Abstract
A fast analytical method by direct analysis in real time ionization coupled to mass spectrometer (DART-MS/MS) has been developed for the estimate the capsaicin (CAP) and dihydrocapsaicin (DHCP) concentration in fermented pepper paste. Firstly, these mass fragmentation schemes of CAP and DHCP by DART-MS/MS are proposed, and compared with those of electrospray ionization source (ESI). After conducting a series of optimizations, the samples were extracted by a simple procedure using acetonitrile, the samples extracts were then directly evaluated by DART-MS/MS without chromatographic separation. A limit of detection (LOD) of 0.0234 and 0.0510 μg/mL was achieved for CAP and DHCP, respectively. The results obtained with the newly developed DART-MS/MS method are in good agreement with those by the conventional ultra-high-performance liquid chromatography-electrospray ionization-mass spectrometry method. The results showed that DART-MS/MS is a high-throughput tool for determination of CAP and DHCP concentration in food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Szechuan cuisine is one of the famous cuisines in China, and is also popular around the word, such as the USA, Canada, Brazil, Peru, and Europe (UK) (Wang 2017). It is characterized by its unique spicy flavor and rich taste. One of the basic seasoning used in the Szechuan cuisine is locally fermented pepper paste (FPP). FPP is a typical product of solid-state fermentation, which is considered an efficient process to improve the flavor of food. As the flowchart outlined in Fig. 1, the preparation of FPP mainly consists of two stages. First, fresh local varieties of red peppers (FRP) are washed, smashed, and soaked in brine, and make the salted peppers (SP). Next, the SP is allowed to undergo aerobic fermentation for about 40 days to make the FPP. The spicy taste in FPP is attributed to the lipophilic alkaloids known as capsaicinoids. Among the many natural capsaicinoids found in hot peppers, two compounds namely capsaicin (CAP) (trans-8-ethyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin (DHCP) (8-methyl-N-vanillylnonanamide) contribute to around 80–90% of the total pungency in most chili peppers (Eich 2008). Interest in capsaicinoids is based on its various biological properties beneficial to human health, such as great antioxidant effect, antitumor, anti-mutagenic, and antibacterial properties (Luo et al. 2011). Positive effects of capsaicinoids against cholesterol and obesity have also been reported (Lu et al. 2017a, b). However, it can also be toxic due to the injury to the nervous system at high dose.
Capsaicinoids are present in peppers and are often regarded as an indicator of aroma quality of FPP. Therefore, a simple, sensitive, and accurate method for the determination of capsaicinoids is of importance for both food quality and health benefit reasons. Several analytical techniques have been used to determine CAP content in hot peppers. For example, high-performance liquid chromatography (Al Othman et al. 2011), gas chromatography (Krajewska and Powers 1987), UV spectrophotometry (Davis et al. 2007), and a colorimetric method based on Gibbs reagent (2,6-dichloroquinone-4-chloroimide) (Ryu et al. 2017) that have been developed for determining capsaicinoid concentrations. The aforementioned chromatographic analysis is the most popularly used method for the quantification of capsaicinoids in food samples. However, chromatographic approaches have drawbacks, such as weak qualitative ability, tedious pretreatment, and long-lasting separation procedure. More recently, an ambient pressure desorption ionization technique, namely direct analysis in real time (DART), has been presented as a simple, high-throughput tool without the use of chromatographic separation (Hajslova et al. 2011). Moreover, the DART ion source provides an open atmospheric pressure interface, which enables direct introduction of samples with minimal pretreatment and increases throughput greatly. Several novel analytical methods based on DART-MS have been proposed to quantify dietary supplements (Kerpel dos Santos et al. 2018), herbs (Rajchl et al. 2018; Xu et al. 2015a), and mycotoxins (Busman and Maragos 2015). However, approaches to determine capsaicinoid levels by DART-MS/MS in food have not been reported until now.
The purpose of the present study was to develop a novel and rapid method to determine capsaicinoid levels in pepper paste by DART-MS/MS during the manufacturing process and to evaluate the quantitative performance of DART-MS/MS in the measurement of CAP and DHCP levels during the pepper paste manufacturing process.
Materials and Methods
Chemicals and Reagents
The CAP and DHCP standards were purchased from Chengdu Herbpurify Co., Ltd. (Chengdu, China). Methanol, acetone, acetonitrile, and formic acid (HPLC grade) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ultra-high purity helium (99.999%), used as ionizing gas, and high purity nitrogen (99.999%), used as MS collision gas, were obtained from Sichuan Messer Gas Co. Ltd. (Chengdu, China). Ultrapure water was filtered through a Milli-Q system (Millipore, Billerica, MA, USA).
Sample Preparation
Fresh red pepper (Ejintiao variety) was procured from Chengdu market in China. FPP samples were prepared according to Fig. 1. In brief, a weight of 1.0 kg of pepper was cleaned with drinking water and then drained. After removing the pepper stems, it was cut into about 4.0-cm pieces. Finally, these peppers were mixed with 12% sodium chloride and 20% water, and ferment in a ceramic pot at 22–25 °C for 40 days. FPP samples were periodically collected at three different stages: FRP (0 day), SP (1 day), and FPP (40 day). Tabasco® pepper sauce and real THAI sweet chilli sauce were obtained from a local market. About 10.0 g of each sample was dehydrated to a constant weight using a LGJ-10N freeze dryer (Yahuashunda Limited Liability Company, Beijing, China). The lyophilized FRP was ground in a C-07129 laboratory mill (Baozhen Limited Liability Company, Xian, China) through a 100-mesh sieve. The powdered samples obtained was stored in a freezer at − 20 °C until use.
Extraction of CAP and DHCP
A weight of 0.5 g of each lyophilized powder sample was extracted with 10 mL of acetonitrile in a 15-mL centrifuge tube. The extraction process was performed in a SB-3200DTDN ultrasonic cleaning bath (Scientz Scientific, Ningbo, China) with an output power of 180 W and irradiation frequency of 40 kHz, followed by centrifugation at × 3000g for 15 min at 4 °C. Subsequently, the extracted supernatant was filtered through a 0.22-μm filter (Jinteng, Tianjin, China).
Preparation of Standard Solution
The CAP stock standard solution (106.0 μg/mL) and DHCP stock standard solution (102.0 μg/mL) were prepared by dissolving each of these compounds in acetonitrile. All the standard solutions were stored at − 20 °C and brought to room temperature before use.
DART-MS Analysis
All DART experiments were carried out on a DART SVP ionization source (IonSense, Saugus, MA, USA) coupled to a 3500 triple quadrupole mass spectrometer (SCIEX, Framingham, MA, USA). A VAPUR interface was used to guide ions from the ion source to the inlet of the mass spectrometer and reduce the influence of turbulence, thus improving sensitivity and reproducibility of the sample analysis. A small diaphragm pump was used to create a vacuum in the VAPUR interface. The direction of the DART source was aligned with the ceramic tube leading into the Vapur interface before the entrance to the mass spectrometer. In this study, the distance between the exit of the DART emitter and the ceramic ion transfer tube connected to mass spectrometer was set at 2.8 cm. The operating conditions of the DART source were as follows: positive mode, helium was used as the ionizing gas and nitrogen was used in standby mode, gas temperature of 450 °C, grid electrode potential of 250 V, the input gas pressure used was 0.5 MPa. An acquiring module with a 12-DIP-it sampler (IonSense) was used to introduce the samples, and 3 μL of liquid was pipetted into the glass sampling tube of the module. Then, the 12-DIP-it module was moved into the MS inlet at a speed of 0.6 mm/s.
The mass spectral parameters were set as follows: curtain gas (CUR) 35 psi, collision gas medium, ion source gas 1 (GS1) 15 psi, ion source gas 2 (GS2) 20 psi, interface heater temperature 500 °C, entrance potential (EP) 10 V, declustering potential (DP) of 80 V, and collision energy (CE) of 17 V. The mass spectra were recorded across the range of 200–400 m/z.
Ultra-High-Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (UHPLC-ESI-MS) Analysis of CAP and DHCP
An ultra-high-performance liquid chromatography (UHPLC) system (Acquity system consisting of a vacuum degasser, autosampler, a binary pump, and a PDA detector, PerkinElmer Technologies, Billerica, MA, USA) was equipped with a PerkinElmer C18 analytical column (2.1 × 50 mm, 1.9-μm particle size) placed in the column temperature which was set at 35 °C. The mobile phase system was a mixture of solvent A (0.1% v/v formic acid in water) and solvent B (acetonitrile) at a flow rate of 0.3 mL/min using the following gradient elution program. The proportion of solvent B was linearly increased from 20 to 100% in 2 min and kept constant for 4 min, then dropped down to 20% in 0.1 min, and eventually led to equilibrate with 20% solvent B for 2 min. Thus, the overall runtime was 8.1 min, and the injection volume was 3 μL.
The mass spectral information of the analytes was obtained using a 3500 triple quadrupole mass spectrometer controlled by the Analyst Software (SCIEX). An electrospray ion (ESI) source interface was used in the positive ion mode using the following settings: curtain gas (CUR) 35 psi, collision gas medium, ion source gas 1 (GS1) 45 psi, ion source gas 2 (GS2) 50 psi, ionspray voitage +5500 V ion source temperature 500 °C, declustering potential (DP) of 80 V, and collision energy (CE) of 17 V. The mass spectra were recorded across the range of 200~400 m/z. In the multiple reaction monitoring (MRM) mode, the ions with the highest responses, namely m/z 306.1/136.9 for CAP and m/z 307.8/136.9 for DHCP, were selected as the quantitative ions and giving the next response, namely m/z 306.1/181.1 for CAP and m/z 307.8/184.1 for DHCP, were selected as the qualitative ions. The MultiQuant 3.0.2 software (AB SCIEX) was used for data analysis.
Assay Validation
In order to ensure the applicability and reliability of the method for routine analysis, the method was validated for specificity, linearity, matrix effects, accuracy, precision, limit of detection (LOD), and limit of quantification (LOQ). To assess the linearity, six-point calibration curves were prepared using standard samples. The CAP working standard solutions ranging from 3.31 to 106 μg/mL were prepared by diluting the CAP stock standard solution with acetonitrile, and the DHCP working standard solutions ranging from 0.80 to 102 μg/mL were prepared through serial dilution with acetonitrile.
Due to the lack of blank matrix solution (without contain CAP or DHCP), we approximately prepared the matrix-matched calibration solution using spiked FPP extraction solution (CCAP = 5.91 μg/mL and CDHCP = 1.95 μg/mL). Finally, the concentration range of CAP and DHCP in matrix-matched calibration solution is 11.54–95.91 and 6.64–76.95 μg/mL, respectively. Matrix effect for FRP matrice was evaluated using the ratio between the calibration curve slopes of matrix-matched calibration standards and solvent-based standards as follows (Lu et al. 2017b):
Signal suppression would occur if the percentage of the difference between these slopes was negative. If it was positive, it would be indicative of signal enhancement. The LOD and LOQ were calculated as the concentrations corresponding to the signal-to-noise ratio (S/N) of 3 and 10, respectively. The precision was determined by analyzing six replicates, including three levels of CAP (6.63, 26.5, and 106 μg/mL), namely high, middle, and low level (n = 6), and three levels of DHCP (1.59, 12.75, and 51 μg/mL) (n = 6). The precision was expressed as the relative standard deviation (RSD %). According to the reference method (Xu et al. 2015b), the recovery of CAP and DHCP were calculated by the following equation:
where Csm is the spiked measurement, Cm is the measurement of the pepper sample, and Cs is the concentration of the spiked CAP or DHCP standard solution.
Statistical Analysis
All experiments were performed in triplicate, and average values with standard deviation are reported. Duncan’s multiple-range test was used to assess the statistical differences in the obtained quantitative data at α = 0.05 using the SPSS statistical analysis software version 22.0 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
The Characterization of CAP and DHCP by DART-MS and UPLC-ESI-MS
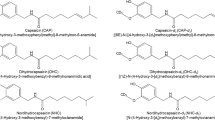
To evaluate the feasibility of quantifying the content of CAP and DHCP in FPP by DART-MS, the results measured by DART-MS were compared with those obtained using a classic UHPLC-ESI-MS method. First, the characterization of CAP and DHCP were carried out by DART-MS and UPLC-ESI-MS in positive ion modes using a standard solution. The full-scan spectra of CAP by the DART-MS method and UPLC-ESI-MS method are shown in Fig. 2. In the DART-MS method spectrum, the primary ion observed was [CAP+H]+(m/z 306) along with several ion adducts as shown in Fig. 2a, the peak at m/z 323 maybe the product ion of m/z 306 plus an ammonium, the peak at m/z 348 in the extract was tentatively identified as the [CAP-2H + COOH]+ ion. The primary ion of DHCP observed from Fig. 2b was [DHCP+H]+ along with some other adducts. The peaks at m/z 325 and m/z 368 are provisionally ascribed to [DHCP+NH4]+ and [DHCP+CH3COOH + H]+ ions.
Meanwhile, with the UPLC-ESI-MS method, the main ion observed was also the parent ion at m/z 306 along with several ion adducts (Fig. 2c), which may include [CAP+Na]+ (m/z 328), [CAP+K]+ (m/z 344), [CAP+MeCN+H]+ (m/z 347), [CAP+MeOH+Na]+ (m/z 360), and [CAP+MeCN+Na]+ ion (m/z 369). The [DHCP+H]+ was the main ion observed in the spectrum shown in Fig. 2d, and there are several ion adducts, probably including [DHCP+H2O + H]+ (m/z 326), [DHCP+Na]+ (m/z 330), and [DHCP+K]+ ion (m/z 346). In general, the MS spectra obtained by UPLC-ESI-MS are more complicated, and the response intensity is much higher than those with the DART-MS method. The ammonium was the typical additive ion with the DART-MS method, whereas the metal additive ions, such as sodium or potassium ion were present with the UPLC-ESI-MS method. It is noteworthy that with the UPLC-ESI-MS method, the m/z 301 and m/z 226 ions were observed both in CAP and DHCP. According to the structural formula of CAP and DHCP, the fragmentation at m/z 301 may be formed though a dimer of the vanillyl moiety (m/z 137) that reacted with a carboxyl group to lose a water molecule. Additionally, the m/z 226 ion may be obtained by the reaction of the adduct ions (MeOH, K+ and H2O) of the background ions with the vanillyl moiety (m/z 137).
The MS2 spectra of CAP and DHCP are shown in Fig. 3. The fragmentation pathway of DART-MS/MS is depicted in Fig. 3a, the fragmentation m/z 137 is a characteristic ion peak of CAP, which is obtained by cleavage of the C7-N8 position and rearrangement of the double bonds of the aromatic ring structure, and the fragmentation at m/z 170 is the part of the acyl chain that results from removing the aromatic ring. Fragmentation m/z 182 is the acyl residue of the molecule resulting from cleavage of the benzylic carbon bond (C1-C7). Also, the m/z 153 is likely obtained by deionizing the ammonium ion from the m/z 170 ion. As shown in Fig. 3b, the characteristic ion peak of DHCP was also present at m/z 137, and the aforementioned CAP fragmentation pathway can also be used for DHCP. These fragmentation patterns of CAP and DHCP obtained by DART-MS/MS are also consistent with those of atmospheric pressure chemical ionization (APCI) (Schweiggert et al. 2006), and ESI (Reilly et al. 2001) as ion sources.
Optimization of the Experimental Parameters of DART-MS/MS
Gas Temperature of the DART Ionization Source
It was found that the helium gas temperature (Nilles et al. 2010) in DART is a key factor for the ionization of analytes. The response intensity can be used as an indicator to evaluate the gas heater temperature on the ionization efficiency of CAP and DHCP. The gas temperature was increased in increments of 50 °C from 200 to 550 °C. The intensity of CAP and DHCP at different gas heater temperatures are shown in Fig. 4a. The response intensities of CAP and DHCP increased significantly with the increase of the temperature from 200 to 300 °C, exhibiting the highest response intensity at 450 °C, but decreased from 450 to 550 °C.
Optimization of the experimental parameters of DART-MS/MS based on the peak intensity of CAP and DHCP in peppers: a gas temperature; b grid voltage; c solvent type. Different lowercase letters mean significant difference (P < 0.05) in capsaicin concentration and with capital letters mean significant difference (P < 0.05) in dihydrocapsaicin concentration
At lower gas temperatures (200–250 °C), the signals of CAP and DHCP were observed, but had low intensity and high background (data not shown). At elevated temperatures (300–450 °C), the rate of evaporation and desorption of the analytes were accelerated, the degree of ionization of the target substance got higher and higher, and the response intensity of the corresponding target substance was also higher. However, at 500–550 °C, the gas temperature is so high that it causes excessive rapid thermal desorption or irreversible degradation resulting in loss of analytes and lower sensitivity. Accordingly, the gas heater temperature was set to 450 °C in the subsequent experiments.
Effect of the Grid Electrode Voltage
The grid voltage is another important factor for the ionization of analytes in DART-MS/MS. The grid electrode has several functions during the ionization of analytes, including acting as an ion repeller and acting to remove ions of the opposite polarity; therefore, it prevents signal loss by ion-ion recombination (Cody et al. 2005). These functions often have a significant effect on eliminating reactant ions in the atmosphere, such as NO+ ion, and in reducing the “chemical background” (Borges et al. 2009). The grid voltage was increased in increments of 50 V from 100 to 400 V. The intensity of CAP and DHCP at different grid voltages is shown in Fig. 4b. The response intensity of CAP increased with the increase of the grid voltage up to 200 V, but decreased from 250 to 400 V. The response intensity of DHCP was also affected by the grid voltages, and the highest intensities was obtained at 200–250 V. Considering that both the highest intensity of CAP and DHCP was obtained at 200 V, the grid voltage was set to 200 V in the subsequent experiments.
Optimization of the Extraction Solvent
The extraction solvent is very important to the response intensity of analytes. On the one hand, the solubility of analytes is influenced by the polarity of extraction solvents. On the other hand, the solvent can significantly affect the ionization of GABA. Accordingly, four common solvents, including acetone, acetonitrile, methanol, and water were used to evaluate the response intensity of CAP and DHCP. The results are shown in Fig. 4c. The results revealed that when the acetonitrile was the extraction solvent, both CAP and DHCP had the highest response intensity, followed by acetone, methanol, and water. Therefore, acetonitrile was chosen as the extraction solvent.
Determination of Samples
Three types of pepper samples were collected at different steps of the fermentation process, and were analyzed using the established method. The contents of CAP and DHCP in the analyzed samples were calculated by the standard calibration curves. The results are listed in Table 1. In the different steps of the fermented pepper production process, the contents of CAP and DHCP were significantly decreased (P < 0.05). This decrease is likely due to the conversion of CAP and DHCP by the action of microorganisms during the fermentation process (Yang et al. 2018). The capsaicinoids can be oxidized by pepper peroxidase and converted into other substances, such as 8-methyl-N-vanillylcarbamoyl-6(E)-octenoic acid, 2-methyl-N-vanillylcarbamoyl-6(Z)-octenoic acid, N-vanillylcarbamoylbutyric acid, ω-hydroxycapsaicin, N-vanillyl-9-hydroxy-8-methyloctanamide, and some highly polymerized dehydrogenation products (Bernal et al. 1993), which resulting in a decrease in the content of capsaicinoids.
The pungency is usually expressed as the Scoville heat unit (SHU). The concentration of CAP and DHCP can be used to calculate SHU values as they are responsible for 95% of the pungency. When the scale of pungency needs to be used in quality control of chili pepper products, rapid analysis of the content of the CAP and DHCP by DART-MS/MS would be a very practical method. As shown in Table 1, Tabasco sauce and Thai chill sauce are the two popular chili sauces in the USA. The pungency of these two kinds of sauces is 132,439 and 2014 SHU, respectively. Therefore, the pungency of FRP (2588 SHU) is between the Tabasco sauce and Thai chill sauce. This discrepancy in pungency could be attributed to differences in the pepper species and processing technology.
Assay Validation
Matrix Effect
When using DART-MS/MS and UPLC-ESI-MS/MS methods for the quantitative analysis of complex samples, the presence of matrix effects tends to adversely affect quantification. Therefore, the evaluation of the matrix effect is of great importance for the analysis of the experimental results. Depending on the value of this percentage, matrix effects were classified into different categories. A strong matrix effect would occur when |matrix effect| > 50%, and a medium matrix effect would be when the values were 20% < |matrix effect| ≤ 50%. |matrix effect| ≤ 20% was considered to be no matrix effect (Kaczyński 2017). As shown in Table 2, the matrix effects of CAP and DHCP on the fermented pepper were − 8.80 and − 7.03%, respectively, using the DART-MS/MS method, while the matrix effects of the CAP and DHCP were − 9.02 and − 14.81%, respectively, by the UPLC-ESI-MS/MS method. Therefore, there was considered to be no matrix suppression effect by both DART and ESI sources. Since the matrix effect almost no influence the quantification by DART-MS/MS and UPLC-ESI-MS/MS approaches, the quantification of each compound was used for the standard calibration. Notably, the DART-MS/MS method possesses a significantly lower matrix effect than the UPLC-ESI-MS/MS method. This may be due to the different ionization mechanisms in these two ion sources.
Calibration, LOD, and LOQ
The equations of the calibration curves for CAP and DHCP with different matrices using the DART-MS/MS and UPLC-MS/MS approaches and their corresponding determination coefficients (R2) are listed in Table 2. Good linearity (R2 > 0.998) was obtained for the calibration curve for each compound. The LOD for CAP and DHCP with the DART method were 0.0234 and 0.0510 μg/mL, respectively, and the LOQ for CAP and DHCP were 0.0859 and 0.2494 μg/mL, respectively, which are 10.68 and 14.71% higher than the ESI method, respectively.
Accuracy and Precision
In order to evaluate the practicability of determining the CAP and DHCP in peppers by the DART-MS/MS method, the results of the DART-MS measurements were compared with those obtained with the UPLC-MS method. As shown in Table 3, with the DART-MS/MS method, the average recovery of CAP was in the range from 86.5–88.8%, with an RSD below 7.0%. The average recovery of DHCP in the three samples was in the range from 85.45–89.20%, with an RSD of less than 4.0%. Thus, there was no significant difference between the accuracy by the two methods, and the RSD values were within an acceptable range. Moreover, the results in Tables 2 and 3 indicate that the validation parameters of the UPLC-ESI-MS/MS method, such as precision, repeatability, and correlation coefficient are better than those of the DART-MS/MS method. Nevertheless, compared with the UPLC-ESI-MS method, the DART-MS/MS method is feasible with minimal sample pretreatment without the chromatographic separation procedure. In this work, it took 24 min (n = 3) to analyze a sample using the UPLC-ESI-MS/MS method and less than 2 min (n = 3) to analyze the same sample by the DART-MS/MS method. Therefore, the DART-MS/MS method can be used to determine the content of CAP and DHCP in large batches of samples. DART-MS/MS provides a simple, rapid, and high-throughput method for the determination of CAP and DHCP.
Conclusion
In this study, a high-throughput method based on DART-MS/MS was developed for the simultaneous determination of CAP and DHCP in pepper samples obtained during the fermentation process. The optimization of the DART-MS/MS parameters enabled the detection of CAP and DHCP at levels as low as 0.0234 and 0.0510 μg/mL, respectively. The CAP and DHCP contents of the pepper samples that were determined by the DART-MS/MS method were consistent with those obtained with the UPLC-ESI-MS method. This study indicated that DART-MS/MS can be used to greatly simplify sample preparation without the need for chromatographic separation, and therefore can be used for large-scale and high-throughput analysis.
References
Al Othman ZA, Ahmed YB, Habila MA, Ghafar AA (2011) Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules 16:8919
Bernal MA, Calderon AA, Pedreno MA, Munoz R, Barcelo AR, Caceres FMD (1993) Capsaicin oxidation by peroxidase from Capsicum annuum (variety Annuum) fruits. J Agric Food Chem 83:151–159
Borges DL, Sturgeon RE, Welz B, Curtius AJ, Mester Z (2009) Ambient mass spectrometric detection of organometallic compounds using direct analysis in real time. Anal Chem 81:9834–9839
Busman M, Maragos CM (2015) Determination of T-2 and HT-2 toxins from maize by direct analysis in real time mass spectrometry. World Mycotoxin J 8:489–497
Cody RB, Laramée JA, Durst HD (2005) Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem 77:2297
Davis CB, Markey CE, Busch MA, Busch KW (2007) Determination of capsaicinoids in habanero peppers by chemometric analysis of UV spectral data. J Agric Food Chem 55:5925–5933
Eich E (2008) Solanaceae and Convolvulaceae: secondary metabolites: biosynthesis, chemotaxonomy, biological and economic significance (a handbook). Springer Science & Business Media, Berlin
Hajslova J, Cajka T, Vaclavik L (2011) Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. TrAC Trends Anal Chem 30:204–218
Kaczyński P (2017) Clean-up and matrix effect in LC-MS/MS analysis of food of plant origin for high polar herbicides. Food Chem 230:524–531
Kerpel dos Santos M, Gleco E, Davidson JT, Jackson GP, Pereira Limberger R, Arroyo LE (2018) DART-MS/MS screening for the determination of 1,3-dimethylamylamine and undeclared stimulants in seized dietary supplements from Brazil. Forensic Chem 8:134–145
Krajewska AM, Powers JJ (1987) Gas chromatography of methyl derivatives of naturally occurring capsaicinoids. J Chromatogr A 409:223–233
Lu M, Ho C-T, Huang Q (2017a) Extraction, bioavailability, and bioefficacy of capsaicinoids. J Food Drug Anal 25:27–36
Lu Z, Fang N, Zhang Z, Wang B, Hou Z, Lu Z, Li Y (2017b) Simultaneous determination of five neonicotinoid insecticides in edible fungi using ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Food Anal Methods 11:1086–1094
Luo X-J, Peng J, Li Y-J (2011) Recent advances in the study on capsaicinoids and capsinoids. Eur J Pharmacol 650:1–7
Nilles JM, Connell TR, Durst HD (2010) Thermal separation to facilitate Direct Analysis in Real Time (DART) of mixtures. Analyst 135:883
Rajchl A, Fernández Cusimamani E, Prchalová J, Ševčík R, Čížková H, Žiarovská J, Hrdličková M (2018) Characterisation of yacon tuberous roots and leaves by DART-TOF/MS. Int J Mass Spectrom 424:27–34
Reilly CA, Crouc DJ, Yost GS, Fatah AA (2001) Determination of capsaicin, dihydrocapsaicin, and nonivamide in self-defense weapons by liquid chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry. J Chromatogr A 912:259–267
Ryu W-K, Kim H-W, Kim G-D, Rhee H-I (2017) Rapid determination of capsaicinoids by colorimetric method. J Food Drug Anal 25:798–803
Schweiggert U, Carle R, Schieber A (2006) Characterization of major and minor capsaicinoids and related compounds in chili pods (Capsicum frutescens L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Anal Chim Acta 557:236–244
Wang S-T (2017) When Chinese cuisine meets western wine. Int J Gastronomy Food Sci 7:32–40
Xu B, Zhang DY, Liu ZY, Zhang Y, Liu L, Li L, Liu CC, Wu GH (2015a) Rapid determination of 1-deoxynojirimycin in Morus alba L. leaves by direct analysis in real time (DART) mass spectrometry. J Pharm Biomed Anal 114:447–454
Xu B et al (2015b) Rapid determination of 1-deoxynojirimycin in Morus alba L. leaves by direct analysis in real time (DART) mass spectrometry. J Pharm Biomed Anal 114:447
Yang HJ, Lee YS, Choi IS (2018) Comparison of physicochemical properties and antioxidant activities of fermented soybean-based red pepper paste, Gochujang, prepared with five different red pepper (Capsicum annuum L.) varieties. J Food Sci Technol 55:792–801
Funding Sources
This work was supported by the key Scientific Research Fund Project of Xihua University (Z1620516, Z1620515), the Talents Plan of Xihua University (0220170105, 21030031), General Financial Grant from the China Postdoctoral Science Foundation (2017M611168), the “Chunhui Plan” of Ministry of Education of China (Z2017061, Z2017092), the Key Project of Sichuan Province Education Department (18ZA0456), the Research on Modern Production Technology Integration of Szechuan Cuisine Seasoning (2016-XT00-00031-NC, 2015-HM02-00066-SF, and 2017NZ0002), and the Science & Technology Innovation Talents of Hunan Province, China (2017TP1021kc1704007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tao Wu declares that he has no conflict of interest. Xv Yuan declares that he has no conflict of interest. Xiaoyu Wu declares that he has no conflict of interest. Yong Tang declares that he has no conflict of interest. Hongbin Lin declares that he has no conflict of interest. Zhenming Che declares that he has no conflict of interest. Wenhua Zhou declares that he has no conflict of interest. Weili Li declares that he has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Wu, T., Yuan, X., Wu, X. et al. Rapid Determination of Capsaicin and Dihydrocapsaicin in Fermented Pepper Paste by Direct Analysis in Real Time Mass Spectrometry. Food Anal. Methods 12, 32–40 (2019). https://doi.org/10.1007/s12161-018-1334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-018-1334-7