Abstract
The use of the direct inlet probe–atmospheric-pressure chemical ionization (DIP-APCI) ion source developed in our laboratory coupled to a high resolution Q-TOF MS for the quantitative analysis of coumarin in different cinnamon samples was demonstrated in this study. Extraction of coumarin from various cinnamon samples was followed by DIP-APCI-mass spectrometry (MS) and liquid chromatography (LC)-MS analysis. For quantification, an external calibration with and without the use of stable isotope-labeled coumarin as internal standard was compared. The results obtained by DIP-APCI-MS and LC-MS were in good agreement. Even without the use of an internal standard satisfying linearity (R 2 > 0.997), recovery (94–104 % for spiking levels between 100 and 5,000 mg/kg) and intra- and interday repeatability (2.2–13.8 %RSD) was demonstrated using DIP-APCI-MS. To reduce the number of samples requiring quantitative analysis, the possibility of semi-quantitative screening of coumarin directly from powdered cinnamon using DIP-APCI-MS was shown. The analysis of woodruff-flavored beverages and cinnamon-flavored chewing gum by DIP-APCI-MS resulted in the formation of an artifact interfering with coumarin detection. As with other ambient ionization methods, special attention has to be paid to possible spectral interferences due to isobaric substances present in the sample matrix or formed from matrix components after ionization. The temperature-programmed vaporization in DIP-APCI-MS combined with the use of stable isotope-labeled coumarin as internal standard helped in recognizing this interference.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coumarin is a naturally occurring secondary plant product with a pleasant flavor that can be found in a variety of plants, for example, in the tonka bean, in woodruff, and especially in Cassia cinnamon [1, 2]. There are two types of cinnamon sold in the European markets, Ceylon and Cassia cinnamon [2, 3]. Coumarin levels are reported to range from below the limit of detection to 190 mg/kg in Ceylon cinnamon and from 700 to 12,230 mg/kg in Cassia cinnamon [4]. More recently, coumarin levels from below the limit of detection (LOD, 30 mg/kg) to 297 mg/kg have been measured in Ceylon cinnamon powder, while the levels in Cassia cinnamon powder ranged from 1,740 to 7,670 mg/kg [2]. From animal experiments, coumarin is deemed to be hepatotoxic and is considered to have a carcinogenic effect [1, 3]. In 2004, the European Food Safety Authority (EFSA) deduced that the carcinogenic effect was caused by a non-genotoxic mode of action and a tolerable daily intake (TDI) of 0.1 mg coumarin/kg body weight based on a no observed adverse effect level for hepatotoxicity in dogs was derived [5]. This TDI was confirmed again in 2008 by EFSA [6] and also based on clinical data on hepatotoxicity from patients treated with coumarin as medicinal drug [7]. In 2011, Germany’s Federal Institute for Risk Assessment (BfR) demonstrated that the bioavailability of coumarin from cinnamon powder is only slightly lower than that of isolated coumarin [8]. Therefore, the TDI derived using isolated coumarin can also be used for coumarin in cinnamon-containing foodstuffs [9]. Since January 2011, new maximum levels for coumarin in certain cinnamon-containing foodstuffs exist in the European Union that were defined in Regulation EC no. 1334/2008, e.g., 50 mg/kg in cinnamon star cookies [9]. There are no maximum levels for coumarin in cinnamon used as a spice. Different methods have been established for the analysis of coumarin, the most widely used being liquid chromatography (LC)-UV [1, 2, 10, 11] and LC-MS (MS) [1, 12]. Here, we report the determination of coumarin using an ambient ionization MS technique, i.e. direct inlet probe–atmospheric-pressure chemical ionization (DIP-APCI)-MS.
Recently, several ambient ionization techniques were introduced for mass spectrometric analyses [13–15]. With these ion sources, solid and/or liquid samples can be brought directly into the ion source without any pretreatment and chromatographic separation and are ionized, for example, by corona discharge (atmospheric solids analysis probe (ASAP) [13]), plasma (direct analysis in real time (DART) [14]) or electrospray processes (desorption electrospray ionization (DESI) [15]). Reported applications of ambient ionization MS cover mainly qualitative confirmation and profiling analyses. There are, however, also a few examples of quantitative determinations by ambient ionization MS found in the literature. ASAP has been used to determine azoxystrobin residues in grain [16], and DART was for example employed for the quantitative analyses of strobilurin fungicides in wheat [17], chemical warfare agents [18], caffeine in coffee samples [19], diazepam in blood [20], geniposide in a Chinese herbal preparation [21], and mycotoxins in cereals [22]. Quantitative DESI applications include the determination of clopazine in animal tissue [23], flunitrazepam in alcoholic beverages [24], diphenhydramine in human urine [25], and cholesterol in serum [26]. In nearly all the reported applications of ambient ionization MS for quantitative analysis, an internal standard was used to compensate the relatively high variation of ion intensities in repeated analyses [17, 19, 20, 22, 23, 26]. The use of stable isotope-labeled standards can also compensate matrix-effects (ion suppression/enhancement) that represent one of the main drawbacks of ambient ionization techniques [19, 20, 27]. The DIP-APCI ion source developed in our laboratory [28] consists of a commercially available temperature-programmed push rod coupled to a homemade APCI ion source. The vaporization of sample components due to the programmed heating of the push rod leads to a certain separation of sample components as a result of their different vapor pressures, which reduces ion suppression and simplifies the evaluation of the resulting mass spectra. For use in routine analysis, DIP-APCI-MS analysis can also be automated using a Combi Pal autosampler.
Experimental section
Materials
Coumarin (≥99 %) and deuterium labeled coumarin-d4 (98.8 atom%D) were purchased from Sigma Aldrich Chemie GmbH (Steinheim, Germany) and Dr. Ehrenstorfer GmbH (Augsburg, Germany), respectively. Methanol (for high-performance liquid chromatography (HPLC); LC-MS grade) was obtained from VWR International (Leuven, Belgium). Water was purified using a TKA ultrapure water system (Thermo Fisher Scientific, Niederelbert, Germany). Formic acid (analytical grade; ∼98 %) was purchased from Fluka (Steinheim, Germany). 4-Coumaric acid (97.5 %) was obtained from Carl Roth GmbH (Karlsruhe, Germany). Potassium ferrocyanide (*3 H2O; analytical grade) and zinc sulfate (*7 H2O; analytical grade) were obtained from Fluka (Steinheim, Germany) and VWR International (Leuven, Belgium), respectively. Purine and HP-0921 (API-TOF Reference Mass Solution Kit) were obtained from Agilent Technologies (Santa Clara, United States). All compounds were used as received.
Preparation of standards
Stock solutions of coumarin (1,000 mg/L) and internal standard coumarin-d4 (460 mg/L) were prepared in methanol. Working standard solutions for the calibration curve (0.1–10 mg/L (0.1, 0.5, 1, 5, and 10 mg/L) coumarin; 0.9 mg/L internal standard coumarin-d4), and spiking of samples were obtained by dilutions using methanol/water (80:20, v/v).
Samples and sample pretreatment
Ten different cinnamon powder samples (five Ceylon, two Cassia, and three samples without specification of botanical origin), two woodruff-flavored beverages, and one cinnamon-flavored chewing gum were purchased from the German retail market. For DIP-APCI-MS and LC-MS analysis, the cinnamon samples were extracted according to method L.00.00-134 of the German official collection of analytical methods (ASU § 64 German Food and Feed Act, LFGB). A portion of 0.5 g of the cinnamon powder was accurately weighed and after addition of internal standard coumarin-d4 stirred with approximately 40 mL of methanol/water (80:20, v/v) for 30 min. The extracts were filled up to 50 mL with methanol/water (80:20, v/v) and filtered through a 0.2 μm PTFE syringe filter. 100 μL of extracts of Cassia cinnamon and cinnamon without specification of botanical origin were diluted with 900 μL methanol/water (80:20, v/v) prior to analysis. The cinnamon-flavored chewing gum was extracted in an analogous manner after grinding in liquid nitrogen. One cinnamon extract was clarified according to Carrez [1, 29] for comparison with the untreated extract. 2 mL each of Carrez I (potassium ferrocyanide *3 H2O, 36 g/L) and Carrez II (zinc sulfate *7 H2O, 72 g/L) were added to the extract before filling up to 50 mL. The clear supernatant was then filtered through a 0.2 μm PTFE syringe filter and analyzed by DIP-APCI-MS. Woodruff-flavored beverages were only diluted with methanol/water (80:20, v/v) after addition of internal standard coumarin-d4 and filtered through a 0.2 μm PTFE syringe filter after dilution. Cinnamon powder samples were also analyzed without any sample preparation using DIP-APCI-MS. For this purpose, approximately 0.1 mg of cinnamon powder was accurately weighed into the probe tips.
Instrumentation
All experiments were performed with an Agilent Technologies 6538 UHD Accurate-Mass Q-TOF operated in high-resolution mode (resolution of at least 20,000 and 40,000 at m/z 322 and m/z 1,522, respectively) in the standard mass range (3,200 m/z).
DIP-APCI-MS
The DIP-APCI ion source described elsewhere [28] was operated in positive atmospheric pressure chemical ionization mode (APCI+) with the following optimized operation parameters: nitrogen as auxiliary gas, 100 L/h; drying-gas flow rate, 6.0 L/min; drying-gas temperature, 300 °C; capillary voltage, 3500 V; corona current, 4 μA; fragmentor voltage, 150 V; skimmer voltage, 65 V; octupole rf, 750 V; vaporizer temperature, 325 °C; nebulizer pressure, 20 psig. Accurate mass spectra were recorded across the range of m/z 50-1,000 with an acquisition rate of 1 spectrum/s. The instrument performed the internal mass calibration automatically, using an automated calibrant delivery system, which introduces, via the vaporizer, a low flow of a calibrating solution containing the internal reference masses m/z 121.050873 ([M + H]+ of purine (C5H4N4)) and m/z 922.009798 ([M + H]+ of HP-0921 (C18H18O6N3P3F24)). The probe tip of the DIP-APCI ion source was moved to a predefined position (4.0 mm from a position directly below the corona needle) in the ion source. The probe tip was heated to 400 °C at 2°/s; the final hold time at 400 °C was 1 min. 1 μL of the standards and samples were injected into disposable probe tips with a GC syringe.
LC-MS
An Agilent Technologies Infinity 1290 UHPLC was coupled to the MS employing an electrospray source. LC separation was performed with a Kinetex 1.7 μm C18 (100 × 2.1 mm) column (Phenomenex, Aschaffenburg, Germany) thermostatted at 40 °C using water and methanol, each with 0.1 % (v/v) formic acid, in the following gradient program with a flow rate of 0.3 mL/min: 0–5 min, 20–55 % methanol; 5–6 min, 55–90 % methanol; 6–7 min, 90 % methanol; 7–8 min, 90–20 % methanol; 8–10 min, 20 % methanol. The injection volume was 1 μL. The electrospray source was operated in positive electrospray ionization mode (electrospray ionization (ESI)+) with the following settings: drying-gas flow rate, 9.0 L/min; drying-gas temperature, 325 °C; capillary voltage, 3500 V; fragmentor voltage, 150 V; skimmer voltage, 65 V; octupole rf, 750 V; nebulizer pressure, 30 psig. Accurate mass spectra were recorded across the range of m/z 50-1,000 with an acquisition rate of 3 spectra/s. The calibrating solution for internal mass calibration was introduced via a second nebulizer.
Method validation
Both the DIP-APCI-MS and LC-MS methods were validated in-house assessing linearity, recovery, repeatability, limit of detection (LOD), and limit of quantification (LOQ) for cinnamon powder. Blank matrices were not available. For this reason, the cinnamon powder with the lowest coumarin content was fortified with four levels (100, 500, 1,000, 5,000 mg/kg) of coumarin for recovery studies. Intraday and interday precision was determined by repeated analysis of the recovery samples. LOD and LOQ were calculated by means of the calibration curve method according to DIN 32645 (α = β = 0.05; k = 3) from the analysis of an extract of the cinnamon powder with the lowest coumarin content spiked with 1–920 μg/L (1, 5, 9, 46, 92, 460, and 920 μg/L) internal standard coumarin-d4.
Results and discussion
Analysis of cinnamon extracts using DIP-APCI-MS and LC-MS
Performance evaluation of DIP-APCI-MS was carried out by comparison to complementary LC-MS data. Therefore, a LC-MS method based on reversed-phase HPLC and electrospray ionization quadrupole time-of-flight (Q-TOF) MS was developed. Figure 1 shows exemplarily a chromatogram of the LC-MS analysis of a calibration standard and an extract of Ceylon cinnamon. A good separation of coumarin and coumarin-d4 from the sample matrix was achieved.
Chromatogram of the LC-MS analysis and structures of coumarin and coumarin-d4; black: total ion chromatogram (TIC), red: extracted ion chromatogram (EIC) of coumarin, green: EIC of coumarin-d4; a calibration standard (coumarin: 5.0 mg/L; coumarin-d4: 0.9 mg/L; absolute peak heights, coumarin: 1.1 × 105 counts, coumarin-d4: 2.5 × 104 counts, TIC at 7–8 min: 1.0–1.1 × 107 counts); b extract of Ceylon cinnamon (coumarin-d4: 0.9 mg/L; absolute peak heights, coumarin: 9.0 × 104 counts, coumarin-d4: 2.5 × 104 counts, TIC at 7–8 min: 1.6–3.1 × 107 counts)
The samples were measured by DIP-APCI-MS. In Fig. 2, DIP-APCI-MS chronograms of a calibration standard and an extract of Ceylon cinnamon are depicted.
Chronogram of the DIP-APCI-MS analysis; black: total ion chronogram (TIC), red: EIC of coumarin, green: EIC of coumarin-d4; a calibration standard (coumarin: 5.0 mg/L; coumarin-d4: 0.9 mg/L; maximum signal heights, coumarin: 5.1 × 105 counts, coumarin-d4: 9.2 × 104 counts, TIC: 1.3 × 107 counts); b extract of Ceylon cinnamon (coumarin-d4: 0.9 mg/L; maximum signal heights, coumarin: 7.0 × 104 counts, coumarin-d4: 1.8 × 104 counts, TIC: 3.1 × 107 counts)
In the DIP-APCI-MS chronogram, the m/z-traces of coumarin and coumarin-d4 show a second rise in their intensity in the cinnamon extract which is not present in the calibration standard (Fig. 2). Coumarin and coumarin-d4 show identical vaporization behavior. This indicates that the second rise in the intensity of the m/z-traces of coumarin and coumarin-d4 arises from the presence of matrix components in the cinnamon extract that partly delay the vaporization of coumarin and coumarin-d4. To eliminate this effect, a cinnamon extract was clarified according to Carrez to remove proteins and other high-molecular-weight substances [1, 29]. Unfortunately, the delay of coumarin vaporization could not be eliminated by the clarification according to Carrez. Due to the different vaporization behavior of coumarin in the calibration standards and the cinnamon extracts, the following approaches for data evaluation were selected: Firstly, for each analysis, the mass spectra obtained were averaged over the whole time range of the analysis, and the heights of the m/z-signals of coumarin and internal standard coumarin-d4 in the resulting mass spectrum (named “average intensity”) were used for evaluation. Secondly, the ion chronograms of the m/z of coumarin and internal standard coumarin-d4 were extracted with ±5 ppm (extracted ion chronogram) and integrated over the whole time range of the analysis (named “area”). Table 1 shows the validation results for both the LC-MS and DIP-APCI-MS methods.
From the validation results, it can be seen that the linearity of both LC-MS and DIP-APCI-MS analyses are satisfactory in the observed concentration range even without the use of an internal standard. The recoveries also fall into an acceptable range for both methods. Interestingly, for DIP-APCI-MS, the recoveries are lower when the internal standard is used for data evaluation. A possible explanation could be the fact that the m/z-signal of coumarin-d4 showed a slightly elevated mass error in the averaged mass spectra of Ceylon cinnamon extracts which could indicate an overestimation of coumarin-d4 due to interference of a not fully resolved matrix component with a very similar m/z. For both LC-MS and DIP-APCI-MS analyses, as expected, the repeatability is generally higher within 1 day and when the internal standard is used for data evaluation. In terms of repeatability, the data evaluation via the averaged mass spectrum (average intensity) leads to better results than the data evaluation via the area. This can be explained by the manual integration performed during the data evaluation via the area. The fact that the LOD and LOQ determined for DIP-APCI-MS are approximately seven times higher than for LC-MS can be attributed to the broad signal spreading over several minutes instead of the sharp chromatographic peak in LC-MS. Furthermore, the more common electrospray ionization was used in LC-MS.
To compare the results of coumarin determination by LC-MS and DIP-APCI-MS and also the different approaches to data evaluation, ten cinnamon samples were analyzed. The results are visualized in Fig. 3.
Contents of coumarin in cinnamon powder determined by LC-MS and DIP-APCI-MS (n = 3); blue: LC-MS, red: DIP-APCI-MS data evaluation via average intensity, green: DIP-APCI-MS data evaluation via area; a internal standard coumarin-d4 used for data evaluation; b data evaluation without the use of the internal standard
When the internal standard is used for data evaluation, it can be seen that the results obtained by both methods are very comparable. For high coumarin contents, the results obtained by DIP-APCI-MS are slightly lower than those obtained by LC-MS, and the data evaluation via the average intensity leads to results closer to those obtained by LC-MS. When the internal standard is not used for data evaluation, high coumarin contents are overestimated in the LC-MS analysis and underestimated in the DIP-APCI-MS analysis. This shows that the use of coumarin-d4 as internal standard is advisable to obtain accurate data with both methods.
Coumarin determination directly from cinnamon powder using DIP-APCI-MS
Figure 4 shows as an example the chronograms of the direct DIP-APCI-MS analysis of one Ceylon cinnamon and one Cassia cinnamon sample.
Chronogram of the direct DIP-APCI-MS analysis of cinnamon powder; black: total ion chronogram (TIC), red: extracted ion chronogram (EIC) of coumarin; a Ceylon cinnamon (maximum signal heights, coumarin: 9.4 × 104 counts, TIC: 6.7 × 107 counts); b Cassia cinnamon (maximum signal heights, coumarin: 1.3 × 106 counts, TIC: 5.8 × 107 counts)
It can be seen that higher temperatures have to be applied to the cinnamon powder to release the contained coumarin than is necessary for the vaporization of coumarin from cinnamon extracts (Figs. 2b and 4). The contents of coumarin in the cinnamon samples derived from the direct DIP-APCI-MS analysis of cinnamon powder and evaluated using the results from the previously analyzed calibration standards are listed in Table 2. It is important to note that when analyzing Cassia cinnamon samples the m/z-signal of coumarin reached detector saturation.
The comparison of the results obtained by direct analysis of the cinnamon powders with the results obtained from the analysis of cinnamon extracts shows an overestimation of low coumarin contents and an underestimation of high coumarin contents by the direct analysis. The underestimation of high coumarin contents could be due to the fact that detector saturation is reached. Since it is not possible to include the addition of an internal standard in the direct analysis of cinnamon powder, reliable quantitative data cannot be obtained from the direct analysis. Another obstacle in gaining reliable quantitative data is the low amount of powdered sample that can be introduced into the probe tips combined with sample inhomogeneity. Even if quantitative determinations of coumarin directly from cinnamon powder are not possible, semi-quantitative information can readily be obtained. A semi-quantitative screening of food samples to be analyzed could be used to reduce the number of samples that require extraction and subsequent quantitative analysis and lead to saving of time and a reduction of solvents used.
Analysis of woodruff-flavored beverages and cinnamon-flavored chewing gum using DIP-APCI-MS and LC-MS
By means of LC-MS, coumarin could not be detected in the woodruff-flavored liquor. In the woodruff-flavored soft drink as well as the extract of the cinnamon-flavored chewing gum, coumarin was detected but not quantified because the signals were below the limit of quantitation. Evaluated as described for the analysis of the cinnamon extracts, the DIP-APCI-MS analysis of the two woodruff-flavored beverages and the extract of the cinnamon-flavored chewing gum would show significant coumarin contents in these samples. Figure 5 shows exemplary chronograms and chromatograms of the analysis of the woodruff-flavored liquor by DIP-APCI-MS (Fig. 5a) and LC-MS (Fig. 5b), respectively.
As can be seen from the DIP-APCI-MS chronogram (Fig. 5 a), the m/z-trace of coumarin shows a delayed rise of its intensity compared with the m/z-trace of internal standard coumarin-d4. The fact that the m/z-traces of coumarin and coumarin-d4 do not behave identically leads to the conclusion that the delayed rise of the intensity of the m/z-trace of coumarin is not due to a hold-up of coumarin vaporization by the presence of matrix. It could rather be caused by the vaporization of an isobaric compound contained in the samples, but this should also appear in the LC-MS chromatogram which is not the case. Another possibility is the formation of coumarin or of an isobaric artifact from matrix components due to the high temperature applied in the DIP-APCI-MS analysis.
Ceylon and Cassia cinnamon contain coumarin mainly in its free form. In other plants like, for example, woodruff, coumarin occurs to a large part in form of a glucosylated precursor and is liberated by enzyme action after disrupture of cells [12, 30]. A glucosylated precursor of coumarin in some plants is 2-coumaric acid β-d-glucopyranoside which is biosynthesized from phenylalanine [30, 31]. To investigate whether the biosynthetic precursor of coumarin could also be the precursor of the artifact formed during DIP-APCI-MS analysis, the m/z-traces of 2-coumaric acid β-d-glucopyranoside and the possible fragmentation product 2-coumaric acid were extracted from LC-MS chromatograms and DIP-APCI-MS chronograms. 2-Coumaric acid β-d-glucopyranoside was not detected by LC-MS analysis (positive (ESI+) and negative (ESI−) electrospray ionization mode) in the woodruff-flavored beverages or the extract of the cinnamon-flavored chewing gum. In LC-ESI-MS, the woodruff-flavored liquor showed a small peak that could correspond to 2-coumaric acid, but the other samples did not. By DIP-APCI-MS the m/z of 2-coumaric acid (m/z 165.0546) was detected in both woodruff-flavored beverages and the extract of the cinnamon-flavored chewing gum and showed an intensity behavior identical to that of the artifact with the m/z of coumarin (m/z 147.0441). The fact that m/z 165.0546 and m/z 147.0441 show identical intensity rises with the temperature program suggests that m/z 147.0441 could be a fragmentation product of m/z 165.0546. Since 2-coumaric acid was not as easily available, 4-coumaric acid was instead analyzed by DIP-APCI-MS and DIP-APCI-MS/MS. 4-Coumaric acid (m/z 165.0546) showed in-source fragmentation to m/z 147.0441 presumably through the loss of water. The resulting intensities of m/z 165.0546 and m/z 147.0441 were almost the same. Figure 6 shows the DIP-APCI-MS and DIP-APCI-MS/MS analyses of 4-coumaric acid, woodruff-flavored liquor, and woodruff-flavored liquor spiked with 4-coumaric acid.
DIP-APCI-MS and DIP-APCI-MS/MS analysis of 4-coumaric acid and woodruff-flavored liquor; black: EIC of m/z 165.0546 (e.g., 4-coumaric acid); red: EIC of m/z 147.0441 (e.g., coumarin); a DIP-APCI-MS analysis of 4-coumaric acid, in-source fragmentation of m/z 165.0546 to m/z 147.0441 is observed; b DIP-APCI-MS analysis of woodruff-flavored liquor, m/z 165.0546 and m/z 147.0441 show the same vaporization behavior; c DIP-APCI-MS analysis of woodruff-flavored liquor spiked with 4-coumaric acid, the two vaporization maxima of 4-coumaric acid and m/z 165.0546 from woodruff-flavored liquor are observed; d MS/MS spectrum of m/z 165 from 4-coumaric acid (isolation width: 4 m/z, CID: 10 eV), fragmentation of m/z 165 to m/z 147 and m/z 119; e MS/MS spectrum of m/z 165 from woodruff-flavored liquor (isolation width: 4 m/z: CID: 10 eV), fragmentation of m/z 165 to m/z 147 and m/z 119 amongst others
It can be seen that 4-coumaric acid (Fig. 6a) is more readily vaporized than m/z 165.0546 from the woodruff-flavored liquor (Fig. 6b) and that the intensity ratios of in-source fragmentation from m/z 165.0546 to m/z 147.0441 from 4-coumaric acid and woodruff-flavored liquor are different. The analysis of woodruff-flavored liquor spiked with 4-coumaric acid (Fig. 6c) clearly confirms that 4-coumaric acid is not the precursor of the artifact with m/z 147.0441 in woodruff-flavored liquor since two separate vaporization maxima of 4-coumaric acid and m/z 165.0546 from woodruff-flavored liquor are observed. 4-Coumaric acid and m/z 165.0546 from the woodruff-flavored liquor show fragmentation toward m/z 147 and m/z 119 in the tandem mass spectrometry (MS/MS) experiment (Fig. 6, d and e). The presence of additional m/z-signals in Fig. 6e could possibly be explained by the chosen isolation width of 4 m/z and the presence of matrix. The similar fragmentation behavior of 4-coumaric acid and m/z 165.0546 in woodruff-flavored liquor as well as the higher temperature required to release m/z 165.0546 from woodruff-flavored liquor suggests that the precursor of m/z 147.0441 could be a substance with a higher molecular weight that contains coumaric acid and fragments to m/z 147.0441 via m/z 165.0546. The analysis of the woodruff-flavored soft drink led to the same conclusions.
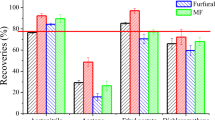
In ambient ionization MS, special attention has to be paid to possible spectral interferences due to isobaric substances present in the sample [27] or formed during the analysis (especially when thermal desorption is used). This limitation of ambient ionization MS was also shown during 5-hydroxymethylfurfural (HMF) quantitation in honey [32]. Due to the decomposition of carbohydrates from the honey matrix to HMF with DART temperatures typically applied, HMF quantitation was impossible. The analysis of the woodruff-flavored beverages and the cinnamon-flavored chewing gum by DIP-APCI-MS and LC-MS shows that even with the use of a high-resolution Q-TOF MS spectral interferences cannot be ruled out. With the retention time as an identification point missing, the presence or formation of isobaric substances can easily lead to false quantification results. The temperature programmed vaporization applied to samples in DIP-APCI-MS analysis leads to a certain separation of sample components according to their boiling points and this way reduces the risk of false quantification results due to the formation of isobaric artifacts, especially when stable isotope-labeled internal standards are used.
Conclusions
In this study, quantitative analyses of coumarin in different cinnamon samples were performed by use of a DIP-APCI ion source coupled to a high-resolution Q-TOF MS following an extraction procedure. The DIP-APCI-MS results showed good agreement with results obtained by LC-MS. Even without the use of an internal standard, the DIP-APCI-MS analysis demonstrated satisfying linearity and repeatability which is an advantage compared with other ambient ionization sources where relatively high variations of ion intensities are often observed in repeated analyses. Although the quantification of coumarin by DIP-APCI-MS following extraction does not result in a significant saving of time compared with UHPLC-MS analyses, DIP-APCI-MS can also be employed for a semi-quantitative screening of coumarin directly from powdered cinnamon. This reduces the number of samples that require extraction and subsequent quantitative analysis. As with other ambient ionization methods, special attention has to be paid to possible spectral interferences due to isobaric substances present in the sample matrix or formed from matrix components during the analysis. This limitation was shown during the analysis of woodruff-flavored beverages and cinnamon-flavored chewing gum. The temperature-programmed vaporization applied to samples in DIP-APCI-MS combined with the use of stable isotope-labeled internal standards can help in recognizing such interferences.
References
Raters M, Matissek R (2008) Analysis of coumarin in various foods using liquid chromatography with tandem mass spectrometric detection. Eur Food Res Technol 227:637–642
Woehrlin F, Fry H, Abraham K, Preiss-Weigert A (2010) Quantification of flavoring constituents in cinnamon: high variation of coumarin in Cassia bark from the german retail market and in authentic samples from Indonesia. J Agr Food Chem 58:10568–10575
Fotland TO, Paulsen JE, Sanner T, Alexander J, Husoy T (2012) Risk assessment of coumarin using the bench mark dose (BMD) approach: children in Norway which regularly eat oatmeal porridge with cinnamon may exceed the TDI for coumarin with several folds. Food Chem Toxicol 50:903–912
Miller KG, Poole CF, Chichila TMP (1995) Solvent-assisted supercritical-fluid extraction for the isolation of semivolatile flavor compounds from the cinnamons of commerce and their separation by series-coupled column gas-chromatography. J High Res Chromatog 18:461–471
EFSA (2004) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to coumarin, adopted on 6 October 2004. The EFSA Journal 104:1–36
EFSA (2008) Coumarin in flavourings and other food ingredients with flavouring properties, scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food (AFC), adopted on 8 July 2008. The EFSA Journal 793:1–15
Abraham K, Woehrlin F, Lindtner O, Heinemeyer G, Lampen A (2010) Toxicology and risk assessment of coumarin: focus on human data. Mol Nutr Food Res 54:228–239
Abraham K, Pfister M, Woehrlin F, Lampen A (2011) Relative bioavailability of coumarin from cinnamon and cinnamon-containing foods compared to isolated coumarin: a four-way crossover study in human volunteers. Mol Nutr Food Res 55:644–653
BfR (2012) Neue Erkenntnisse zu Cumarin in Zimt. Stellungnahme Nr. 036/2012 des BfR vom 27. September 2012. http://www.bfr.bund.de/cm/343/neue-erkenntnisse-zu-cumarin-in-zimt.pdf
Sproll C, Ruge W, Andlauer C, Godelmann R, Lachenmeier DW (2008) HPLC analysis and safety assessment of coumarin in foods. Food Chem 109:462–469
Lungarini S, Aureli F, Coni E (2008) Coumarin and cinnamaldehyde in cinnamon marketed in Italy: a natural chemical hazard? Food Addit Contam A 25:1297–1305
Rychlik M (2008) Quantification of free coumarin and its liberation from glucosylated precursors by stable isotope dilution assays based on liquid chromatography-tandem mass spectrometric detection. J Agr Food Chem 56:796–801
McEwen CN, McKay RG, Larsen BS (2005) Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal Chem 77:7826–7831
Cody RB, Laramee JA, Durst HD (2005) Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem 77:2297–2302
Takats Z, Wiseman JM, Gologan B, Cooks RG (2004) Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306:471–473
Fussell RJ, Chan D, Sharman M (2010) An assessment of atmospheric-pressure solids-analysis probes for the detection of chemicals in food. Trac-Trends Anal Chem 29:1326–1335
Schurek J, Vaclavik L, Hooijerink H, Lacina O, Poustka J, Sharman M, Caldow M, Nielen MWF, Hajslova J (2008) Control of strobilurin fungicides in wheat using direct analysis in real time accurate time-of-flight and desorption electrospray ionization linear ion trap mass spectrometry. Anal Chem 80:9567–9575
Nilles JM, Connell TR, Durst HD (2009) Quantitation of chemical warfare agents using the direct analysis in real time (DART) technique. Anal Chem 81:6744–6749
Danhelova H, Hradecky J, Prinosilova S, Cajka T, Riddellova K, Vaclavik L, Hajslova J (2012) Rapid analysis of caffeine in various coffee samples employing direct analysis in real-time ionization-high-resolution mass spectrometry. Anal Bioanal Chem 403:2883–2889
Mirnaghi FS, Pawliszyn J (2012) Reusable solid-phase microextraction coating for direct immersion whole-blood analysis and extracted blood spot sampling coupled with liquid chromatography-tandem mass spectrometry and direct analysis in real-time tandem mass spectrometry. Anal Chem 84:8301–8309
Li YJ, Wang ZZ, Bi YA, Ding G, Sheng LS, Qin JP, Xiao W, Li JC, Wang YX, Wang X (2012) The evaluation and implementation of direct analysis in real time quadrupole time-of-flight tandem mass spectrometry for characterization and quantification of geniposide in Re Du Ning Injections. Rapid Commun Mass Spectrom 26:1377–1384
Vaclavik L, Zachariasova M, Hrbek V, Hajslova J (2010) Analysis of multiple mycotoxins in cereals under ambient conditions using direct analysis in real time (DART) ionization coupled to high resolution mass spectrometry. Talanta 82:1950–1957
Vismeh R, Waldon DJ, Teffera Y, Zhao ZY (2012) Localization and quantification of drugs in animal tissues by use of desorption electrospray ionization mass spectrometry imaging. Anal Chem 84:5439–5445
D’Aloise P, Chen H (2012) Rapid determination of flunitrazepam in alcoholic beverages by desorption electrospray ionization-mass spectrometry. Sci Justice 52:2–8
Thunig J, Flo L, Pedersen-Bjergaard S, Hansen SH, Janfelt C (2012) Liquid-phase microextraction and desorption electrospray ionization mass spectrometry for identification and quantification of basic drugs in human urine. Rapid Commun Mass Spectrom 26:133–140
Wu CP, Ifa DR, Manicke NE, Cooks RG (2009) Rapid, direct analysis of cholesterol by charge labeling in reactive desorption electrospray ionization. Anal Chem 81:7618–7624
Hajslova J, Cajka T, Vaclavik L (2011) Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. Trac-Trends Anal Chem 30:204–218
Krieger S, von Trotha A, Leung KS, Schmitz OJ (2013) Development, optimization, and use of an APCI source with temperature-controlled vaporization of solid and liquid samples. Anal Bioanal Chem 405:1373–1381
Matissek R, Steiner G, Fischer M (2010) Lebensmittelanalytik. Springer, Germany
Edwards KG, Stoker JR (1967) Biosynthesis of coumarin—isomerization stage. Phytochemistry 6:655–661
Yang ZY, Kinoshita T, Tanida A, Sayama H, Morita A, Watanabe N (2009) Analysis of coumarin and its glycosidically bound precursor in Japanese green tea having sweet-herbaceous odour. Food Chem 114:289–294
Chernetsova ES, Morlock GE (2012) Assessing the capabilities of direct analysis in real time mass spectrometry for 5-hydroxymethylfurfural quantitation in honey. Int J Mass Spectrom 314:22–32
Acknowledgment
The authors thank Scientific Instruments Manufacturer GmbH (SIM) for providing the direct inlet probe and Phenomenex Inc. for providing the Kinetex column. Sonja Krieger also thanks the Zentrum fuer Graduiertenstudien of the University of Wuppertal for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krieger, S., Hayen, H. & Schmitz, O.J. Quantification of coumarin in cinnamon and woodruff beverages using DIP-APCI-MS and LC-MS. Anal Bioanal Chem 405, 8337–8345 (2013). https://doi.org/10.1007/s00216-013-7238-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7238-x