplementary Material Figure S1).
Method validation
We used the FDA “Bioanalytical Method Validation” and IUPAC validation guidelines [21, 22] as well as our internal division document “Policies and Procedures Manual for Bioanalytical Measurements” to design our method validation experiments. We evaluated calibrator accuracy for 10 runs by calculating the proportion of measured to nominal calibrator value. We assessed the effect of the presence of matrix on the calibration curve from a 10-point calibration curve (0–2500 nmol/L, three independent calibration curves) prepared both in water and in human serum. Slopes that agreed within ±5 % were considered to be equivalent. We determined method imprecision using three levels of serum QC pools: within-run, 10 replicates/level in one run; total and between-run, two replicates/level over 10 runs. We assessed method accuracy through spike recovery: a serum pool was spiked with MMA at four levels (two runs, two replicates/level: 50, 250, 700, and 1000 nmol/L). We also measured the unspiked serum pool for endogenous MMA concentration (two runs, two replicates each). To assess spike recovery, we added the ISTD to the samples and carried them through the extraction process. To assess extraction efficiency, we added the ISTD after extraction was completed. The spike recovery and the extraction efficiency were calculated as the measured concentration difference between the spiked and the unspiked sample divided by the nominal concentration of the spike. We estimated the limit of detection (LOD) by serially diluting the low QC pool with charcoal stripped serum (Aalto Scientific, Carlsbad, CA, USA) and calculating the SD at concentrations of zero (σ0) from the extrapolation of repeat measurements (three runs, three replicates/dilution) [23]. We defined the LOD as 3σ0.
MMA stability
We exposed three serum QC pools (97.1, 247, and 548 nmol/L) to room temperature for 1, 2, 3, and 7 d to assess short-term MMA stability. The vials were returned to –70 °C storage and later all samples were processed and analyzed together with pristine serum QC pools kept at –70 °C as a reference. We also subjected the same QC pools to repeated freeze-thaw (up to three cycles, 2 h/cycle at room temperature in closed vials). We studied the stability of processed serum extracts for the above set of serum QC pools kept in the autosampler compartment at 7 °C for 3 d. In a separate experiment, we kept the 96-well plate containing processed serum QC pools and 25 unknown samples at –20 °C for 1 wk and compared the results with the initial results.
We assessed mid-term (6 mo and 1 y) MMA stability using three plasma QC pools (179, 515, and 2007 nmol/L, three runs, one replicate/condition) stored at suboptimal temperatures (5 °C and –20 °C). The vials were returned to –70 °C storage and later all samples were processed and analyzed together with pristine plasma QC pools kept at –70 °C as a reference. Long-term (14 y) MMA stability at ≤ –70 °C was assessed from six plasma QC pools (120, 137, 1079, 1825, 10230, and 10295 nmol/L, two runs, five replicates/level).
Effect of specimen type and anticoagulant
To study the effect of different anticoagulants, we used matched serum and plasma samples from 14 anonymous blood donors [serum, serum separator, K2EDTA plasma, Na citrate plasma, and Na heparin plasma (5-mL blood collection tubes with 0.5 mL anticoagulant), Tennessee Blood Bank Services (Memphis, TN, USA)]. Plasma was obtained within 2–4 h of blood collection (blood held at room temperature), serum after overnight clotting (leading to higher serum yield and less residual fibrinogen clots) at room temperature through centrifugation at 4 °C, following standard operating procedures. All specimens were refrigerated, shipped on cold packs, and frozen at –70 °C within 48 h of blood collection. Serum was used as a reference. Because of the diluent in the plasma citrate tubes, we multiplied results for this specimen type by 1.1.
Method comparison studies
We performed three comparison studies to assess how the new LC-MS/MS method compared with other methods. First, we subjected randomly selected residual NHANES plasma samples (n = 336; MMA: 60–2320 nmol/L) previously analyzed with our GC/MS method to analysis by our new LC-MS/MS method. We excluded seven extreme outlier results (difference between the two procedures >40 %) and three samples with MMA concentrations above 1500 nmol/L to avoid an undue influence of very high concentrations. The final set (n = 326; MMA: 60–1180 nmol/L) was evaluated for Pearson correlation, Deming regression, and Bland-Altman analysis using Analyse-it, a statistical plug-in for Microsoft Excel (Analyse-it ver. 2.20; Analyse-it Software, Ltd., Leeds, UK). Second, we subjected eight serum and 11 plasma samples (stored at –70 °C for 14 y) from a previous international round-robin study [24] to analysis by our new LC-MS/MS method and compared the results with the consensus mean of all 13 participating laboratories and to our previous GC/MS results. Third, we characterized the plasma-based NIST Standard Reference Material (SRM) 1950 “Metabolites in Human Plasma” by our LC-MS/MS and GC/MS procedures (replicate analyses of six vials over 3 d). This material was also characterized by a NIST GC/MS measurement procedure that has been used in value assignment of NIST reference materials. For all three methods, we calculated the mean±SD and the coefficient of variation (CV). This evaluation was another accuracy assessment of the new LC-MS/MS method.
Results and discussion
The sample preparation procedure (adapted from Pedersen et al. with some minor modifications) involved liquid–liquid extraction of MMA from serum, followed by derivatization of MMA to dibutyl ester and analysis by positive ion ESI-LC-MS/MS.
We optimized and tested critical sample preparation steps. Because the addition of acid to the organic solvent improves the transfer of organic acid from the aqueous to the organic phase, we tested extraction efficiency with different concentrations of o-phosphoric acid (0.1, 0.25, and 0.5 M) on a set of calibrators and QC samples. We obtained poor MMA extraction with 0.1 M o-phosphoric acid in MTBE and selected the 0.5 M acid concentration because it provided optimum MMA extraction from the aqueous phase (data not shown). Placing the tubes into a dry ice/ethanol bath was a fast and efficient way to separate the organic from the aqueous layer by simply decanting the top (MTBE) layer after the bottom (aqueous) layer froze. This process happened very quickly, and small variations in the amount of time the samples were kept in the ice bath (1, 2, or 3 min) did not influence the MMA areas in a set of calibrators and QC samples. Because the derivatization of extracted MMA to the dibutyl ester (at 60 °C) is a critical step, we studied the timing on the heating block to monitor reaction completion (20, 30, and 40 min). The use of excess of derivatization reagent and a 30 min incubation time assured that even samples with high MMA concentrations (above 10000 nmol/L) completed the reaction. The derivatized samples have to be subjected to evaporation to remove the excess of the high boiling derivatization reagent (3 M HCl in n-butanol). In our experience, longer evaporation times, involving vacuum and heat, can reduce the recovery of the volatile MMA dibutyl ester (optimum evaporation time is 30 min at 45 °C under vacuum, data not shown).
One important challenge in the analysis of serum MMA is the potential interference from other low-mass carboxylic acids, especially the structural isomer succinic acid, which is present in serum in high concentrations. Because the mass spectra of the two acids are nearly identical, MMA and succinic acid have to be separated chromatographically. To achieve the best resolution, we tested several reversed phase analytical columns (pentafluorophenyl, cyano, and C18). We selected the C18 UHPLC column because it allowed excellent resolution between MMA and succinic acid with relatively fast isocratic chromatography, narrow analyte peaks, and excellent column life (typically ~2000 injections) (Fig. 2). Our LC-MS/MS method offers an additional degree of specificity because it features data collection from two specific MMA transitions (QISRM and CISRM). We obtained fairly low (±8 %) variation of the confirmation ion ratio when we monitored these two ions in all six calibrators over 20 runs, particularly compared with common “industry standards” (FDA: ±10 % [25]; Society of Forensic Toxicologists: ±20 % to ±30 % [26]; European Commission: ±20 % to ±50 % depending of the abundance of CISRM vs. QISRM [27]). When we applied the confirmation ion ratio limits to MMA results >150 nmol/L obtained from 600 serum samples, 99 % of samples met the predetermined limits. This indicates that our confirmation ion ratio limits, although narrow, can be used in routine applications to allow identification of a potentially interfering compound that may be present in a sample.
Our previous GC/MS procedure [19], although performing reliably for the measurement of thousands of NHANES samples, was not an efficient method. The sample volume requirement was 275 μL/test, the GC/MS run time was 15 min, the throughput was limited to 36 samples/run, and the sample preparation time for one run was ~12 h (Table 1). With the new LC-MS/MS method, we achieved our main goal to improve the method efficiency. The LC-MS/MS method used a much reduced sample volume (75 μL), allowed a notably faster sample preparation time (~4 h) and LC-MS/MS run time (6 min), and had a much higher sample throughput (up to 160 unknown samples/run).
We subjected our LC-MS/MS method to complete method validation. Ion suppression was evaluated by post-column infusion (using a T-connection) of 50 nmol/L MMA-dibutyl solution while injecting a reagent blank, a blank, standards, and unknown and QC samples. No baseline drifts were observed around the elution time of MMA upon injection of matrix and non-matrix samples, indicating lack of ion suppression. We verified and confirmed that aqueous calibration produced an equivalent slope (0.0025, r 2 = 0.999) compared with calibration in serum (0.0024, r 2 = 0.994). The average slopes differed by 4.1 %. Due to matrix equivalency, we prepared many aliquots of aqueous calibrators, stored them at –70 °C, and used a new set with each run, which greatly simplified method implementation and daily routine preparations for the analyst. The aqueous calibration curve showed linearity over two orders of magnitude (25–2500 nmol/L) with a high correlation coefficient (r 2 = 0.999). Some investigators reported storage at –20 °C as preferred over storage at –80 °C (for aqueous calibrators [16] or for serum-based calibrators [12]) because of possible MMA absorption to the storage container surface. We have not observed any change in method performance with aqueous calibrators stored in 2-mL Nalgene vials for up to 2 y at –80 °C (data not shown).
Our LC-MS/MS method achieved good precision with a total CV of 4.9 %–7.9 % for three serum QC pools analyzed over 22 runs (Table 2), similar to the precision of the GC/MS method (CV of 5.4 % for three plasma QC pools analyzed over 56 runs, [19]) and to other LC-MS/MS methods [10–18]. We can rate our method precision more objectively by using generally applicable quality goals based on biologic variation [28]. As such, the analytical variation CVa should be a fraction of the within-person biologic variation CVw; optimum performance, CVa = 0.25 × CVw, desirable performance, CVa = 0.5 × CVw, minimum performance, CVa = 0.75 × CVw. Lacher et al. reported a within-person biologic variation of 18.7 % for MMA using data from NHANES 1999–2002 [29]. Therefore, the optimum, desirable, and minimum precision performance criteria for MMA are <4.7 %, <9.4 %, and <14 %, respectively. Our LC-MS/MS method achieved desirable precision.
We evaluated method accuracy through spike recovery. The use of an isotopically-labeled ISTD is expected to correct for any loss of analyte during sample handling. The high accuracy assessed through spike recovery for the LC-MS/MS method (94 % ± 5.5 %) was comparable to that reported for the GC/MS method (96 % ± 1.9 %, [19]) and to other LC-MS/MS methods [10–18]. Our LC-MS/MS method also achieved a good extraction efficiency of 81 %, as assessed from post-extraction addition of ISTD.
The LC-MS/MS method sensitivity, as expressed by the LOD (22.1 nmol/L), was improved compared with our previous GC/MS method (50 nmol/L) and commensurate or better compared with other LC-MS/MS methods [11, 13–15]. The use of MRM in our LC-MS/MS method allowed us to monitor a cumulative signal for quantitation (sum of quantitation and confirmation ion areas). This produced a notable increase in signal, while keeping the background approximately the same. The confirmation ion area contributed ~60 %–85 % of the quantitation ion area. Thus, MRM helped to improve our method detection limit. The improved sensitivity of our new LC-MS/MS method and the small sample volume requirement (75 μL) allowed the accurate measurement of MMA at normal concentrations encountered in population surveys. Low-volume samples (<100 μL) that require confirmation can be diluted with deionized water and reanalyzed.
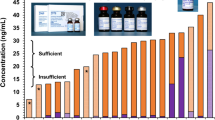
Plasma can form micro-fibrinogen clots during long-term storage and, therefore, serum is generally preferred. After we adjusted for the dilution in the plasma citrate tube, we found interchangeable results among the matched serum (plain and serum separators tubes) and plasma (EDTA, Na heparin, and citrate) samples (n = 14) with a mean difference of ±3 % (Fig. 3). This was generally consistent with other reports [11, 18]; however, Kushnir et al. reported an interference with the citrate tube, causing a significant increase of the confirmation ion ratio [11].
Effect of specimen type and anticoagulant on MMA. Fourteen matched serum and plasma samples were analyzed with the LC-MS/MS method. Error bars represent standard error. Because Na citrate was a liquid (0.5 mL/5 mL vacutainer tube) anticoagulant, MMA results were multiplied by 1.1 to correct for the dilution
We assessed the short-term stability of MMA in serum and in extracted samples, mid- and long-term stability of MMA in refrigerated and/or frozen serum, and the stability of MMA during repeated freeze/thaw of serum, and the short-term stability of MMA in extracted samples. We confirmed previous reports [16, 18] that MMA was stable for up to three freeze/thaw cycles and for up to 1 wk of serum storage at room temperature (data not shown). Given these results, it was not surprising that the short-term stability of MMA in extracted samples (3 d at 7 °C and 1 wk at –20 °C) was also very good (±3 % and ±1 %, respectively, from the original value). Furthermore, storage of serum at –20 °C for 1 y also showed excellent stability with no significant change in MMA concentrations from baseline [mean percent difference (95 % CI) among the three serum QC pools: 0.98 % (–1.46 % to –3.47 %), P = 0.42]. Storage of serum at 5 °C for 1 y showed a small but significant increase in MMA [6.59 % (2.19 % to 11.2 %), P = 0.0048], possibly due to slight sample evaporation. However, the 6 mo time point for storage at 5 °C showed no significant changes. This excellent MMA stability makes it possible to ship serum at ambient temperature to laboratories around the world participating in external quality assessment schemes. Lastly, the long-term (up to 14 y) stability of frozen (≤ –70 °C) plasma QC pools that were originally value-assigned by our GC/MS method was also excellent with MMA concentrations being within ±1 % of the original target values.
The second objective of this report was to compare our new LC-MS/MS method with the previous GC/MS method so as to provide continuity in interpreting NHANES data. The mean±SD (nmol/L) MMA concentration in the final set of 326 residual NHANES samples was 236 ± 191 nmol/L by LC-MS/MS compared with 235 ± 186 nmol/L by GC/MS. The two methods showed excellent correlation (Pearson r = 0.99). Deming regression analysis showed a significant but small deviation from a slope of 1 and an intercept of 0 and a nonsignificant bias (Table 3 and Fig. 4). Therefore, no adjustments will be needed to compare the NHANES 1999–2004 GC/MS data with the new NHANES 2011–2014 LC-MS/MS data. Using a much smaller sample set from a previously conducted international round-robin (n = 19, concentration range: 70–10500 nmol/L, [24]), we also found excellent correlation (Pearson r = 1.0) and no bias between our LC-MS/MS method and the earlier generated GC/MS or consensus results from 13 laboratories (Table 3).
At present, there is no serum-based reference material available with certified MMA concentrations. As an additional accuracy evaluation of the new LC-MS/MS method and as part of our third objective, we compared our results by both the LC-MS/MS and the GC/MS method with those obtained by NIST on a future NIST SRM material. SRM 1950 “Metabolites in Human Plasma” is the first reference material that has been characterized for MMA by the NIST GC/MS and CDC GC/MS and LC-MS/MS measurement procedures. The mean±SD (ng/mL) MMA concentrations obtained by the three procedures were virtually identical: CDC LC-MS/MS, 18.47 ± 0.71 (n = 17); CDC GC/MS, 18.18 ± 0.67 (n = 11); NIST GC/MS, 18.41 ± 0.70 (n = 15). The variability (CV) of the three procedures was also very comparable: 3.8 %, 3.7 %, and 3.8 %, respectively.
In summary, with this new LC-MS/MS method, we have implemented important features for methods used in large population surveys: low sample volume and high throughput, high accuracy, low imprecision, and good sensitivity. Time will show how stable the method will perform long-term. However, so far an intermittent use of the method did not pose any problems in bringing the method back online after several months of pausing. The excellent comparability and bias-free agreement between this method and the NIST GC/MS measurement procedure as well as our previous GC/MS method will facilitate future time trend analyses using NHANES data.
References
Institute of Medicine, Food, and Nutrition Board (1998) Dietary Reference Intakes: Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academy Press, Washington, DC
Baik HW, Russel RM (1999) Vitamin B12 deficiency in the elderly. Annu Rev Nutr 19:357–377
Allen LH (2008) Causes of vitamin B12 and folate deficiency. Food Nutr Bull 29(2 Suppl):S20–S34
Moelby L, Rasmussen K, Jensen MK, Pedersen KO (1990) The relationship between clinically confirmed cobalamin deficiency and serum methylmalonic acid. J Intern Med 228:373–378
Rasmussen K, Moller J, Lyngbak M, Holm Pedersen A-M, Dybkjaer L (1996) Age- and gender-specific reference intervals for total homocysteine and methylmalonic acid in plasma before and after vitamin supplementation. Clin Chem 42(4):630–636
Stabler S, Marcell PD, Podell ER, Allen RG, Lindenbaum J (1986) Assay of methylmalonic acid in the serum of patients with cobalamin deficiency using capillary gas chromatography-mass spectrometry. J Clin Invest 77:1606–1612
Rasmussen K (1989) Solid-phase sample extraction for rapid determination of methylmalonic acid in serum and urine by a stable-isotope-dilution method. Clin Chem 35(2):260–264
Windelberg A, Årseth O, Kvalheim G, Ueland PM (2005) Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin Chem 51(11):2103–2109
Chen J, Bennett M (2010) Quantitaion of methylmalonic acid in serum or plasma using isotope dilution-selected ion gas chromatography-mass spectrometry. Clin Appl Mass Spec Methods Mol Biol 603:365–370
Magera MJ, Helgeson JK, Matern D, Rinaldo P (2000) Methylmalonic acid measured in plasma and urine by stable-isotope dilution and electrospray tandem mass-spectrometry. Clin Chem 46(11):1804–1810
Kushnir MM, Komaromy-Hiller G, Shushan B, Urry FM, Roberts W (2001) Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma and urine. Clin Chem 47(11):1993–2002
Schmedes A, Brandslund I (2006) Analysis of methylmalonic acid in plasma by liquid chromatography-tandem mass spectrometry. Clin Chem 52(4):754–757
Blom HJ, van Rooij A, Hogeveen M (2007) A simple high-throughput method for determination of plasma methylmalonic acid by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med 45(5):645–650
Hempen C, Wanschers H (2008) A fast liquid chromatographic tandem mass spectrometric method for the simultaneous determination of total homocysteine and methylmalonic acid. Anal Bioanal Chem 391:263–270
Lakso H-A, Appelblad P, Scgneede J (2008) Quantification of methylmalonic acid in plasma with hydrophilic interaction liquid chromatography separation and mass spectrometric detection. Clin Chem 54(12):2028–2035
Pedersen TL, Keyes WR, Shahab-Ferdows S, Allen LH, Newman JW (2011) Methylmalonic acid quantification in low serum volumes by UHPLC-MS/MS. J Chromatogr B 879:1502–1506
Ouweland JMW, Kema IP (2012) The role of liquid chromatography-tandem mass spectrometry in the clinical laboratory. J Chromatogr B 883(884):18–32
Yaun C, Gabler J, El-Khoury JM, Spatholt R, Wang S (2012) Highly sensitive and selective measurement of underivatized methylmalonic acid in serum and plasma by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 404:133–140
Pfeiffer CM, Gunter EW (1999) Automated assay for methylmalonic acid (MMA) in plasma. 51st Annual Meeting of the American Association of Clinical Chemistry. Clin Chem 45(S6):A166 (Abstract 593)
Yetley EA, Pfeiffer CM, Phinney KW, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizu RA et al (2011) Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 94:313S–321S
Food and Drug Administration (2011) Guidance for industry: bioanalytical method validation. Food and Drug Administration, US Department of Health and Human Services, Roackville. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070336.pdf. Accessed 11 Sept 2014
International Union of Pure and Applied Chemistry (2002) Harmonized guidelines for single laboratory validation of method of analysis (IUPAC Technical Report). Available at: http://www.iupac.org/publications/pac/2002/pdf/7405x0835.pdf. Accessed 11 Sept 2014
Taylor JK (1987) Quality Assurance of Chemical Measurement. Lewis Publishers, Michigan
Pfeiffer CM, Smith SJ, Miller DT, Gunter EW (1999) Comparison of serum and plasma methylmalonic acid measurements of 13 laboratories: an international study. Clin Chem 45(12):2236–2242
Food and Drug Administration (2003) Guidance for industry: mass spectrometry for confirmation of the identity of animal drug residues. Food and Drug Administration, US Department of Health and Human Services, Rockville. Available at: http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm052658.pdfAccessed 11 Sept 2014
Forensic Toxicology Laboratory Guidelines (2002) Society of Forensic Toxicology. American Academy of Forensic Science, Washington, DC
European Union Decision (2002) 2002/657/EC 17.08.2002: commission decision laying down performance criteria for the analytical methods to be used for certain substances and residues thereof in live animals and animal products. Off J Eur Commun (221):8–32
Fraser CG, Harris EK (1989) Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 27:409–437
Lacher DA, Hughes JP, Carroll MD (2010) Biological variation of laboratory analytes based on the 1999–2002 National Health and Nutrition Examination Survey. Natl Health Stat Report 21(1):1-7
Acknowledgments
The Division of Laboratory Sciences at the National Centers for Environmental Health contributed to this project. The authors thank Michael Welch and Lorna Sniegoski, from NIST, for their involvement in MMA measurements in SRM 1950. They thank Hui Ping Chen and Bridgette Toombs, members of Nutritional Biomarkers group at the CDC, for their contribution in sample supply for stability and collection tube studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Reference Materials for Chemical Analysis with guest editors Hendrik Emons and Stephen A. Wise.
No specific sources of financial support. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry. Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 338 kb)
Rights and permissions
About this article
Cite this article
Mineva, E.M., Zhang, M., Rabinowitz, D.J. et al. An LC-MS/MS method for serum methylmalonic acid suitable for monitoring vitamin B12 status in population surveys. Anal Bioanal Chem 407, 2955–2964 (2015). https://doi.org/10.1007/s00216-014-8148-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8148-2