Abstract
Two new Standard Reference Materials (SRMs), SRM 3672 Organic Contaminants in Smokers’ Urine (Frozen) and SRM 3673 Organic Contaminants in Non-Smokers’ Urine (Frozen), have been developed in support of studies for assessment of human exposure to select organic environmental contaminants. Collaborations among three organizations resulted in certified values for 11 hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs) and reference values for 11 phthalate metabolites, 8 environmental phenols and parabens, and 24 volatile organic compound (VOC) metabolites. Reference values are also available for creatinine and the free forms of caffeine, theobromine, ibuprofen, nicotine, cotinine, and 3-hydroxycotinine. These are the first urine Certified Reference Materials characterized for metabolites of organic environmental contaminants. Noteworthy, the mass fractions of the environmental organic contaminants in the two SRMs are within the ranges reported in population survey studies such as the National Health and Nutrition Examination Survey (NHANES) and the Canadian Health Measures Survey (CHMS). These SRMs will be useful as quality control samples for ensuring compatibility of results among population survey studies and will fill a void to assess the accuracy of analytical methods used in studies monitoring human exposure to these organic environmental contaminants.

Metabolites of PAHs, Phthalates, Phenols, Parabens, and VOCs in Urine SRMs
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urine sampling is widely used to assess exposure to environmental pollutants because it is non-invasive, easily repeatable, and poses minimal or no collection risk to the donor [1]. In addition, urine is the bodily fluid used for the biomonitoring of compounds with short biological half-lives. Many environmental pollutants, including certain nicotine metabolites, polycyclic aromatic hydrocarbon (PAH), phthalate, phenol, paraben, and volatile organic compound (VOC) metabolites, are monitored in urine as part of studies such as the National Health and Nutrition Examination Survey (NHANES) [2] and the Canadian Health Measures Survey (CHMS) [3]. Quantitation of urinary metabolites has been used to assess exposure to the parent compounds [4, 5].
As part of ongoing collaborations with the Centers for Disease Control and Prevention (CDC) [6], the National Institute of Standards and Technology (NIST) has developed two new Standard Reference Materials (SRMs) for organic contaminants in urine, SRM 3672 Organic Contaminants in Smokers’ Urine (Frozen) and SRM 3673 Organic Contaminants in Non-Smokers’ Urine (Frozen). These two materials have been characterized for hydroxylated PAHs (OH-PAHs), phthalate metabolites, phenols, parabens, VOC metabolites, creatinine, and the free forms of caffeine, theobromine, ibuprofen, nicotine, cotinine, and 3-hydroxycotinine. In addition to CDC and NIST, the Institut National de Santé Publique du Québec (INSPQ) provided data for OH-PAHs.
As noted in the titles of the two SRMs, the sources of the two materials are from smokers (SRM 3672) and non-smokers (SRM 3673). Nicotine is the primary addictive compound in tobacco smoke, and exposure to tobacco products is often assessed by measuring cotinine, one of the major metabolites of nicotine. Cotinine can be easily detected in urine, plasma, and saliva [7]. In addition to nicotine, tobacco smoke contains over 8000 chemicals [8], such as PAHs and VOCs.
People are exposed to mixtures of PAHs through the incomplete combustion of organic compounds from sources such as vehicle emissions, charbroiled foods, and tobacco smoke. Upon exposure, PAHs are metabolized to hydroxylated metabolites (OH-PAHs) [2]. The OH-PAHs are conjugated to glucuronic acid or sulfate for elimination through urine [9]. The urinary concentrations of OH-PAHs have been used to assess the possible sources of human exposure to PAHs [10].
Phthalates are used as plasticizers to make plastics more flexible for use in packaging film and sheets, garden hoses, inflatable toys, blood-storage containers, and medical tubing; phthalates may also be present in personal care products. Once phthalates enter a person’s body, they are converted into hydrolytic monoesters which may undergo further phase I and phase II transformations before urinary excretion [2]. Studies quantifying urinary metabolites have been used to identify possible routes of exposure [11].
Humans are exposed to phenols through industrial pollution, pesticide use, food consumption, and use of personal care products [2]. For example, bisphenol A (BPA) is used in the manufacture of polycarbonate plastics and epoxy resins; benzophenone-3 is used as an ultraviolet filter in cosmetic products and plastics; triclosan is used as a bactericide in soap and other personal care products; and parabens are common antimicrobial preservatives used in cosmetics, pharmaceuticals, and food and beverage processing. Similar to PAHs and phthalates, these environmental phenolic compounds are metabolized (e.g., conjugated) for urinary excretion [12].
Exposure to VOCs is ubiquitous, and chronic exposure to high levels of some VOCs may lead to cancer and neurocognitive dysfunction [2]. Most of the mercapturic acid metabolites of VOCs found in urine are specific to environmental exposure and have longer physiological half-lives compared to the parent compounds [13]. The concentrations of selected VOC metabolites in urine have been investigated as a potential tool for early detection of lung cancer [14].
SRMs 3672 and 3673 are the first frozen urine reference materials characterized for organic environmental contaminants. Exposure assessment relying on the urinary metabolites characterized in these materials can be used to identify exposure sources, follow exposure trends, and study potential health effects associated with exposures. Similar to the two serum and two human milk SRMs previously characterized for organic environmental contaminants [6, 15], these urine materials will be useful in validating methods used for exposure assessment and developing methods for future chemicals of concern.
Experimental
Sample preparation
In 2009 Solomon Park Research Laboratory (Kirkland, WA) acquired a 50 L pool of non-smokers’ urine from donors who were not exposed to secondhand cigarette smoke and a 25 L pool of smokers’ urine from donors who smoked more than a pack of cigarettes per day. The urine was filtered prior to aliquotting into amber glass bottles that are capable of withstanding ultra-cold temperatures. Each bottle was filled with 10 mL of urine and stored at −80 °C prior to shipping on dry ice to NIST.
OH-PAHs
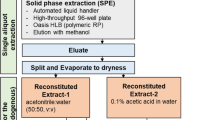
The approach used for the value assignment of OH-PAHs consisted of combining results from analyses of the material at NIST, CDC, and INSPQ using three different methods all based on gas chromatography with mass spectrometry (GC/MS). At NIST, duplicate test portions of approximately 3 g from 10 vials of each SRM were gravimetrically transferred to centrifuge tubes, spiked with a known amount of internal standard solution containing 1-naphthol-d 7 and 3-hydroxyphenanthrene-13C6, followed by the addition of sodium acetate and β-glucuronidase/aryl sulfatase. The samples were incubated at 37 °C for 18 h and then eluted through a Strata X (Phenomenex, Torrance, CA) solid-phase extraction (SPE) column. Following washing and elution, the samples were concentrated to 0.5 mL in toluene and transferred to an autosampler vial. N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) was added, and samples were heated at 60 °C for 30 min and analyzed using GC/MS with a 0.25 mm i.d. × 60 m fused silica capillary column with a 5 % (mole fraction) phenyl methylpolysiloxane phase (0.25 μm film thickness; DB-5, Agilent Technologies, Wilmington, DE).
The CDC laboratory used GC coupled with isotope dilution high resolution MS (GC/ID-HRMS) for the measurement of the OH-PAHs as described in Li et al. [9]. At INSPQ, the urine samples were spiked with internal standards (1-naphthol-13C6, 2-naphthol-13C6, 2-hydroxyfluorene-d 9, 3-hydroxyphenanthrene-13C6 and 1-hydroxypyrene-13C6). The urinary metabolites were hydrolyzed with β-glucuronidase followed by extraction with hexane at neutral pH. These extracts were then evaporated, derivatized with MSTFA, and analyzed using GC coupled with tandem mass spectrometry (GC/MS/MS) on a proprietary non-polar column (DB-XLB, 30 m × 0.25 mm × 0.10 μm film thickness). Multiple reaction monitoring (MRM) mode was used to quantify the OH-PAHs following an electronic impact (EI mode) [16]. A total of 20, 24 and 4 aliquots were analyzed by NIST, CDC, and INSPQ, respectively.
Metabolites of phthalates, phenols and parabens, and volatile organic compounds
The on-line SPE high performance liquid chromatography/ isotope dilution tandem mass spectrometry (LC/MS/MS) methods used at CDC for the quantification of phthalate (18 aliquots of each SRM) and phenol and paraben (6 aliquots of each SRM) metabolites are described in Silva et al. [17], Ye et al. [12], and Ye et al. [18]. BPA was quantified at NIST using a method adapted from Arakawa et al. [19]. Aliquots of approximately 1 g were taken from six vials of each SRM. An internal standard solution containing 13C12-BPA was added followed by the addition of β-glucuronidase. Samples were incubated at 37 °C for 120 min. An amino SPE column was used to remove some of the potential interferences. MSTFA containing 1 % (volume fraction) trimethylchlorosilane (TMCS) was added to the concentrated sample followed by incubation at 60 °C for 20 min prior to GC/MS analysis on a 0.25 mm i.d. × 30 m fused silica capillary column with a 5 % (mole fraction) phenyl methylpolysiloxane phase (0.25 μm film thickness; HP-5MS). The ultra-high performance liquid chromatography - electrospray ionization tandem mass spectrometry method used at CDC for the quantification of VOC metabolites is described in Alwis et al. [13]. Three aliquots of each SRM were quantified for VOC metabolites.
Additional analytes
Creatinine was quantified at NIST using liquid chromatography with mass spectrometry (LC/MS) [20]. Samples of the thawed urine were spiked with creatinine-d 3 followed by the addition of water and a hydrochloric acid (HCl) solution to bring the final concentration to 0.01 mol/L HCl. LC/MS measurements utilized a Luna C18 column, 0.25 cm × 4.6 mm, 5 μm particle (Phenomenex, Torrance, CA) with single-ion monitoring.
Free (i.e., non-conjugated) levels of ibuprofen, caffeine and theobromine, nicotine, cotinine and 3-hydroxycotinine were measured at NIST using liquid/liquid extraction with chloroform followed by GC/MS analysis similar to that described in Man et al. [7]. Isotopically-labeled nicotine, ibuprofen, caffeine, cotinine, hydroxycotinine, and theobromine were used as the internal standards in the NIST modification of the method. The GC/MS analysis used a 0.25 mm i.d. × 30 m fused silica capillary column with a 50 % (mole fraction) trifluoropropyl methylpolysiloxane phase (0.25 μm film thickness; DB-210).
Results and discussion
Currently certified, reference, or information values are provided in NIST SRMs that are characterized for chemical composition. The categorization of the assigned value is based on the number and independence of the analytical methods used, source of the results, and degree of confidence in the accuracy of the assigned value [21]. For natural-matrix SRMs, the assignment of certified mass fraction values is typically based on the combination of results from two or more independent methods [21].
For the two SRMs evaluated in the current study, SRM 3672 and SRM 3673, the certified mass fraction values for the OH-PAHs (Table 1) are based on combining data from three institutes: NIST, CDC, and INSPQ. The agreement among the three data sets was good, as shown in the Electronic Supplementary Material (ESM) Tables S1 and S2, with the relative uncertainties ranging from 1 to 24 % with the majority <15 %. Each method used a hydrolysis step followed by extraction and derivatization with the final analysis using GC with either low-resolution MS (NIST), high-resolution MS (CDC), or tandem MS (INSPQ). There are other published methods using LC with fluorescence detection [22] or MS/MS [23]; however, these LC-based methods have some analytical challenges, such as interfering peaks, particularly for the hydroxynaphthalenes, and coelution of several hydroxyphenanthrenes.
The values for the OH-PAHs in SRM 3672 and 3673 fall within the ranges reported in NHANES [2] and the CHMS [3]. The mass fraction of 4-hydroxyphenanthrene is the lowest in both materials (<0.05 μg/kg) and that of 1-hydroxynaphthalene is the highest. Except for 1-hydroxynaphthalene, the mass fraction of each hydroxylated PAH is higher (3 times to 84 times) in the smokers’ urine (SRM 3672) than in the non-smokers’ urine (SRM 3673). Interestingly, 1-hydroxynaphthalene is 6 times lower in SRM 3672 than in SRM 3673, while 2-hydroxynaphthalene is 6.5 times higher in SRM 3672 than in SRM 3673, following an expected and similar pattern as the remaining PAH metabolites. This suggests that a source other than naphthalene contributed to the levels of 1-hydroxynaphthalene in the non-smokers’ urine. It has been reported that 1-hydroxynaphthalene is also the main metabolite of carbaryl (1-naphthyl-N-methylcarbamate), a major active ingredient in commonly used broad spectrum pesticides accounting for more than 85 % of urinary carbaryl metabolites [24, 25]. However, the inconsistency in levels of 1-hydroxy naphthalene could also be caused by the relatively small number of subjects donating urine to the two SRMs (fewer than 5 subjects per SRM).
In 2008, CDC published geometric means for urinary OH- PAHs in US adults over 20 years of age for both smokers and non-smokers [26]. Other than 1-hydroxynapthalene and 1-hydroxypyrene, the certified values for the OH-PAHs in the smokers’ urine material, SRM 3672, are just slightly higher (within 2 %) or lower than the geometric means for smokers in the US population. The certified value for 1-hydroxynapthalene is 500 % higher and for 1- hydroxypyrene 66 % higher in SRM 3672 than the US smokers. Other than 1-hydroxynaphthalene, the certified values for the OH-PAHs in the non-smokers’ urine material, SRM 3673, are lower than the geometric means for the non-smokers’ urine in the US population. The certified value for 1-hydroxynapthalene is 140 times higher in SRM 3673 than the geometric mean for non-smokers in the US. This comparison is another indication of exposures to sources other than cigarette smoke contributing to the concentrations of some PAH metabolites, particularly 1-hydroxynapthalene. Chetiyanukornkul et al. [22] attributed some of the differences that they noted in their study to exposure to traffic exhaust and biomass burning. Because we do not have information on the donors in this study, it is impossible to draw any conclusions regarding the nature of the exposure sources. With SRMs 3672 and 3673 being the first reference materials characterized for OH-PAHs, they will be useful in future studies to verify the methods used. In a study recently published by CDC, Li et al. [27] used SRMs 3672 and 3673 to validate a new GC-MS/MS method for quantifying 21 metabolites of methylnapthalenes and PAHs in human urine. In the development of the method, they confirmed the certified values for the OH-PAHs [27].
The urine SRMs are the third in a series of biological fluids that were prepared as spiked and unspiked pairs. (The other two materials are human serum and milk [6].) As was done for the serum and milk, a spiked non-smokers’ urine was prepared; the spiking solution contained 25 OH-PAHs in acetonitrile. When the OH-PAHs were quantified in the spiked material, however, the mass fractions were not statistically different than those in the unspiked non-smokers’ urine. A separate stability study was then conducted to assess stability of unconjugated OH-PAHs spiked into urine. This was done by combining the contents from 10 vials of the non-smokers’ urine chosen at random and spiked with a solution of the OH-PAH standards gravimetrically prepared in acetonitrile. The spiked urine was then aliquoted into 2 mL vials and stored at −80 °C. Three aliquots of the spiked urine were removed from the freezer and the mass fractions of the OH-PAHs measured at day 0, 1, 3, 7, and 14. As can be seen in Fig. 1 the OH-PAHs spiked to urine in their free unconjugated form are not stable and are decreasing in concentration up to day 7 after which the concentrations appear to have stabilized. Due to the inherent instability of free form OH-PAHs spiked into urine during storage at −80 °C, the decision was made to not release an SRM spiked with the free un-conjugated OH-PAHs. The naturally occurring conjugates (sulfate and/or glucuronic acid conjugates) present in urine have been stable during storage.
INSPQ and CDC have also investigated the stability of the OH-PAHs in other pooled urine samples (free and conjugated forms). In the study at INSPQ, 19 OH-PAH compounds were relatively stable at −20 and −80 °C over the course of a year. However, at 4 °C and room temperature the free and conjugated species are less stable (degrading in less than 1 to 27 weeks depending on the compound). In the studies at CDC, degradation of the free OH-PAHs was noted but not to the same extent as for this pooled urine sample (data not shown). These differences in stability may be due to other constituents in the pooled urine samples; however, this cannot be proven by the limited studies done.
Reference values are provided for phthalate metabolites (Table 2), phenol and paraben metabolites (Table 3), VOC metabolites (Table 4), and additional compounds, including creatinine and non-conjugated caffeine, theobromine, ibuprofen, nicotine, cotinine, and 3-hydroxycotinine (Table 5). The reference values for the metabolites of phthalates, phenols (except BPA), parabens, and VOCs are based on measurements made at CDC, and the reference values for the additional compounds are based on measurements made at NIST. The reference value for BPA is based on data using LC/MS/MS (CDC) and GC/MS (NIST). The agreement between the two sets of data for BPA is good with an expanded uncertainty for the reference values of 5 % (SRM 3672) and 6 % (SRM 3673).
As for the OH-PAHs, the mass fractions of the phthalate, phenol, paraben, and VOC metabolites are generally higher in the smokers’ urine than in the non-smokers’ urine. There are some exceptions including mono-n-butyl phthalate, benzophenone-3, ethyl and propyl paraben, and 2-thioxothiazolidine-4-carboxylic acid. Smoking is not a known relevant source of exposure for the phthalates, phenols, and parabens measured so it is not surprising that the differences in the mass fractions between the two materials are smallest for the phthalate metabolites (a factor of 2 or less). The differences in the mass fractions for the VOC metabolites in the two materials are generally larger (up to approximately a factor of 6). These differences likely represent a larger contribution to the VOC concentrations from smoking compared to a more diverse contribution, such as exposure to plastics or use of personal care products for the phthalate concentrations. Similar to the OH-PAHs, the mass fractions of these metabolites fall within the ranges reported in NHANES [2, 28, 29] and CHMS [3].
The mass fractions for the additional analytes in Table 5 are based on analyses performed at NIST using LC/MS for creatinine and GC/MS for the remaining analytes. The mass fractions for nicotine, ibuprofen, caffeine, cotinine, theobromine, and 3-hydroxycotinine are for the free (non-conjugated) species only. The free ibuprofen, caffeine, and theobromine mass fractions are higher in the non-smokers’ urine than in the smokers’ urine.
Conclusion
SRM 3672 Organic Contaminants in Smokers’ Urine (Frozen) and SRM 3673 Organic Contaminants in Nonsmokers’ Urine (Frozen) are the first urine reference materials characterized for organic contaminants of interest in biomonitoring studies including PAH metabolites, VOC, phthalate, phenol, paraben, caffeine, theobromine, ibuprofen, creatinine, and tobacco markers. These materials will be useful for method development and for ensuring comparability among survey studies done throughout the world to assess human exposure to multiple classes of compounds including PAHs, phthalates, phenols, and VOCs.
References
Protano C, Andreoli R, Manini P, Vitali M (2012) Sci Total Environ 435–436:115–123
CDC (2013) Biomonitoring summaries. http://www.cdc.gov/biomonitoring/biomonitoring_summaries.html
Second Report on Human Biomonitoring of Environmental Chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 2 (2009–2011). April 2013. www.healthcanada.gc.ca/biomonitoring
Jacob J, Seidel A (2002) J Chromatogr B 778:31–47
Soeborg T, Frederiksen H, Andersson AM (2014) Reproduction 147:455–463
Schantz MM, Eppe G, Focant J-F, Hamilton C, Heckert NA, Heltsley RM, Hoover D, Keller JM, Leigh SD, Patterson DG Jr, Pintar AL, Sharpless KE, Sjödin A, Turner WE, Vander Pol SS, Wise SA (2013) Anal Bioanal Chem 405:1203–1211
Man CN, Gim L-H, Ismail S, Lajis R, Awang R (2006) J Chromatogr B 844:322–327
Rodgman A, Perfetti TA (2009) The chemical components of tobacco and tobacco smoke. CRC Press, New York
Li Z, Romanoff LC, Trinidad DA, Hussain N, Jones RS, Porter EN, Patterson DG Jr, Sjödin A (2006) Anal Chem 78:5744–5751
Shin HM, McKone TE, Bennett DH (2013) Atmos Environ 69:148–155
Koch HM, Lorber M, Christensen KLY, Palmke, Koslitz, Bruening T (2013) Int J Hyg Environ Health 216:672–681
Ye X, Kuklenyik Z, Needham LL, Calafat AM (2005) Anal Chem 77:5407–5413
Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL (2012) Anal Chim Acta 750:152–160
Hanai Y, Shimono K, Matsumura K, Vachani A, Slbelda S, Yamazaki K, Beauchamp GK, Oka H (2012) Biosci Biotech Biochem 76:679–684
Keller JM, Calafat AM, Kato K, Ellefson ME, Reagen WK, Strynar M, O’Connell S, Butt CM, Mabury SA, Small J, Muir DC, Leigh SD, Schantz MM (2010) Anal Bioanal Chem 397:439–451
Gaudreau É, Bienvenu J-F, Bérubé R, Daigle É, Chouinard S, Kim M (2012) Using the agilent 7000B triple quadrupole GC/MS for parts per trillion detection of PAH metabolites in human urine, http://www.chem.agilent.com/Library/applications/5991-0991EN.pdf
Silva MJ, Samander E, Preau JL, Reidy JA, Needham LL, Calafat AM (2007) J Chromatogr B 860:106–112
Ye X, Kuklenyik Z, Bishop AM, Needham LL, Calafat AM (2006) J Chromatogr B 844:53–59
Arakawa C, Fujimaki K, Yoshinaga J, Imai H, Serizawa S, Shiraishi H (2004) Environ Health Prev Med 9:22–26
Fan Z, Xie F, Qiaoling X, Wang S, Ding L, Lui H (2008) Chromatographia 68:623
May W, Parris R, Beck C, Fassett J, Greenberg R, Guenther F, Kramer G, Wise S, Gills T, Colbert J, Gettings R, MacDonald B (2000) NIST special publication 260–136, U.S. Government Printing Office: Gaithersburg, MD. http://www.nist.gov/manuscript-publication-search.cfm?pub_id=200172Accessed 9 July 2014
Chetiyanukornkul T, Toriba A, Kameda T, Tang N, Hayakawa K (2006) Anal Bioanal Chem 386:712–718
Onyemauwa F, Rappaport SM, Sobus JR, Gajdosova D, Wu RA, Waidyanatha S (2009) J Chromatogr B 877:1117–1125
Maroni M, Colosio C, Ferioli A, Fait A (2000) Toxicology 143:5–118
Meeker JD, Barr DB, Serdar B, Rappaport SM, Hauser R (2007) J Expo Sci Environ Epidemiol 17:314–320
Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjödin A, Needham LL, Patterson DG Jr (2008) Environ Res 107:320–331
Li Z, Romanoff LC, Trinidad DA, Pittman EN, Hilton D, Hubbard K, Carmichael H, Parker J, Calafat AM, Sjödin A (2014) Anal Bioanal Chem 406:3119–3129
Alwis KU, deCastro BR, Morrow JC, Blount BC (2014) Tobacco smoke as a major source of acrolein exposure in the US population: NHANES 2005–2006 (submitted to Environmental Health Perspectives)
Alwis KU, deCastro BR, Blount BC (2014) Tobacco smoke as a significant source of exposure to 1,3-butadiene in the US Population: NHANES 2005–2006 (submitted to Environmental Health Perspectives)
Dersimonian R, Laird N (1986) Control Clin Trials 7:177–188
Rukhin AL (2009) Metrologia 46:323–331
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman & Hall, London
JCGM 100:2008; Joint Committee for Guides in Metrology (2008). Available at http://www.bipm.org/utils/common/documents/jcgm/JCGM_100_2008_E.pdf. Accessed 10 April 2013; see also Taylor, B.N.; Kuyatt, C.E.; Guidelines for Evaluating and Expressing the Uncertainty of NIST Measurement Results; (1994) NIST Technical Note 1297; U.S. Government Printing Office: Washington, DC. Available at http://www.nist.gov/physlab/pubs/tn1297/index.cfm. Accessed 10 April 2013
JCGM 101:2008; Joint Committee for Guides in Metrology (BIPM, IEC, IFCC, ILAC, ISO, IUPAC, IUPAP and OIML) (2008) International Bureau of Weights and Measures (BIPM), Sèvres, France. Available at http://www.bipm.org/utils/common/documents/jcgm/JCGM_101_2008_E.pdf. Accessed 10 April 2013
Horn RA, Horn SA, Duncan DB (1975) J Am Stat Assoc 70:380–385
Disclaimer
Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Reference Materials for Chemical Analysis with guest editors Hendrik Emons and Stephen A. Wise.
Contribution of the US Government; not subject to copyright
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 13 kb)
Rights and permissions
About this article
Cite this article
Schantz, M.M., Benner, B.A., Heckert, N.A. et al. Development of urine standard reference materials for metabolites of organic chemicals including polycyclic aromatic hydrocarbons, phthalates, phenols, parabens, and volatile organic compounds. Anal Bioanal Chem 407, 2945–2954 (2015). https://doi.org/10.1007/s00216-014-8441-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8441-0