Abstract
2-Hydroxy-4-methoxybenzophenone (HMB), which is one of the most commonly used UV filters in sunscreen cosmetics to protect skin from the deleterious effects of the sun, can be percutaneously absorbed, further metabolized, and finally excreted or bioaccumulated. An analytical method for the sensitive determination of HMB and its three metabolites in both human urine and semen is developed. The presented analytical method is based on a solid-phase extraction (SPE) procedure to clean-up and preconcentrate the target analytes from the urine and semen samples followed by liquid chromatography–tandem mass spectrometry (LC-MS/MS) detection. The methodology was fully validated and the standard addition calibration method was used to quantify the target analytes in order to correct the matrix effects observed. Considering this approach, the accuracy of the method was evaluated and the recoveries ranged from 98% to 115% and from 86% to 111% in urine and semen samples, respectively, depending on the analyte. For urine samples, the limits of detection ranged between 0.027 and 0.103 ng mL−1 and the repeatability of the method, expressed as relative standard deviation, was in the range of 7.2–9.2%, depending on the analyte. In the case of semen samples, the limits of detection ranged between 1 and 3 ng mL−1 whereas the repeatability was in the range of 2.2–6.4%, depending on the analyte. The described SPE-LC-MS/MS method was satisfactorily applied to both urine and semen samples from a male volunteer who applied a sunscreen cosmetic product containing HMB. HMB and its metabolites were found and quantified in the low ng mL−1 range in both urine and semen samples, although at a different extent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2-Hydroxy-4-methoxybenzophenone (HMB), also called benzophenone-3, is commonly used as a broad-band UV filter in sunscreen cosmetic products alone or in combination with other UV filters to protect from the deleterious effects of the UV radiation coming from the sun. This substance is currently regulated in cosmetic products by different legislations around the world. Hence, HMB can be used up to a maximum authorized content, which may be 10%, 6%, and 5% (w/w) in the final product, according to legislation in force in the European Union, the United States, and Japan, respectively [1]. Moreover, the legislation in the European Union forced to include a warning on the label about its presence in the cosmetic. Based on the available data related with safety considerations [2], HMB is considered safe for topical application on human skin in the specified practices of use and concentrations in cosmetics.
Nevertheless, some observations suggest that HMB is not as harmless as it may seem. A number of evident positive reactions to HMB clearly shows that this cosmetic ingredient can be considered as a potential allergen [3] and contact photoallergen [4]. On the other hand, both in vitro and in vivo studies clearly show that HMB is absorbed through the skin to a certain extent [5]. In fact, sufficiently high penetration through human epidermis for HMB, among other UV filters, has been demonstrated to warrant further investigation of its continued application [6]. In studies concerning the hormonal activity, it was found that daily exposure to sunscreen formulations including HMB may have estrogenic effects in humans [7–9]. In addition, a certain kind of endocrine disruption that is not assessed by classical estrogenic markers is indicated for HMB [10, 11].
As an immediate consequence of this percutaneous penetration, a series of biotransformation reactions take place in the organism involving changes in the physical properties of the external chemical that enters the body. These processes are performed by a limited number of enzymes, which are divided into two classes depending on the catalyzed reaction, namely Phase I and Phase II enzymatic reactions [12]. Thus, toxicokinetic studies carried out in rats [13] and humans [14] indicate that HMB is readily biotransformed into its three Phase I metabolites, namely 2,4-dihydroxybenzophenone (DHB), 2,2′-dihydroxy-4-methoxybenzophenone (DHMB), and 2,3,4-trihydroxybenzophenone (THB). Concerning Phase II enzymatic reactions, HMB and its Phase I metabolites have been identified in their free and conjugated forms, mainly via glucuronidation [15]. Due to the fact that the concentration of these metabolites in human fluids decrease much more slowly over time compared to that of the parent compound, it has been pointed out that metabolites might have more long-term adverse effects than HMB [16]. In vitro studies identified DHB as an estrogenic metabolite of HMB and showed that it had more anti androgenic activity than the parent compound [17]. Furthermore, it was confirmed that HMB and DHMB display estrogenic activity [18] and that both DHB and THB present stronger activity than potent and well-established endocrine disruptors, such as bisphenol A [19].
Studies about the pharmacokinetics of HMB after oral administration in rats showed that urine was the major route of excretion [20]. Different methods have been proposed to determine HMB in urine [21]. The most commonly used techniques are both liquid (LC) and gas chromatography (GC) coupled to mass spectrometry (MS) detection with previous sample pretreatment procedures, such as solid-phase microextraction (SPME) [22], on-line solid-phase extraction (SPE) [23], stir bar sorptive extraction [24] and hollow-fiber-assisted liquid-phase microextraction [25]. LC-UV detection has also been used after the preconcentration and clean-up steps based on single-drop microextraction [26] or on-line SPE by means of the principle of sequential injection analysis [27].
In the literature, methodologies that provide adequate limits of detection (i.e., in the low ng mL−1 range) to monitor the dermal penetration and excretion processes of HMB can be found. However, most of them do not consider the contribution of its metabolites [23–25]. Moreover, the methods that take into account this contribution have considerably high limits of detection [14, 28] or do not consider all the main primary metabolites (i.e., THB) [22]. On the other hand, both LC-MS and GC-MS methodologies have also been extensively applied to the sensitive analysis of HMB in water samples [21, 29]. Nevertheless, the more complex urine matrix in comparison to water makes more difficult to obtain validated methods to determine simultaneously HMB and all its metabolites in urine with low limits of detection. Finally, it should be mentioned that HMB has been also determined in other biological fluids, such as plasma [14, 16, 28], human milk [30, 31], and serum [32, 33], but the determination of its metabolites is ignored.
On the other hand, some controversial results have been obtained when specific reproductive toxicity parameters were studied after HMB administration to rats and/or mice. Thus, the potential of HMB to cause reproductive toxicity in male mice was assessed and the results indicated that topically applied HMB had no reproductive toxic potential under the conditions tested [34]. However, on the contrary, consistent findings from a toxicity report [35] showed that HMB produced generally similar effects following topical and oral administration to rats and mice, including decreases in epididymal sperm density and lengthening estrous cycle. Direct lines of evidence showing that the most potent estrogen (17β-estradiol) and other environmental estrogens significantly affect the function of mature sperm in mice by stimulating its capacitation and its fertilizing ability have been provided [36]. Thus, considering the potential estrogenic activity of HMB, and especially of its metabolites, further investigation focused on human semen could be of great interest, since relationships between semen quality and contents of these compounds could be found. However, to our knowledge, no analytical methods have been developed to determine HMB and/or its metabolites in either human or animal semen.
This report focuses on the development and validation of an analytical method based on SPE prior to liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis that allows the sensitive determination of the parent compound (HMB) and its three metabolites (i.e., DHB, DHMB, and THB) in human urine and, for the first time, in human semen. In order to determine the content of the analytes in their free and glucuronide-conjugated forms, urine and semen samples were treated with β-glucuronidase to carry out an enzymatic hydrolysis.
Experimental
Reagents and samples
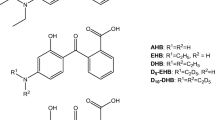
HMB 98%, DHB 99%, DHMB 98%, and THB 99% were all purchased from Sigma-Aldrich (Steinheim, Germany) and used as standards. 2,2′-dihydroxy-4,4’-dimethoxybenzophenone 98% (DHDMB) was also from Sigma-Aldrich and used as internal standard. It should be mentioned that this compound was chosen as internal standard due to DHDMB presenting a chemical structure similar to the target analytes and analogous chromatographic behavior with analytes may be expected. Besides this, preliminary experiments showed that DHDMB is not potentially present in the analyzed samples. The chemical structures and some properties of relevance of these compounds are given in Table 1.
Absolute ethanol (EtOH) LC grade, methanol (MeOH) LC grade, acetone extrapure, hydrochloric acid (HCl) ca. 37% reagent grade and sodium hydroxide (NaOH) reagent grade were from Scharlab (Barcelona, Spain). Formic acid was from Fluka Chemie (Steinheim, Germany). β-glucuronidase solution (116,300 IU mL−1), type HP2, from Helix pomatia was also from Sigma-Aldrich. Deionized water was obtained by using a NANOpure II device from Barnstead (Boston, USA).
Multicomponent (HMB, DHB, DHMB, and THB) and internal standard (DHDMB) stock solutions were prepared separately in EtOH at 200 μg mL−1 and kept at 4 °C in the refrigerator.
Typical cosmetic-grade ingredients from Guinama S.L. (Valencia, Spain) such as emollients, surfactants, smoothing agents, hydrating agents, preservatives, perfumes, etc. were used to prepare laboratory-made sunscreen creams, according to an adapted protocol provided by Guinama S.L. [37].
Urine and semen samples used for the method development and validation were pooled from different male volunteers who did not use any cosmetic product containing HMB. On the other hand, a laboratory-made sunscreen cosmetic product containing HMB (10%) was topically applied to the body of a male volunteer after informed consent and his urine and semen excretions were collected at different times after cosmetic application.
All urine and semen samples were kept at −20 °C in the freezer until analysis.
Liquid chromatography–tandem mass spectrometry
The LC-MS/MS system consisted of a LC-10AD liquid chromatography system from Shimadzu (Duisburg, Germany) and a Quattro triple quadrupole mass spectrometer from Micromass (Beverly, USA).
The column used was a Mediterranea SEA 18 column 5 × 0.21 cm, 3 μm (Teknokroma, Barcelona, Spain) with an Ultraguard SEA 18 precolumn 10 × 3.2 mm (Teknokroma, Barcelona, Spain). Mobile phase consisted of solvent A (water with 0.1% of formic acid) and solvent B (MeOH with 0.1% of formic acid). The pumps supplied the following gradient at 0.2 mL min−1 and room temperature: 0–1 min, 40% solvent B; 1–9.5 min linear gradient to 95% solvent B, held for 5 min. The injection volume was 20 μL if not otherwise stated.
The MS detector operated in positive electrospray ionization mode (ESI+) by multiple reaction monitoring (MRM). The ESI probe and ion source in positive ion mode were operated at 3.5 kV of capillary voltage. Nitrogen (99.9%), used as nebulizing and desolvation gas in the ESI source, was provided by a high-purity generator (CLAN Tecnológica, Sevilla, Spain). Source temperature was set at 120 °C, desolvation temperature was 350 °C, nebulizer gas flow rate was 33 L h−1, and desolvation gas flow rate was 600 L h−1.
The MS/MS spectra were produced by collision-induced dissociation (CID) of the selected precursor ions with Premier helium (Carburos Metálicos, Paterna, Spain) as the collision gas.
Standard addition calibration
For urine analysis, an internal standard solution (DHDMB) at 5 μg mL−1 in water and a multicomponent solution of HMB, DHB, DHMB, and THB at 5 μg mL−1 in water were daily prepared from their respective stock solutions. The pH of urine samples was measured and adjusted between 5 and 6 with either HCl (1 M) or NaOH (1 M; if necessary). To prepare a standard addition calibration, five aliquots of 5 mL of urine were spiked with 0, 20, 40, 60, and 80 μL of the multicomponent solution (5 μg mL−1), to which 100, 80, 60, 40, and 20 μL of water were added, respectively, in order to reach the same content of water in the calibration solutions; 100 μL of the internal standard solution (5 μg mL−1) was also added to each calibration solution.
For semen analysis, an internal standard solution (DHDMB) at 1 μg mL−1 in water and a multicomponent solution of HMB, DHB, DHMB, and THB at 1 μg mL−1 in water were daily prepared from their respective stock solutions. Two milliliters of semen were acidified in a 15-mL conical tube by adding 300 μL of HCl (1 M) to denature proteins present in the matrix. Then, the mixture was vortexed and centrifuged at 6,000×g for 3 min at room temperature. To prepare a standard addition calibration, five aliquots of 340 μL of the semen supernatant were spiked with 0, 20, 40, 60, and 80 μL of the multicomponent solution (1 μg mL−1), to which 100, 80, 60, 40, and 20 μL of water were added, respectively, in order to reach the same content of water in the calibration solutions. To adjust the pH to 6.5 in order to obtain the adequate conditions for the enzymatic hydrolysis, 60 μL of NaOH (1 M) were added followed by 100 μL of the internal standard solution (1 μg mL−1).
Enzymatic hydrolysis
In order to determine the total content of the analytes (i.e., both free and glucuronide-conjugated forms), an aliquot of a β-glucuronidase solution was added to sample solutions. In the case of urine samples, 50 μL of the commercial β-glucuronidase solution were added. In the case of semen samples, 250 μL of a β-glucuronidase aqueous solution obtained by 50-fold dilution of the commercial solution were added. To determine the analytes just in their free forms, an aliquot of water (i.e., 50 or 250 μL for urine or semen analysis, respectively) instead of the β-glucuronidase solution was used. Then, sample solutions were vortex mixed and incubated at 37 °C for 12 h. Once incubation was accomplished, 30 μL and 20 μL of formic acid (resulting pH = 3) were added to the urine and semen samples, respectively, and afterwards they were loaded to the SPE cartridges. The final volume values of both urine and semen solutions were 5,280 μL and 870 μL, respectively.
Then, semen and urine solutions were subjected to the respective SPE procedures described below.
Solid-phase extraction procedure
Bond Elut™ C18 SPE cartridges (100 mg, 5 mm i.d.) from Varian (Barcelona, Spain) were conditioned with 2 mL of EtOH followed by 2 mL of water.
For urine analysis, cartridges were then loaded with 5 mL of the incubated samples at a flow rate of about 0.5 mL min−1, washed with 8 × 1 mL of water and dried under full vacuum for 10 min by using a SPE vacuum manifold from Varian (Barcelona, Spain). The analytes were eluted with 3 × 0.35 mL of acetone.
For semen analysis, cartridges were loaded with 0.7 mL of the incubated samples at a flow rate of about 0.5 mL min−1, washed with 3 × 0.7 mL of water and dried under full vacuum for 10 min. The analytes were eluted with 3 × 0.35 mL of acetone.
For both urine and semen analysis, eluates were evaporated to dryness under a gentle stream of air and redissolved in 60 μL of a 1:1 (v/v) mixture of LC solvents A and B. Finally, the redissolved extracts were placed in a conical insert and injected by means of a conventional LC autosampler into the LC-MS/MS system with the conditions aforementioned. The ratio of the peak area of each target analyte to DHDMB (internal standard) was plotted versus the added analyte concentration, and the obtained concentration values were obtained by extrapolation of the standard addition calibration line [38]. Results were expressed in absolute mass by considering the corresponding dilution factor and the total volume of sample collected.
Results and discussion
Optimization of LC-MS/MS parameters
The ESI-MS/MS collision-induced dissociation (CID) of all considered compounds was investigated by infusion experiments of single standards of 500 μg mL−1 prepared in EtOH, operating the electrospray source in both positive (ESI+) and negative (ESI−) modes. However, the most intense products appeared in the positive mode in all cases, yielding the corresponding protonated ([M + H]+) parent ions. Figure 1 shows the MS/MS fragmentation pattern of the targeted compounds and the internal standard, showing the molecular structure of the fragments.
Chromatographic separation was achieved for all compounds within a runtime of 17 min. The respective retention times were as follows: THB at 12.4 min, DHB at 14.2 min, DHMB at 14.6 min, DHDMB at 15.7 min, and HMB at 16.1 min.
Study of the experimental variables involved in the SPE procedure
According to their pK a values (Table 1) corresponding to the ionization of the –OH moiety, the targeted compounds are quantitatively not ionized (i.e., uncharged) at pH values lower than 5, which correspond approximately to a pH of 2 units below their pK a values. As not ionized analytes are more likely to be affected by hydrophobic interactions, they can be retained more satisfactorily in C18 cartridges. Hence, pH was adjusted to 3 by adding formic acid to the sample solution before being loaded to the SPE cartridges.
Thus, preliminary studies showed that the employed Bond Elut™ C18 cartridges presented a good capacity to retain adequately and simultaneously the most lipophilic (HMB) and the most polar (DHB, DHMB, and THB) analytes.
Then, SPE conditions were studied for the extraction and preconcentration processes of the targeted compounds. Different volumes (1–5 mL) of an aqueous multicomponent standard solution (1,000 ng mL−1) adjusted to pH 3 with formic acid were passed through the SPE cartridges to study the loading capacity. Cartridges were dried and eluted with 3 × 0.35 mL of acetone, which were enough to elute the retained analytes, according to preliminary experiments. Extracts from the SPE cartridges were then evaporated to dryness and reconstituted with 60 μL of 1:1 (v/v) mixture of LC solvents A and B.
The obtained extraction yields in the SPE cartridges were estimated against external calibration when 5 mL of an aqueous multicomponent standard solution (1,000 ng mL−1) adjusted to pH 3 with formic acid were loaded. They were around 100% for HMB, DHB, DHMB, and around 40% for THB. The lowest extraction yield obtained for the most polar compound (i.e., THB) stands in good accordance with the nature of retention by the C18 SPE cartridge, which is based on reversed-phase and non-polar interactions.
Similar results were obtained by using the polymer-based SPE cartridges Bond Elut™ Plexa™ (100 mg, 5 mm i.d.) from Varian (Barcelona, Spain). Taking into account the greater availability of C18 cartridges, this type of sorbent was preferred.
Study of matrix effects
Matrix effects, which can be caused by the extraction and/or detection processes, were assessed by comparing the differences between responses obtained for a set calibration of five aqueous multicomponent standard solutions (20–100 ng mL−1; aqueous calibrate) with those prepared in free-analytes urine or semen from male volunteers (standard addition calibrate) according to the procedure described above. All solutions were also spiked with the internal standard solution at 100 ng mL−1 and the pH was set to 3 by adding formic acid before the SPE procedure.
For both urine and semen samples, statistically different slopes were obtained when both aqueous and standard addition calibrates were compared for all the analytes (Table 2). The recoveries obtained (as a ratio of the slopes) ranged from 14% to 82% and from 24% to 86% for urine and semen samples, respectively, depending on the analyte. On the basis of these data, the standard addition calibration method is recommended as quantification approach to correct the matrix interferences and to assess the levels of targeted compounds in urine and semen samples.
Study of the accuracy
The accuracy was evaluated by applying the developed method to the analysis of urine and semen samples from male volunteers who were known not to use any cosmetic product containing HMB (i.e., analyte-free samples). Urine and semen samples were fortified with known amounts of HMB, DHB, DHMB, and THB, and were used as quality control (QC) samples in order to evaluate the accuracy of the proposed method. Then, they were analyzed with the proposed SPE-LC-MS/MS method. Values obtained for each sample in urine and semen with their standard deviation values are shown in Tables 3 and 4, respectively. Standard deviations were obtained as the standard deviation of the extrapolated value in the standard addition calibration line [38]. As can be seen, the recoveries ranged from 98% to 115% and from 86% to 111% in urine and semen samples, respectively, depending on the analyte.
The Student’s t test [38] (Tables 3 and 4) confirmed that there were no significant differences between the found concentrations values obtained by the application of the developed method and the added concentrations values for both urine and semen samples, thereby showing the accuracy of the methodology.
Extracted ion chromatograms of non-fortified and fortified (120 ng mL−1) analyte-free urine and semen samples subjected to the described SPE-LC-MS/MS method are shown in Figs. 2 and 3, respectively.
Multiple reaction monitoring chromatograms obtained from a non-fortified (dotted line) and a fortified (120 ng mL−1; solid line) analyte-free urine samples subjected to the described SPE-LC-MS/MS method for all the analytes. Internal standard (DHDMB) was included in both types of samples. See text for experimental conditions. Transitions correspond to: m/z precursor ion (capillary voltage (V)) → m/z product ion (collision energy (eV))
Multiple reaction monitoring chromatograms obtained from a non-fortified (dotted line) and a fortified (120 ng mL−1; solid line) analyte-free semen samples subjected to the described SPE-LC-MS/MS method for all the analytes. Internal standard (DHDMB) was included in both types of samples. See text for experimental conditions. Transitions correspond to: m/z precursor ion (capillary voltage (V)) → m/z product ion (collision energy (eV))
Analytical figures of merit
The calibration graphs (n = 5) were linear for HMB, DHB, DHMB, and THB over a concentration range from 20 to 100 ng mL−1 (working range) with a regression coefficient higher than 0.995 in all cases. The limit of detection (LOD) and limit of quantification (LOQ) of the studied analytes were determined according to International Conference on Harmonization (ICH) guidelines [39]. All results are given in Table 3 and Table 4 for urine and semen samples, respectively. It should be noted that low values were obtained in the case of sample LOD, which ranged from 0.027 to 0.103 ng mL−1 in urine samples and from 1 to 3 ng mL−1 in semen samples depending on the compound.
The instrumental precision of both urine and semen methodologies was determined by a repeated injection of a worked up sample at an analyte concentration of 100 ng mL−1 (n = 5). The method precision was evaluated by extracting the analytes from five aliquots of the same sample of urine or semen, fortified with the analytes at 100 ng mL−1 (Table 5).
Real samples analysis
An amount of 13 g of a laboratory-made sun cream containing HMB (10%) was topically applied to the body of a male volunteer after informed consent. This dose is included in the usual range of thickness application for sunscreens (0.5–1 mg of cream per cm2 of skin), which is usually below the recommended dose for a maximum sun protection (2 mg). The total sample volume of both biological fluids was measured to determine the excreted amount of analytes and was kept at −20 °C in the freezer until be analyzed. Samples were analyzed according to the SPE-LC-MS/MS method described above.
In the case of urine, sample 1 (U1), which corresponded to the excretion collected 20 h after the application of the cream, was divided in two fractions. Each fraction was treated with either water or β-glucuronidase solution (see “Enzymatic hydrolysis” section) in order to determine the content of the analytes in their free forms or in both free and glucurunonate-conjugated forms (i.e., total content), respectively. Analogous procedure was followed for sample 2 (U2), which was collected 30 h after the application of the cream.
In the case of semen, sample 1 (S1), which corresponded to the pooled excretions obtained during the first 24 h after the application of the cream, was treated only with the β-glucuronidase solution to determine the total analytes content due to the lack of sufficient sample. Sample 2 (S2), which corresponded to the pooled excretions obtained during the second 24 h after the application of the cream, was treated with either water or β-glucuronidase solution, analogously to the urine samples.
As can be seen in Fig. 4, the amount of HMB and its metabolites is different depending on the biological fluid. Furthermore, the contribution of the conjugated content to the total excreted amount is highly dependent on the analyte and the considered sample.
Amounts of HMB, DHB, DHMB, and THB in urine (a) and semen (b) samples from a male volunteer after topical application of a sunscreen product containing HMB (10%). Error bars show the standard deviation of the results. Results are expressed in absolute mass by considering the corresponding dilution factor and the total volume of sample collected. Both samples of urine (U1 and U2) and semen (S1 and S2) were collected at different times, which are specified along the text
Despite a higher number of samples from diverse people at different periods of time should be analyzed by using the proposed method to get a more consistent idea of the toxicokinetics of HMB and its metabolites, some interesting points can be extracted from this study. For example, it should be pointed out that HMB and DHB were found to be predominant in both urine samples when compared with the found contents of DHMB and THB. Moreover, except to DHMB, the free and glucuronate-conjugated contents are different for both urine samples. For semen samples, the trend is different, that is, HMB and THB are excreted largely than DHB and DHMB, although the excreted mass tends to increase with the time for all the analytes, being the found contents in S2 higher than in S1.
Conclusions
A sensitive analytical method based on SPE combined with LC-MS/MS for the determination of HMB, DHB, DHMB, and THB in both human urine and semen is presented. The standard addition calibration method was used as a quantitative approach to correct the matrix interferences. Full validation of the method was carried out, giving statistically accurate results for fortified analyte-free samples. Moreover, the methodology was satisfactorily applied to the analysis of urine and semen samples collected from a volunteer that had applied a sunscreen cosmetic containing HMB.
The described analytical method can be applied to carry out further required in vivo studies concerning the pharmacokinetics of HMB, and especially of its metabolites, which might have more long-term adverse effects than the parent compound. Furthermore, another point of interest might be the study of how do the estrogenic metabolites of this widely used UV filter affect the variation of specific reproductive toxicity parameters by establishing relationships between the found amounts and semen quality.
In comparison to existing methods, the developed method is the one that allows the simultaneous determination of the parent compound (HMB) and all its three metabolites (DHB, DHMB, and THB) in human urine in the low ng mL−1 range. Moreover, this is the first time that a UV filter is determined in human semen.
References
Chisvert A, Salvador A (2007) UV filters in sunscreens and other cosmetics. Regulatory aspects and analytical methods. In: Salvador A, Chisvert A (eds) Analysis of cosmetic products. Elsevier, Amsterdam
Scientific committee on consumer products (2006), COLIPA N° S38, SCCP/1069/06
Berne B, Ros AM (1998) Contact Dermat 38:61–64
Darvay A, White IR, Rycroft RGJ, Jones AB, Hawk JL, McFadden JP (2001) Br J Dermatol 145:597–601
Treffel P, Gabard B (1996) Pharm Res 13:770–774
Jiang R, Roberts MS, Collins DM, Benson HAE (1999) Brit J Clin Pharmacol 48:635–637
Schlumpf M, Cotton B, Conscience M, Haller V, Steinmann B, Lichtensteiger W (2001) Environ Health Perspect 109:239–244
Ma RS, Cotton B, Lichtensteiger W, Schlumpf M (2003) Toxicol Sci 74:43–50
Henewer M, Muusse M, Van den Berg M, Sanderson JT (2005) Toxicol Appl Pharmacol 208:170–177
Schlecht C, Klammer H, Jarry H, Wuttke W (2004) Toxicology 205:123–130
Coronado M, De Haro H, Deng X, Rempel MA, Lavado R, Schlenk D (2008) Aquat Toxicol 90:182–187
Parkinson A, Olgivie B (2008) Biotransformation of xenobiotics. In: Klaassen C (ed) Casarett and Doull's toxicology, the basic science of poisons, 7th edn. McGraw-Hill, New York
Okereke CS, Kadry AM, Abdel-Rhaman MS, Davis RA, Friedman RA (1993) Drug Metab Dispos 21:778–791
Sarveiya V, Risk S, Benson HAE (2004) J Chromatogr B 803:225–231
Kadry AM, Okereke CS, Abderrahman MS, Friedman MA, Davis RA (1995) J Appl Toxicol 15:97–102
Jeon HK, Sarma SN, Kim YJ, Ryu JC (2008) Toxicology 248:89–95
Molina-Molina JM, Escande A, Pillon A, Gomez E, Pakdel F, Cavailles V, Olea N, Ait-Aissa S, Balaguer P (2008) Toxicol Appl Pharmacol 232:384–395
Ogawa Y, Kawamura Y, Wakui C, Mutsuga M, Nishimura T, Tanamoto K (2006) Food Addit Contam 23:422–430
Kawamura Y, Ogawa Y, Nishimura T, Kikuchi Y, Nishikawa J, Nishihara T, Tanamoto K (2003) J Health Sci 49:205–212
El Dareer SM, Kalin JR, Tillery KF, Hill DL (1986) J Toxicol Environ Health A 19:491–502
Giokas DL, Salvador A, Chisvert A (2007) Trends Anal Chem 26:360–374
Felix T, Hall BJ, Brodbelt JS (1998) Anal Chim Acta 371:195–203
Ye XY, Zsuzsanna K, Needham LL, Calafat AM (2005) Anal Bioanal Chem 383:638–644
Kawaguchi M, Ito R, Honda H, Endo N, Okanouchi N, Saito K, Seto Y, Nakazawa H (2008) Anal Sci 24:1509–1512
Kawaguchi M, Ito R, Honda H, Koganei Y, Okanouchi N, Saito K, Seto Y, Nakazawa H (2009) J Chromatogr B 877:298–302
Vidal L, Chisvert A, Canals A, Salvador A (2007) J Chromatogr A 1174:95–103
León Z, Chisvert A, Balaguer A, Salvador A (2010) Anal Chim Acta 664:178–184
Kasichayanula S, House JD, Wang T, Gu X (2005) J Chromatogr B 822:271–277
Díaz-Cruz MS, Llorca M, Barceló D (2008) Trends Anal Chem 27:873–887
Hany J, Nagel R (1995) Dtsch Lebensm Rundsch 91:341–345
Ye XY, Bishop AM, Needham LL, Calafat AM (2008) Anal Chim Acta 622:150–156
Jiang R, Hayden CGJ, Prankerd RJ, Roberts MS, Benson HAE (1996) J Chromatogr B 682:137–145
Ye XY, Tao LJ, Needham LL, Calafat AM (2008) Talanta 76:865–871
Daston GP, Gettings SD, Carlton BD, Chudkowski M, Davis RA, Kraus AL, Luke CF, Ouellette RE, Re TA, Hoberman AM, Sambuco CP (1993) Fund Appl Toxicol 20:120–124
National Toxicology Program (1992), Toxicity report series number 21, U.S. Department of Health and Human Services
Adeoya-Osiguwa SA, Markoulaki S, Pocock V, Milligan SR, Fraser LR (2003) Hum Reprod 18:100–107
Jordán MC, Jordán AM (1991) Formulario de Cosmética. NAU Llibres, Valencia
Miller JC, Miller JN (2005) Statistics and chemometrics for analytical chemistry, 5th edn. Prentice Hall, New Jersey
ICH validation of analytical procedures methodology: text and methodology Q2(R1) (2005), ICH harmonised tripartite guidelines
Acknowledgments
The authors acknowledge the financial support of the Spanish Ministry of Education and Science (Project no CTQ2009-12709). Zacarías León and Isuha Tarazona are also grateful to Spanish Ministry (MICINN) and Generalitat Valenciana, respectively, for their FPI predoctoral grants. The authors want to acknowledge the collaboration of the volunteer, Adrián Cambres.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
León, Z., Chisvert, A., Tarazona, I. et al. Solid-phase extraction liquid chromatography–tandem mass spectrometry analytical method for the determination of 2-hydroxy-4-methoxybenzophenone and its metabolites in both human urine and semen. Anal Bioanal Chem 398, 831–843 (2010). https://doi.org/10.1007/s00216-010-3947-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3947-6