Abstract
Estimation of postmortem interval (PMI) is an important goal in judicial autopsy. Although many approaches can estimate PMI through physical findings and biochemical tests, accurate PMI calculation by these conventional methods remains difficult because PMI is readily affected by surrounding conditions, such as ambient temperature and humidity. In this study, Sprague-Dawley (SD) rats (10 weeks) were sacrificed by suffocation, and blood was collected by dissection at various time intervals (0, 3, 6, 12, 24, and 48 h; n = 6) after death. A total of 70 endogenous metabolites were detected in plasma by gas chromatography-tandem mass spectrometry (GC-MS/MS). Each time group was separated from each other on the principal component analysis (PCA) score plot, suggesting that the various endogenous metabolites changed with time after death. To prepare a prediction model of a PMI, a partial least squares (or projection to latent structure, PLS) regression model was constructed using the levels of significantly different metabolites determined by variable importance in the projection (VIP) score and the Kruskal-Wallis test (P < 0.05). Because the constructed PLS regression model could successfully predict each PMI, this model was validated with another validation set (n = 3). In conclusion, plasma metabolic profiling demonstrated its ability to successfully estimate PMI under a certain condition. This result can be considered to be the first step for using the metabolomics method in future forensic casework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estimation of time since death is a practical task in daily forensic casework. The physical changes that occur after death, including cooling of the body, rigor mortis, and development of postmortem lividity, are well recognized as early postmortem phenomena. These phenomena continue to be the main basis for estimating the time since death, also known as the postmortem interval (PMI). Accurate estimation of PMI is an important medicolegal issue, as it remains a difficult problem for forensic pathologists. For more than a century, various approaches have been used to determine the PMI, including examining various physical changes, changes in biochemical constituents in various body tissues and fluids, DNA/RNA degradation, and forensic entomology [1–14]. Despite extensive studies on this issue, accurate PMI estimation remains a challenge. Thus, a more reliable and accurate method to estimate PMI is in demand.
Metabolomics broadly aims to measure the systemic metabolic changes over time in multicellular systems and can be defined as the comprehensive analytical approach for the study of all low molecular weight biochemicals, including sugars, amino acids, organic acids, nucleotides, and lipids from biological specimens. Metabolic profiling has also been used in studies investigating the metabolic response to various diseases and drug abuse [15–19]. Previous studies using nuclear magnetic resonance (NMR) spectroscopy have shown that levels of endogenous metabolites, such as amino acids and saccharides, in a dead body change with time after death, suggesting the potential for NMR as a useful tool in PMI estimation [20–23]. However, there are no reports on a PMI prediction model using mass spectrometry (MS)-based metabolic profiling.
To the best of our knowledge, this is the first trial to estimate PMI in a suffocated rat model using gas chromatography-tandem mass spectrometry (GC-MS/MS)-based metabolic profiling by constructing a prediction model with analytical validation. The aim of the present study was therefore to assess the applicability of GC-MS/MS-based metabolic profiling to estimate PMI and to construct a prediction model based on experiments with suffocated rats.
Materials and methods
Chemicals
All chemicals and reagents were of analytical grade or better quality and purchased from Wako Pure Chemical Industries (Osaka, Japan), except where stated otherwise. Methoxyamine hydrochloride and 2-isopropyl-malic acid were supplied from Sigma-Aldrich (St. Louis, MO, USA). N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) was purchased from GL Sciences (Tokyo, Japan).
Animal experiments
Training set
Thirty-six male Sprague-Dawley (SD) rats (10 weeks old, weight 282–320 g) were obtained from Japan SLC, Inc. (Hamamatsu, Japan). The rats were kept in an animal facility in a specific and opportunist pathogen-free environment at 22–24 °C with a 12/12 h light-dark cycle. The animals were supplied with water and food ad libitum. After fasting for 16 h, they were sacrificed by CO2 inhalation and cardiac blood was collected by dissection at six time intervals (0, 3, 6, 12, 24, and 48 h after death; 0 h: T1–T6, 3 h: T7–T12, 6 h: T13–T18, 12 h: T19–T24, 24 h: T25–T30, 48 h: T31–T36). Until sample collection, bodies were preserved at 16 °C and 40–45 % humidity and separately bagged in a plastic bag to prevent drying. The body weight was recorded at 0 h and each sample collection time. The blood samples were rapidly prepared to plasma and quickly frozen under liquid nitrogen and stored at −80 °C until analysis.
Validation set
Another 18 male SD rats (10 weeks old, weight 282–319 g) were used under the same experimental course as above. The blood samples were collected at six time intervals (0, 3, 6, 12, 24, and 48 h after death; 0 h: V1–V3, 3 h: V4–V6, 6 h: V7–V9, 12 h: V10–V12, 24 h: V13–V15, 48 h: V16–V18).
Experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Animal Research Laboratory, Osaka Medical College).
Sample preparation
Frozen plasma samples were thawed on an ice bath prior to use. The extraction protocol was the same as described in previous reports with slight modification [19]. In brief, an internal standard aqueous solution (25 μl, 2.3 mM 2-isopropyl-malic acid) and chloroform-methanol (2:5, v/v) solvent mix (225 μl) was added to 50 μl each of the plasma samples, and samples were vortexed for 5 min. After centrifugation at 16,000×g at 4 °C for 5 min, 225 μl of the upper layer was removed to another Eppendorf tube, and 200 μl distilled water was added. After vortexing for 5 min, the mixed solution was centrifuged at 16,000×g at 4 °C for 10 min, and 300 μl of the upper layer was moved to another tube. We then collected 4 μl of solution from the tube and added 196 μl of distilled water. This diluted sample was concentrated by a centrifugal concentrator (Concentrator 5301, Eppendorf AG, Hamburg, Germany) for 1 h, followed by freeze-drying (VD-400F, TAITEC, Saitama, Japan) overnight. Methoxyamine hydrochloride pyridine solution (60 μl, 20 mg/ml) was added to the residue, and the sample was incubated at 30 °C for 90 min for methoximation. MSTFA (40 μl) was added to the solution, and the sample was incubated at 37 °C for 30 min for trimethylsilylation.
Instrumental analysis
A GCMS-TQ8030 triple quadrupole gas chromatograph mass spectrometer (Shimadzu, Kyoto, Japan) was used for analysis. A DB-5 capillary column (30 m × 0.25 mm i.d., film thickness 1 μm, Agilent, Santa Clara, CA, USA) was used for the chromatographic separation. The column oven temperature was maintained at 100 °C for 4 min, and then increased by 10 °C/min to 320 °C, with a final hold for 9 min. The transfer line temperature and carrier gas (He) flow rate were set at 280 °C and 1.1 ml/min, respectively. The electron ionization (EI) operating parameters were set as follows: the ion-source and interface temperatures, 200 and 280 °C, respectively, and electron energy, 70 eV. Mass spectra were collected from m/z 35–600 at scan rate of 2,000 units/s. GC-MS/MS was conducted in the positive ion mode. Samples were automatically injected in the splitless mode, and the injection volume was set at 1 μl. Sequence of the sample injection was randomly arranged. In a single analysis, we performed scan mode for qualitative analysis and selected reaction monitoring (SRM) detection for quantitative analysis at the same time. The SRM transitions and collision energies are shown in the Electronic Supplementary Material (ESM; Table S1). Peak detection and peak area calculation of the data were carried out by the built-in software (GCMS solution Ver4.11) and a metabolite database (Shimadzu, ver. 2.0). Peak areas of each metabolite were normalized by that of 2-isopropyl-malic acid.
Statistical analysis
Multivariate analysis including principal component analysis (PCA), partial least squares (or projection to latent structure, PLS) analysis, and calculation for variable importance in the projection (VIP) score were performed using SIMCA P+ statistical software (ver. 13, Umetrics AB, Umea, Sweden). Kruskal-Wallis test was performed for the evaluation of statistically significant metabolites using JMP Pro software (ver. 11, SAS Institute Japan Ltd., Japan). Hotelling’s T 2 is a multivariate generalization of Student’s t distribution. It provides a tolerance region for the data in a two-dimensional score plot that represented a 95 % confidence interval [24]. VIP shows the contribution of each predictor variable to the model and presents the influence of each predictor on response variables [25]. In SIMCA P+, VIP plots are sorted based on the importance of variables. Variables with importance value greater than 1 are usually considered as the most important value for explaining Y-variables, and VIP value is often used for variable selection. To construct a more highly accurate model of PMI estimation, the metabolites were selected with a threshold of VIP score >1.2. The predictive ability of the developed model was evaluated using the permutation test. To ensure the stability of the probability estimates, 50 permutations were carried out.
Results
Body weights of animals
We recorded the weight of animals at the time of sacrifice and at each sample collection. The weights in grams at the times of sacrifice (3, 6, 12, 24, and 48 h after death groups) were 304.6 ± 11.9, 297.4 ± 7.6, 305.5 ± 10.9, 300.4 ± 10.6, and 291.4 ± 6.0, respectively, and the weights at sample collection were 304.4 ± 11.9, 297.3 ± 7.6, 305.4 ± 10.9, 300.1 ± 10.6, and 291.1 ± 5.9.
Metabolite identification
A total of 70 endogenous metabolites were detected in plasma by GC-MS/MS (Table 1). As data from the T16 sample could not be obtained due to injection failure, statistical analysis was performed excluding sample T16.
PCA analysis and PLS regression model
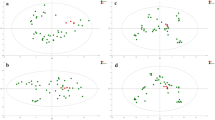
On the PCA score plot, each time group was separated from each other with the exception of a considerable overlap of time points 3 and 6 h and a minor overlap of 12 and 24 h, suggesting that the various endogenous metabolites changed with time after death (Fig. 1). To prepare a prediction model of a PMI, a PLS regression model was constructed using the levels of significantly different metabolites determined by the VIP score (VIP score >1.2) and Kruskal-Wallis test (P < 0.05) (see ESM Fig. S1). As a result, 25 metabolites were selected (Table 1). Figure S2 in the ESM shows the concentration changes of the 25 metabolites over time, and these were used for the PLS regression model. In the regression model, the R 2 and Q 2 values were 0.98 and 0.96, respectively (Fig. 2a). In the permutation test plot, the y-intercept values of the regression line were 0.11 for R 2 and −0.21 for Q 2 (Fig. 2b).
Validation analysis
Since the constructed PLS regression model could successfully predict each PMI, this model was validated with a new validation set (n = 3). In the validation set, the same 70 metabolites from the previous analysis were detected. This result showed the satisfactory prediction accuracy of the regression model from the metabolic profiling (Table 2).
Discussion
In this study, we investigated the GC-MS/MS-based metabolic profiling of a suffocated rat model after a certain period of time after death and constructed a prediction model of PMI estimation with satisfactory prediction accuracy, substantiated by another validation analysis.
In forensic practice, PMI estimation is an important subject. Various strategies are currently used to determine the PMI, including evaluation of various physical changes, changes in biochemical constituents in various body tissues and fluids, DNA/RNA degradation, and forensic entomology [1–14]. Despite broad research, accurate PMI estimation remains a problem.
Recently, analytical methods have been developed to generate multivariate profiles of metabolites, mainly using NMR or MS [26]. To examine the mechanism of various diseases and conditions, 1H-NMR-based metabolomics studies have been carried out using a variety of tissues and body fluids [27–29]. Several studies have estimated PMI using 1H-NMR. Scheurer et al. measured concentrations of 27 metabolites or metabolite groups in a sheep head model and selected five (acetate, alanine, butyrate, free trimethyl-ammonium, and propionate) for further statistical analysis and modeling by analytical functions [21]. Furthermore, to evaluate the temperature effect, the same research group performed the simultaneous determination of 25 different biochemical compounds at four different temperatures between 4 and 26 °C. The authors performed a linear regression analysis based on the data from eight metabolites (acetate, alanine, aspartic acid, butyrate, γ-aminobutyric acid, myoinositol and glycine, succinic acid, and valine) and showed a high correlation between the estimated and true postmortem times at the investigated temperatures [22]. Hirakawa et al. investigated the metabolic changes in rat femoral muscles using 1H-NMR pattern recognition. Rats killed by suffocation, cocaine overdose, and induced respiratory failure were classified into three groups according to the time of sampling (within 15 min, after 1–4 h and ≥8 h after death). As the principal component scores of each group clustered in discrete regions, the authors concluded that the metabolic profiles correlated with PMI [23]. However, compared with 1H-NMR, MS yields relatively high sensitivity measurements and allows for reliable metabolite identification [26, 30].
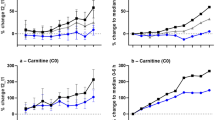
In our study, we detected 70 metabolites and found that among these, 25 metabolites had a statistically strong correlation with PMI. The metabolites enabled us to collect more detailed information about the metabolites concerning PMI relative to previous reports using 1H-NMR. The levels of 25 metabolites continuously increased over time. As a cadaver generally begins to dry over time after death, we placed the rats individually in bags to prevent drying. Weight in grams at the times of sacrifice and sample collection (3, 6, 12, 24, and 48 h after death) changed from 304.6 ± 11.9, 297.4 ± 7.6, 305.5 ± 10.9, 300.4 ± 10.6, and 291.4 ± 6.0 to 304.4 ± 11.9, 297.3 ± 7.6, 305.4 ± 10.9, 300.1 ± 10.6, and 291.1 ± 5.9, respectively. This result suggested that the concentrations of metabolites in plasma were not affected by evaporation. In addition, lactic acid is known to increase with time elapsed after death for anaerobic metabolism. We found that lactic acid level increased at the 6 h point, as previously reported (Fig. 3) [31]. Therefore, we believe that the levels of these 25 metabolites increased because of the influence of PMI.
The 25 metabolites consisted of 18 amino acids, 5 sugars, a carboxylic acid, and a phosphate. Usually, processes accruing at postmortem such as the following should be considered: metabolic processes relating to enzyme activities, autolysis, and microbial processes. Among the 18 amino acids detected in our study, eight were essential amino acids that are suspected to be produced by autolysis or microbial cell metabolism [32]. With regard to sugars, l-arabinose is released by fungi from plant polymers [33]. Thus, we speculate that the metabolites may have been partly affected by microbial processes.
In the regression model, the R 2 and Q 2 values were 0.98 and 0.96, respectively. The R 2 value reflects the goodness of fit and measures the strength of the regression model. The Q 2 value indicates the predictive ability of the model. The R 2 and Q 2 values are considered to be good when the values are closer to unity. Thus, the performance of the two-component regression model was considered to be satisfactory. In the permutation test plot, the y-intercept values of the regression line were 0.11 for R 2 and −0.21 for Q 2. The y-intercept of the regression line shows a measure of the overfit; a near-zero slope of the regression line and a high y-intercept value suggest the inadequacy of the model. Therefore, this result indicated that the two-component regression model was sufficient to predict PMI.
Our data are currently preliminary, as most postmortem parameters are influenced by several influencing factors of ambient conditions (i.e., temperature, humidity). Furthermore, there may be additional differences depending on the kinds of microorganisms existing in the body. However, it is notable that we performed the validation analysis using another validation set and confirmed the satisfactory prediction accuracy of the regression model. We thus expect that the collected metabolites in our study would be informative for further progress in detecting PMI in combination with the various postmortem parameters already reported.
In conclusion, this is the first report to investigate the PMI of suffocated rat models by MS-based metabolic profiling. The plasma metabolic profiling demonstrated its ability to successfully estimate PMI under a certain condition. Although further experiments are needed for future routine forensic casework, this result can be considered to be the first step for using the metabolomics method for PMI estimation.
References
Althaus L, Henssge C (1999) Rectal temperature time of death nomogram: sudden change of ambient temperature. Forensic Sci Int 99:171–178
Mall G, Eckl M, Sinicina I, Peschel O, Hubig M (2005) Temperature-based death time estimation with only partially known environmental conditions. Int J Legal Med 119:185–194
Warther S, Sehner S, Raupach T, Püschel K, Anders S (2012) Estimation of the time since death: post-mortem contractions of human skeletal muscles following mechanical stimulation (idiomuscular contraction). Int J Legal Med 126:399–405
Kaliszan M (2013) Studies on time of death estimation in the early post mortem period—application of a method based on eyeball temperature measurement to human bodies. Leg Med (Tokyo) 15:278–282
Biermann FM, Potente S (2011) The deployment of conditional probability distributions for death time estimation. Forensic Sci Int 210:82–86
Madea B, Rodig A (2006) Time of death dependent criteria in vitreous humor: accuracy of estimating the time since death. Forensic Sci Int 164:87–92
Singh D, Prashad R, Sharma SK, Pandey AN (2006) Estimation of postmortem interval from human pericardial fluid electrolytes concentrations in Chandigarh zone of India: log transformed linear regression model. Leg Med (Tokyo) 8:279–287
Mao S, Fu G, Seese RR, Wang ZY (2013) Estimation of PMI depends on the changes in ATP and its degradation products. Leg Med (Tokyo) 15:235–238
Zapico S C, Menendez ST, Nunez P (2013) Cell death proteins as markers of early postmortem interval. Cell Mol Life Sci 71:2957–2962
Poloz YO, O’Day DH (2009) Determining time of death: temperature-dependent postmortem changes in calcineurin A, MARCKS, CaMKII, and protein phosphatase 2A in mouse. Int J Legal Med 123:305–314
Sampaio-Silva F, Magalhaes T, Carvalho F, Dinis-Oliveira RJ, Silvestre R (2013) Profiling of RNA degradation for estimation of post mortem interval. PLoS One 8:e56507
Hansen J, Lesnikova I, Funder AM, Banner J (2014) DNA and RNA analysis of blood and muscle from bodies with variable postmortem intervals. Forensic Sci Med Pathol 10:322–328
Kimura A, Ishida Y, Hayashi T, Nosaka M, Kondo T (2011) Estimating time of death based on the biological clock. Int J Legal Med 125:385–391
Wells JD, LaMotte LR (2010) Estimating the postmortem interval. In: Byrd JH, Castner JL (eds) Forensic entomology: the utility of arthropods in legal investigations. CRC, Boca Raton, pp 367–388
Rasmiena AA, Ng TW, Meikle PJ (2013) Metabolomics and ischaemic heart disease. Clin Sci (Lond) 124:289–306
Hu X, Wu GP, Zhang MH, Pan SQ, Wang RR, Ouyang JH, Liu JG, Chen ZY, Tian H, Liu DB (2012) GC-MS-based metabolic profiling reveals metabolic changes in anaphylaxis animal models. Anal Bioanal Chem 404:887–893
Kobayashi T, Yoshida T, Fujisawa T, Matsumura Y, Ozawa T, Yanai H, Iwasawa A, Kamachi T, Fujiwara K, Kohno M, Tanaka N (2014) A metabolomics-based approach for predicting stages of chronic kidney disease. Biochem Biophys Res Commun 445:412–416
Shima N, Miyawaki I, Bando K, Horie H, Zaitsu K, Katagi M, Bamba T, Tsuchihashi H, Fukusaki E (2011) Influences of methamphetamine-induced acute intoxication on urinary and plasma metabolic profiles in the rat. Toxicology 287:29–37
Zaitsu K, Miyawaki I, Bando K, Horie H, Shima N, Katagi M, Tatsuno M, Bamba T, Sato T, Ishii A, Tsuchihashi H, Suzuki K, Fukusaki E (2014) Metabolic profiling of urine and blood plasma in rat models of drug addiction on the basis of morphine, methamphetamine, and cocaine-induced conditioned place preference. Anal Bioanal Chem 406:1339–1354
Banaschak S, Rzanny R, Reichenbach JR, Kaiser WA, Klein A (2005) Estimation of postmortem metabolic changes in porcine brain tissue using 1H-MR spectroscopy—preliminary results. Int J Legal Med 119:77–79
Scheurer E, Ith M, Dietrich D, Kreis R, Husler J, Dirnhofer R, Boesch C (2005) Statistical evaluation of time-dependent metabolite concentrations: estimation of post-mortem intervals based on in situ 1H-MRS of the brain. NMR Biomed 18:163–172
Ith M, Scheurer E, Kreis R, Thali M, Dirnhofer R, Boesch C (2011) Estimation of the postmortem interval by means of 1H MRS of decomposing brain tissue: influence of ambient temperature. NMR Biomed 24:791–798
Hirakawa K, Koike K, Uekusa K, Nihira M, Yuta K, Ohno Y (2009) Experimental estimation of postmortem interval using multivariate analysis of proton NMR metabolomic data. Leg Med (Tokyo) 11(Suppl 1):S282–S285
Hotelling H (1947) Multivariate quality control-illustrated by the air testing of sample bombsights. In: Eisenhart C, Hastay MW, Wallis WA (eds) Tech Stat Anal. McGraw-Hill, New York, pp 111–184
Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikstrom C, Wold S (2001) Multi- and Megavariate Data Analysis Part I: Basic Principles and Applications, 2nd edn. Umetrics Academy
Nicholson JK, Holmes E, Kinross JM, Darzi AW, Takats Z, Lindon JC (2012) Metabolic phenotyping in clinical and surgical environments. Nature 491:384–392
Le Moyec L, Mille-Hamard L, Triba MN, Breuneval C, Petot H, Billat VL (2012) NMR metabolomics for assessment of exercise effects with mouse biofluids. Anal Bioanal Chem 404:593–602
Smolinska A, Posma JM, Blanchet L, Ampt KA, Attali A, Tuinstra T, Luider T, Doskocz M, Michiels PJ, Girard FC, Buydens LM, Wijmenga SS (2012) Simultaneous analysis of plasma and CSF by NMR and hierarchical models fusion. Anal Bioanal Chem 403:947–959
Kim IY, Jung J, Jang M, Ahn YG, Shin JH, Choi JW, Sohn MR, Shin SM, Kang DG, Lee HS, Bae YS, Ryu do H, Seong JK, Hwang GS (2010) 1H NMR-based metabolomic study on resistance to diet-induced obesity in AHNAK knock-out mice. Biochem Biophys Res Commun 403:428–434
Pan Z, Raftery D (2007) Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Anal Bioanal Chem 387:525–527
Katsumata Y, Sato K, Yada S, Uematsu T, Oya M, Yoshino M (1983) Anaerobic metabolism in dogs after organismal death. Jpn J Legal Med 37:75–78
Mitsuhashi S (2014) Current topics in the biotechnological production of essential amino acids, functional amino acids, and dipeptides. Curr Opin Biotechnol 26:38–44
Seiboth B, Metz B (2011) Fungal arabinan and l-arabinose metabolism. Appl Microbiol Biotechnol 89:1665–1673
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology, Japan (grant No. 25460880).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1167 kb)
Rights and permissions
About this article
Cite this article
Sato, T., Zaitsu, K., Tsuboi, K. et al. A preliminary study on postmortem interval estimation of suffocated rats by GC-MS/MS-based plasma metabolic profiling. Anal Bioanal Chem 407, 3659–3665 (2015). https://doi.org/10.1007/s00216-015-8584-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8584-7