Abstract
Human exposure to polycyclic aromatic hydrocarbons (PAHs) can be assessed through monitoring of urinary mono-hydroxylated PAHs (OH-PAHs). Gas chromatography (GC) has been widely used to separate OH-PAHs before quantification by mass spectrometry in biomonitoring studies. However, because GC requires derivatization, it can be time consuming. We developed an on-line solid phase extraction coupled to isotope dilution-high performance liquid chromatography-tandem mass spectrometry (on-line-SPE-HPLC-MS/MS) method for the quantification in urine of 1-OH-naphthalene, 2-OH-naphthalene, 2-OH-fluorene, 3-OH-fluorene, 1-OH-phenanthrene, the sum of 2-OH and 3-OH-phenanthrene, 4-OH-phenanthrene, and 1-OH-pyrene. The method, which employed a 96-well plate platform and on-line SPE, showed good sensitivity (i.e., limits of detection ranged from 0.007 to 0.09 ng/mL) and used only 100 μL of urine. Accuracy, calculated from the recovery percentage at three spiking levels, varied from 94 to 113 %, depending on the analyte. The inter- and intra-day precision, calculated from 20 repeated measurements of two quality control materials, varied from 5.2 to 16.7 %. Adequate method performance was also confirmed by acceptable recovery (83–102 %) of two NIST standard reference materials (3672 and 3673). This high-throughput on-line-SPE-HPLC-MS/MS method can be applied in large-scale epidemiological studies.

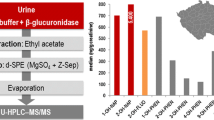
Example LC-MS chromatogram of urinary mono-hydroxylated PAH metabolites
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants linked to a variety of adverse health effects [1–3]. Humans may be exposed to PAHs through occupation, such as in work involving diesel fuels and coal tars [4, 5], as well as through diet and other lifestyle activities (e.g., smoking) [6–8]. The urinary concentrations of PAH metabolites, specifically mono-hydroxylated PAHs (OH-PAHs), have been used as biomarkers of human exposure to PAHs [7, 9, 10].
The pioneering quantification of OH-PAHs in urine was conducted by high performance liquid chromatography (HPLC) coupled with fluorescence detection [4]. Thanks in part to technology advances of the last few decades, isotope dilution gas chromatography-mass spectrometry (GC-MS) became widely used for the determination of urinary OH-PAHs [11–16]. GC-MS showed improved accuracy, sensitivity, and precision, mainly because of the high specificity of mass spectrometry detection. However, sample preparation involved derivatization and solvent evaporation steps, and was, therefore, labor intensive and time consuming. More recently, HPLC-tandem MS (HPLC-MS/MS), introduced to measure OH-PAHs [17–26], eliminated the derivatization step and yet maintained the high specificity of mass spectrometry. Still, a relatively large volume of urine, e.g., 2–5 mL, was required in HPLC-MS/MS methods, and automated sample preparation was not fully applied [20, 21]. These conditions have limited HPLC-MS from application in large-scale biomonitoring studies when matrix volume is often limited.
For the present study, we developed a fully automatic on-line solid phase extraction coupled with isotope dilution-high performance liquid chromatography-tandem mass spectrometry (on-line SPE-HPLC-MS/MS) method for the accurate and reliable measurement of nine OH-PAHs in human urine.
Experimental section
Materials and methods
All solvents were HPLC grade, and chemicals were reagent grade. We purchased acetonitrile, ethanol, 0.1 % formic acid in water, methanol, water, and ammonium fluoride from Thermo Fisher Scientific (Waltham, MA, USA); ascorbic acid, sodium acetate, and Helix pomatia β-glucuronidase type H-1 (β-glucuronidase ≥300,000 units/g, sulfatase ≥10,000 units/g) from Sigma-Aldrich (St. Louis, MO, USA). We obtained 1-hydroxynaphthalene (1-OH-NAP), 2-hydroxynaphthalene (2-OH-NAP), 2-hydroxyfluorene (2-OH-FLU), 3-hydroxyfluorene (3-OH-FLU), 1-hydroxyphenanthrene (1-OH-PHE), 2-hydroxyphenanthrene (2-OH-PHE), 3-hydroxyphenanthrene (3-OH-PHE), 4-hydroxyphenanthrene (4-OH-PHE), 1-hydroxypyrene (1-OH-PYR), and their corresponding 13C-labeled internal standards (IS, listed in Table 1) from Cambridge Isotope Laboratories (Andover, MA, USA).
We purchased smokers’ urine samples from BioreclamationIVT (Westbury, NY, USA). We also collected urine anonymously in 2015 from non-smoker adult volunteers with no documented occupational exposure to PAHs in Atlanta, GA. We obtained two Standard Reference Materials® (SRMs), SRM 3672 (smoker urine) and SRM 3673 (non-smoker urine), from the US National Institute of Standards and Technology (NIST) (Gaithersburg, MD, USA). All urine specimens were stored upon collection or arrival at −70 °C until use.
Appropriate safety control measures (including engineering, administrative, and personal protective equipment) were used for all procedures based on a site-specific risk assessment that identified physical, health, and procedural hazards.
Preparation of standard stock solutions and quality control materials
We prepared the stock solutions of individual analytical standard in ethanol. Standards with all nine OH-PAHs were generated by serial dilution of the individual stock in 40 % ethanol/60 % water. The final concentrations of the mixed stock standards ranged from 0.08 to 200 ng/mL (1-OH-NAP and 2-OH-NAP) and 0.005 to 50 ng/mL (all other analytes). The standard solutions were aliquoted into 2 mL silanized amber glass vials and stored at 4 or -70 °C until use. The internal standard solution with 13C-labeled analytes was prepared in water with 0.2 % acetonitrile so that a 50-μL spike would result in approximate concentrations of 32 ng/mL (13C-1-OH-NAP and 13C-2-OH-NAP) or 8 ng/mL (other 13C-labeled analytes). Internal standards were aliquoted into 15 mL amber glass vials and stored at −70 °C.
Two levels of quality control (QC) materials, QC low (QCL) and QC high (QCH), were prepared by pooling urines from smokers and non-smokers. The QC concentrations were fortified, as needed, with native target compounds to encompass the ranges described for the US general population [27]. All QC materials were stored in 4 mL amber glass vials at −70 °C until used. The stability of spiked material stored at −70 °C has been previously evaluated for up to 1 year (data not shown), and no obvious degradation of OH-PAHs was observed. The QC materials stored at −70 °C for more than 1 year will be re-evaluated for their stability.
Sample preparation
Urine samples were thawed and mixed at room temperature. QC samples, reagent blanks, and standards were processed the same way as urine samples, going through all of the sample preparation steps. Sample preparation was automatically conducted on a Perkin-Elmer Staccato® System (controlled by the Perkin-Elmer iLink and Maestro software) (Waltham, MA, USA). The robotic system included six main components: Sciclone G3/G3T, Fluidx CESD-24PRO decapper, Hettich Rotanta 460 centrifuge, ThermoScienfic ALPS 3000 sealer, IVD Inheco Incubator shaker DWP, and Mitsubishi robotic arm. We programmed this system to aliquot urine samples, standards, QCs, and reagent blanks (100 μL) into a 96-well plate (Corning, NY, USA), and subsequently add ascorbic acid solution (20 μL, ∼12.5 mg/mL), internal standards solution (50 μL), and sodium acetate buffer (50 μL, ∼1 mol/L, pH 5.5) containing ∼10 mg/mL β-glucuronidase/arylsulfatase. The accuracy and precision of automatic aliquating by Sciclone robotic system were previously evaluated, and good aliquoting accuracy (recovery rate from 95 to 105 %) and precision (CV <3 %) were achieved from current procedures. Details regarding enzymatic de-conjugation were previously described [16]. The robotic system then sealed and transferred the plate for overnight incubation at 37 ± 2 °C. After enzymatic hydrolysis, the robotic system automatically added methanol (175 μL) to all sample wells, mixed the solution, resealed the plate, and centrifuged for ∼15 min at 5000 rpm (5900 rcf). Finally, the robotic system transferred 200 μL of the supernatant in each well to a new 96-well plate, and added 350 μL of water to each well before on-line SPE-HPLC-MS/MS analysis. Non-spiked and spiked synthetic urine [28] used for determining the limit of detection (LOD) was prepared as study samples.
On-line SPE-HPLC-MS/MS
The on-line SPE-HPLC-MS/MS system consisted of a Sciex 5500 or 6500 triple quadrupole mass spectrometer (Foster City, CA, USA) equipped with an electrospray ion source and controlled by AB Sciex AnalystTM software, and one Agilent 1260 pump and one degasser (Santa Clara, CA, USA), and an on-line SPE Spark Holland system (Glassboro, NJ, USA) controlled by the Sparklink® software (iChrom Symbiosis system).
After injection (300 μL), the sample was loaded onto an Oasis WAX on-line SPE cartridge with 0.1 % formic acid in water (1.5 mL), and the cartridge was washed with acetonitrile/methanol/water (∼0.4 mL, 1/1/2, v/v/v). The target analytes were eluted with methanol (350 μL) and focused on a Chromolith HighResolution RP-18 endcapped guard column (5 × 4.6 mm, Merck KGaA, Darmstadt, Germany) with the initial HPLC gradient.
We separated the target analytes on a pair of Chromolith HighResolution RP-18 endcapped HPLC columns (100 × 4.6 mm, Merck KGaA) by a programmed HPLC gradient (Table 2). The mobile phases were water with 0.1 mM ammonium fluoride (A) and methanol with 0.1 mM ammonium fluoride (B).
The ionspray voltage and source temperature were −3.0 kV and 500 °C, respectively. Curtain gas, ion source gas 1, ion source gas 2, and collision gas were 35, 50, 70, and 9 psi, respectively. The representative decluster, entrance and exit potentials were −120, −3, and −12 V, respectively. We quantified OH-PAHs by selected reaction monitoring in the negative ion mode by the ion transitions listed in Table 1 and optimized the collision energies for all ion transitions (Table 1).
Data analysis
We used Analyst (version 1.6.2, Sciex, Foster City, CA, USA) and MultiQuant (version 3.0, Sciex, Foster City, CA, USA) for data processing. We defined quality control limits and evaluated analytical runs using SAS (version 9.3, SAS Institute Inc.; Cary, NC, USA) with a multi-rule quality control approach [29].
Results and discussion
OH-PAHs were enriched and extracted from the urine matrix by on-line SPE. We separated the target analytes on a pair of monolithic RP-18 column by using a gradient of water and methanol, including ammonium fluoride (0.1 mM) in both mobile phases. We used ammonium fluoride to improve method sensitivity in the negative ion mode [30]. An example of LC-MS/MS selected ion chromatogram is shown in Fig. 1. Within 27 min, we were able to separate several pair of isomers: 2-OH-NAP, 1-OH-NAP, 3-OH-FLU, 2-OH-FLU, 1-OH-PHE, 4-OH-PHE, and 1-OH-PYR, but 2-OH-PHE and 3-OH-PHE were eluted together so we had to measure these two analytes as a sum (∑2,3-OH-PHE). 9-Hydroxyphenanthrene and 3-hydroxyfluoranthene could also be separated from the other target metabolites (data not shown).
Method accuracy was assessed by repeated analyses (n = 7) of synthetic urine spiked with the target analytes at three spiking concentrations. Accuracy, expressed as a percentage of recovery, was 105–113 % (level 1), 94–100 % (level 2), and 98–102 % (level 3), depending on the analyte (Table 3). Furthermore, accuracy was evaluated by analyzing two NIST SRMs: SRM 3672 and SRM 3673. The calculated OH-PAHs concentrations were in good agreement with the certified concentrations [31], and accuracy ranged from 83 to 102 %, depending on the analyte (Table 3).
We determined the method precision from repeated measurements of low and high QC pools by following the CLSI protocol EP5-A2 [32] on 51 different days (two results from each of two daily runs) over a period of 8 months that involved multiple analysts. The relative standard deviations (RSDs), which reflect the within- and between-run variability, ranged from 3.2 to 12.1 % (within-run) and 4.8 to 13.0 % (within and between runs) for all analytes (Table 4).
The LOD was determined according to procedures previously described [33] from 60 repeated measurements of non-spiked and spiked synthetic urine analyzed by multiple operators and using four different mass spectrometers (Sciex 5500 or 6500). The LODs ranged from 0.007 to 0.09 ng/mL for all analytes (Table 4), indicating the good sensitivity of the method, especially considering the relatively low volume of urine used (100 μL). The method also provided wide dynamic ranges, with upper linearity of the method at 200 ng/mL (2-OH-NAP, 1-OH-NAP), 25 ng/mL (2-OH-FLU), 20 ng/mL (∑2,3-OH-PHE), and 10 ng/mL (3-OH-FLU, 1-OH-PHE, 4-OH-PHE, 1-OH-PYR).
SPE recovery was calculated as previously described [34]. The mean recoveries of three repeated measurements were 67 ± 4 % (2-OH-NAP), 67 ± 5 % (1-OH-NAP), 81 ± 4 % (3-OH-FLU), 64 ± 2 % (2-OH-FLU), 63 ± 2 % (∑2,3-OH-PHE), 72 ± 5 % (1-OH-PHE), 55 ± 2 % (4-OH-PHE), and 49 ± 6 % (1-OH-PYR). These recoveries, which are comparable with those reported before using a GC-MS method [16], are adequate for quantitative measurement of OH-PAHs in urine from both smokers and non-smokers. We also evaluated matrix effects using a matrix factor, defined as the ratio of IS peak area in the presence of urine matrix to the IS peak area in the absence of urine matrix [34]. The matrix factor, calculated from two different QC concentrations of three repeated measurements varied from 63 to 101 %, depending on the analyte.
To validate the method, we measured OH-PAHs in 36 non-smokers and 36 self-identified smokers’ urine samples (Table 5). Among non-smokers, detection frequencies were 56 % for 1-OH-PYR, 14 % for 4-OH-PHE, 94 % for 3-OH-FLU, and 100 % for the other analytes. For smokers, detection frequencies were 97 % for 1-OH-PYR, 53 % for 4-OH-PHE, and 100 % for the rest of analytes. The geometric mean concentrations of OH-PAHs were 2.5–12.4 (average 6.6) times higher in smokers than non-smokers (Table 5). 2- and 3-OH-FLU and 1- and 2-OH-NAP showed the largest concentration differences between smokers and non-smokers.
Compared with our previous GC-MS method [13, 16], the current approach eliminated the derivatization and solvent evaporation steps, simplified the sample processing procedure, and greatly improved throughput, while using 10 times less urine volume (0.1 vs 1.0 mL). This robust, sensitive, and highly automated on-line SPE-HPLC-MS/MS method is suitable for the quick analysis of human samples in national surveys and other large epidemiological studies to evaluate human exposure to PAHs.
References
Toxicological profile for polycyclic aromatic hydrocarbons. Agency for Toxic Substances and Disease Registry. Atlanta, GA. 1995. http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf.
Polycyclic aromatic hydrocarbons—occurrence in foods, dietary exposure, and health effects. European Commission, Scientific Committee on Food. 2002. http://ec.europa.eu/food/fs/sc/scf/out154_en.pdf.
A review of human carcinogens: chemical agents and related occupations. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol 100 F, International Agency for Research on Cancer. 2012. http://monographs.iarc.fr/ENG/Monographs/vol100F/.
Jongeneelen FJ, Anzion RB, Leijdekkers CM, Bos RP, Henderson PT. 1-Hydroxypyrene in human urine after exposure to coal tar and a coal tar derived product. Int Arch Occup Environ Health. 1985;57(1):47–55.
Rodrigues EG, Smith K, Maule AL, Sjodin A, Li Z, Romanoff L, et al. Urinary polycyclic aromatic hydrocarbon (OH-PAH) metabolite concentrations and the effect of GST polymorphisms among US Air Force personnel exposed to jet fuel. J Occup Environ Med. 2014;56(5):465–71.
Jacob J, Grimmer G, Dettbarn G. Profile of urinary phenanthrene metabolites in smokers and non-smokers. Biomarkers. 1999;4(5):319–27.
Suwan-ampai P, Navas-Acien A, Strickland PT, Agnew J. Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol Biomarkers Prev. 2009;18(3):884–93.
Hagedorn HW, Scherer G, Engl J, Riedel K, Cheung F, Errington G, et al. Urinary excretion of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in nonsmokers and in smokers of cigarettes with different ISO tar yields. J Anal Toxicol. 2009;33(6):301–9.
Huang W, Caudill SP, Grainger J, Needham LL, Patterson Jr DG. Levels of 1-hydroxypyrene and other monohydroxy polycyclic aromatic hydrocarbons in children: a study based on U.S. reference range values. Toxicol Lett. 2006;163(1):10–9.
Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res. 2008;107(3):320–31.
Smith CJ, Huang W, Walcott CJ, Turner W, Grainger J, Patterson Jr DG. Quantification of monohydroxy-PAH metabolites in urine by solid-phase extraction with isotope dilution-GC-MS. Anal Bioanal Chem. 2002;372(1):216–20.
Smith CJ, Walcott CJ, Huang W, Maggio V, Grainger J, Patterson Jr DG. Determination of selected monohydroxy metabolites of 2-, 3- and 4-ring polycyclic aromatic hydrocarbons in urine by solid-phase microextraction and isotope dilution gas chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778(1–2):157–64.
Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, et al. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal Chem. 2006;78(16):5744–51.
Romanoff LC, Li Z, Young KJ, Blakely 3rd NC, Patterson Jr DG, Sandau CD. Automated solid-phase extraction method for measuring urinary polycyclic aromatic hydrocarbon metabolites in human biomonitoring using isotope-dilution gas chromatography high-resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;835(1–2):47–54.
Campo L, Rossella F, Fustinoni S. Development of a gas chromatography/mass spectrometry method to quantify several urinary monohydroxy metabolites of polycyclic aromatic hydrocarbons in occupationally exposed subjects. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875(2):531–40.
Li Z, Romanoff LC, Trinidad DA, Pittman EN, Hilton D, Hubbard K, et al. Quantification of 21 metabolites of methylnaphthalenes and polycyclic aromatic hydrocarbons in human urine. Anal Bioanal Chem. 2014;406(13):3119–29.
Xu X, Zhang J, Zhang L, Liu W, Weisel CP. Selective detection of monohydroxy metabolites of polycyclic aromatic hydrocarbons in urine using liquid chromatography/triple quadrupole tandem mass spectrometry. Rapid Commun Mass Spectrom. 2004;18(19):2299–308.
Fan R, Dong Y, Zhang W, Wang Y, Yu Z, Sheng G, et al. Fast simultaneous determination of urinary 1-hydroxypyrene and 3-hydroxybenzo[a]pyrene by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;836(1–2):92–7.
Pigini D, Cialdella AM, Faranda P, Tranfo G. Comparison between external and internal standard calibration in the validation of an analytical method for 1-hydroxypyrene in human urine by high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20(6):1013–8.
Onyemauwa F, Rappaport SM, Sobus JR, Gajdosova D, Wu R, Waidyanatha S. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(11–12):1117–25.
Ramsauer B, Sterz K, Hagedorn HW, Engl J, Scherer G, McEwan M, et al. A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method for the determination of phenolic polycyclic aromatic hydrocarbons (OH-PAH) in urine of non-smokers and smokers. Anal Bioanal Chem. 2011;399(2):877–89.
Fan R, Ramage R, Wang D, Zhou J, She J. Determination of ten monohydroxylated polycyclic aromatic hydrocarbons by liquid-liquid extraction and liquid chromatography/tandem mass spectrometry. Talanta. 2012;93:383–91.
Zhao G, Chen Y, Wang S, Yu J, Wang X, Xie F, et al. Simultaneous determination of 11 monohydroxylated PAHs in human urine by stir bar sorptive extraction and liquid chromatography/tandem mass spectrometry. Talanta. 2013;116:822–6.
Liu M, Zhao S, Wang Z, Wang Y, Liu T, Li S, et al. Identification of metabolites of deoxyschizandrin in rats by UPLC-Q-TOF-MS/MS based on multiple mass defect filter data acquisition and multiple data processing techniques. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;949–950:115–26.
Zhang X, Hou H, Xiong W, Hu Q. Development of a method to detect three monohydroxylated polycyclic aromatic hydrocarbons in human urine by liquid chromatographic tandem mass spectrometry. J Anal Methods Chem. 2015;2015:514320.
Lankova D, Urbancova K, Sram RJ, Hajslova J, Pulkrabova J. A novel strategy for the determination of polycyclic aromatic hydrocarbon monohydroxylated metabolites in urine using ultra-high-performance liquid chromatography with tandem mass spectrometry. Anal Bioanal Chem. 2016.
CDC. http://www.cdc.gov/exposurereport/; 2015. [Page last updated: February 2015].
Gustafsson JE, Uzqueda HR. The influence of citrate and phosphate on the Mancini single radial immunodiffusion technique and suggested improvements for the determination of urinary albumin. Clin Chim Acta. 1978;90(3):249–57.
Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27(20):4094–106.
Fiers T, Casetta B, Bernaert B, Vandersypt E, Debock M, Kaufman JM. Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;893–894:57–62.
Schantz MM, Benner Jr BA, Heckert NA, Sander LC, Sharpless KE, Vander Pol SS, et al. Development of urine standard reference materials for metabolites of organic chemicals including polycyclic aromatic hydrocarbons, phthalates, phenols, parabens, and volatile organic compounds. Anal Bioanal Chem. 2015;407(11):2945–54.
CLSI. Evaluation of precision performance of quantitative measurement methods; Approved Guideline—Second Edition. NCCLS document EP5-A2. 2004.
Taylor J. Quality assurance of chemical measurements. Boca Raton: CRC Press; 1987.
Zhou X, Kramer JP, Calafat AM, Ye X. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;944:152–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This research involved human participants. The Centers for Disease Control and Prevention (CDC) Institutional Review Board approved the anonymous collection of urine, and waived informed consent under 45 CFR46.116 (d).
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare they have no actual or potential competing financial interests. The authors complied with all needed research requirements regarding human subjects.
Rights and permissions
About this article
Cite this article
Wang, Y., Meng, L., Pittman, E.N. et al. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 409, 931–937 (2017). https://doi.org/10.1007/s00216-016-9933-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9933-x