Abstract
Uvinul® A plus (DHHB) is a synthetic benzophenone derivative mainly used in sunscreens, and also in other skin care products. The compound is authorized by the EU as UV filter and a maximum concentration of 10% in consumer products is permitted. Despite its high production volume and usage in consumer products,to date, no information about the systemic exposure to Uvinul® A plus in humans is available. Therefore, we developed a human biomonitoring method which allows the simultaneous determination of three major metabolites of Uvinul® A plus in human urine samples. Furthermore, three minor metabolites of Uvinul® A plus were identified by ion trap experiments. Urine samples were enzymatically hydrolyzed, extracted via liquid-liquid extraction with ethyl acetate, and analyzed by means of UPLC-MS/MS. The final method was validated according to FDA guidelines and applied to 58 urine samples retrieved from the general German population. The three major and specific metabolites of Uvinul® A plus were found in about 36% of the samples, proving the suitability of the method for future human biomonitoring studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hexyl 2-[4-(diethylamino)-2-hydroxybenzoyl]benzoate (DHHB; CAS No. 302776-68-7), better known under the trading name Uvinul® A plus, is a synthetic, organic UV filter possessing its maximum of absorption at 354 nm [1]. It is mainly used in sunscreen formulations as protection against UV-A radiation, but it is also present in other skin care products [2, 3]. Since 2005 DHHB is approved as UV filter by the European Commission with a maximum concentration in consumer products of 10% [4]. The compound is registered under the EC number 443-860-6 at the European Chemicals Agency and is described to be manufactured in 100–1000 tons per year within the European Economic Area [5].
The high production volume of the substance as well as the long skin contact time after application of cosmetic products along with the relatively high concentrations of DHHB in the skin care products led to the assumption that the general population might be exposed significantly to this compound. Therefore, Uvinul® A plus was selected as a chemical of interest as part of a 10-year human biomonitoring (HBM) project conducted in cooperation with the German Federal Ministry of the Environment, Nature Conservation and Nuclear Safety (BMU) and the German Chemical Industry Association (VCI). This joint cooperation aims to establish new HBM methods for 50 chemicals with an assumed wide range of exposure to the general population [6].
A method for the simultaneous determination of three major and specific metabolites of DHHB (Fig. 1) was developed and validated: 2-(4-amino-2-hydroxybenzoyl)benzoic acid (AHB; CAS No.: 67414-64-6), 2-(4-ethylamino-2-hydroxybenzoyl)benzoic acid (EHB), and 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (DHB; CAS No.: 5809-23-4). The targeted metabolites were postulated based on metabolism pathways of compounds with structures similar to DHHB [7, 8] and identified as urinary metabolites of DHHB as part of a human metabolism study after oral and dermal application (publication in preparation).
The validated method was applied to 58 urine samples from healthy volunteers in order to estimate DHHB exposure within the population.
Experimental
Chemicals
2-(4-(Diethylamino)-2-hydroxybenzoyl)benzoic acid (DHB) was purchased from Sigma-Aldrich (Taufkirchen, Germany). 2-(4-Amino-2-hydroxybenzoyl)benzoic acid (AHB) was synthesized in our lab (for details, see Electronic Supplementary Material (ESM)); 2-(4-(ethylamino)-2-hydroxybenzoyl)benzoic acid (EHB) and the deuterated internal standards D4-AHB, D5-EHB, and D10-DHB were custom synthesized (for additional information, see ESM). 4-Methylumbelliferyl-β-d-glucuronide dihydrate (MUG) was obtained from Santa Cruz Biotechnology (Dallas, TX, USA).
Ethyl acetate and methanol were purchased from Th. Geyer (Renningen, Germany). Ethanol, hexane, heptane, dichloromethane, and chloroform were from LGC Standards (Wesel, Germany), LC-MS grade acetonitrile with 0.1% formic acid and formic acid were obtained from Biosolve Chimie (Dieuze, France). Aqueous phosphoric acid (85%), potassium dihydrogen phosphate, and disodium hydrogen phosphate were supplied by VWR International (Leuven, Belgium). The water used was filtered through a Sartorius arium (Göttingen, Germany) water system. For enzymatic hydrolysis β-glucuronidase from E. coli was obtained from Megazyme (Bray, Ireland). During method development SPE was tested with the following cartridges: Oasis MAX, 3 mL, 60 mg sorbent per cartridge (Waters, Eschborn, Germany); Oasis MCX, 3 mL, 60 mg sorbent per cartridge (Waters); Oasis HLB 3 mL, 60 mg sorbent per cartridge (Waters); and Chromabond C18, 6 mL, 1000 mg sorbent per cartridge (Macherey-Nagel, Düren, Germany).
Urine sample preparation
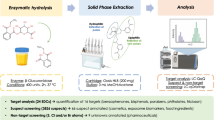
Urine samples were stored at − 20 °C, thawed at room temperature, and shaken for homogenization prior to analysis. To 1.0 mL of urine, 10 μL of an internal standard solution, containing 1 μg/mL D4-AHB, D5-EHB, and D10-DHB in water and 10 μL of an aqueous solution of 4-methylumbelliferyl-β-d-glucuronide dihydrate (MUG; 50 μg/mL), was added. For enzymatic hydrolysis the sample was diluted with 0.5 mL phosphate buffer (1/9 M, pH = 6.4) and 10 μL of β-glucuronidase was added. Subsequently, the samples were kept at 37 °C for 1.5 h. After incubation, pH adjustment of the samples was achieved by adding 25 μL of phosphoric acid (8.5% v/v). The sample was mixed with 2 mL of ethyl acetate and centrifuged for 10 min at 1900×g. The organic layer was separated and evaporated to dryness using a SpeedVac centrifuge (Christ, Osterode am Harz, Germany) and the residue was reconstituted in 100 μL of methanol and transferred into a 1.5-mL HPLC microvial with a 0.3-mL insert. Reagent blank samples were prepared following the sample preparation described under the “Urine sample preparation” section, by replacing 1 mL of urine with 1 mL of deionized water.
Urine samples for method validation were obtained from volunteers at ABF. Additionally, 58 samples from the general population were collected in the greater Munich area. For our metabolism study five subjects were given DHHB once oral and once dermal and all urine fractions were collected for the following 2 days.
UPLC-MS/MS analysis
Five microliters of the extract was injected into a liquid chromatography Nexera X2 UHPLC system (Shimadzu, Neufahrn, Germany). Chromatography was performed on an Acquity UPLC BEH Phenyl column (1.7 μm, 2.1 × 100 mm; Waters, Eschborn, Germany) equipped with an Acquity UPLC BEH Phenyl VanGuard (1.7 μm, 2.1 × 5 mm) at 40 °C and at a flow rate of 0.5 mL/min. Water (eluent A) and acetonitrile (eluent B) each containing 0.1% formic acid were used for gradient elution. Gradient elution was achieved starting at 90% of A for 2 min and then decreasing A linearly to 0% A over two more minutes. 0% of A was maintained for 1.5 min followed by an increase immediately to 90% A and held for 1 min until the end of the run at 6.5 min. For the detection of DHHB metabolites, a 6500+ QTrap® from Sciex (Darmstadt, Germany) was used in ESI positive mode and scheduled MRM. All modules were controlled via Analyst software (Sciex, Version 1.6.3).
Nitrogen was used for all instrument gas flows. Curtain gas was set to 50 psi; ion source gas 1 and 2 were set to 45 psi. Source was heated to 500 °C, ion spray voltage was 5000 V, and entrance potential (EP) was set to 10 V for all analytes. Detailed information for the MRM transitions is summarized in Table 1.
Calibration
Calibration standards were freshly prepared and treated similar to urine samples as described in the “UPLC-MS/MS analysis” section by using an analyte-free urine pool mixed from at least three different analyte-free urine samples and measured immediately after sample work-up. A total of eleven calibrators were prepared by spiking increasing amounts of analytes to urine from 0.1 to 200 ng/mL (0.1; 0.2; 0.5; 1; 2; 5; 10; 20; 50; 100; and 200 ng/mL) for AHB and EHB and 0.05 to 100 ng/mL (0.05; 0.1; 0.2; 0.5; 1; 2; 5; 10; 20; 50; and 100 ng/mL) for DHB. Linear calibration was achieved by applying 1/x weighting. Linearity of all calibrators was proven during method validation.
Method validation
Method validation was performed according to the guidelines issued by the US Food and Drug Administration (FDA) [9]. Quality control samples (QCs) were prepared by pooling native human urine samples with known concentrations of AHB, EHB, and DHB. Where appropriate, analytes were spiked to the QC material. Three different concentration levels (low, medium, and high) were prepared to cover the entire range of expected concentrations. QCs were stored at − 20 °C until analysis. Two QC samples of each level were analyzed in combination with a calibration every day to monitor the robustness of the method. The target values of the QC samples for each analyte were determined prior to the method validation by analyzing six QC samples per level.
Selectivity was proven for the applied MRM transitions for each analyte and the corresponding internal standard. Therefore six analyte-free urine samples (i.e., not containing AHB, EHB, or DHB) were cleaned up as described in the “Urine sample preparation” section and analyzed by LC-MS/MS. The respective mass transitions were screened for interfering peaks. Additionally, the same six urine samples were spiked with 10 ng/mL AHB, EHB, and DHB and accuracy was determined. Accuracy and precision were determined by spiking analyte-free native human urine samples at four different concentration levels (LOQ, low, medium, high). Inter-day accuracy and precision were determined by analyzing five spiked urine samples for each concentration level on three different days. Intra-day accuracy and precision values were derived from five spiked samples per level of 1 day. Intra- and inter-day precisions were evaluated calculating the relative coefficients of variation (CVs), which should not exceed 15% for concentrations > three times the LOQ and 20% for concentrations up to three times of the LOQ, respectively. Acceptance criteria were met with accuracy rates of 85–115% (80–120% at levels up to three times the LOQ). Sample work-up-dependent losses of analytes were expressed by the recovery. Recovery rates were determined by comparing the analyte concentrations at three levels, measured when analyte-free urine samples (N = 3) were spiked after sample work-up (reference, corresponding to 100%), and when the same urine samples were spiked before the sample work-up procedure. LOQs were validated within accuracy and precision experiments and were set to the lowest concentrations fulfilling the requirements of accuracy and precision. The LODs were calculated by dividing the LOQ by 3. Accuracy after dilution was investigated by spiking three different analyte-free urine samples with concentrations of AHB, EHB, and DHB above their highest calibration level (ULOQ) and diluting the samples with water. Three different dilutions (1:100, 1:10, and 1:5) were tested and accuracy was found to be acceptable in the range between 85 and 115%.
Occurring matrix effects (MEs) were evaluated by comparing spiked and processed urine samples at low and high concentrations of the analytes with aqueous reference standards possessing the same concentrations of analytes (reference, corresponding to 100%). MEs are described as ratios from processed samples to references. Ratios > 1 indicate a signal enhancing ME; signals < 1 indicate a signal suppressing ME. Carryover effects were tested by repeated injection (N = 5) of the highest calibrator followed by injection of methanol as a blank sample. Stability of analytes during storage was investigated at room temperature, 10 °C, and − 20 °C over different time periods ranging from 1 day up to several months.
Application of the method to human urine samples
The validated method was applied to 58 human urine samples collected in the greater Munich area from volunteers not occupationally exposed to DHHB. In total, 27 female and 31 male healthy volunteers aged between 18 and 83 years participated in this study. The study was approved by the Ethic Committee of the national Medical Association of Bavaria.
UPLC-QTRAP experiments for the identification of minor metabolites of DHHB
Samples from a human metabolism study (manuscript currently in preparation) were processed with a preliminary method (see ESM) and at first analyzed for the major metabolites of DHHB (AHB, EHB, DHB). Samples with high concentrations of AHB, EHB, and DHB (<ULOQ = 100 μg/L) were selected for the investigation of further additionally hydroxylated metabolites.
Samples were chromatographically separated using the gradient described in the “UPLC-MS/MS analysis” section. At first an Enhanced MS Scan (EMS) was conducted with focus on m/z of + 16 or + 32 of the parent ions from AHB, EHB, and DHB, in order to screen for additionally mono- or dihydroxylated species. Afterwards Enhanced Product Ion Scans (EPIs) based on the molecular weights found in EMS were carried out. False positive transitions were excluded by comparison with a sample showing levels of AHB, EHB, and DHB below LOQ. MRM transitions for additionally hydroxylated metabolites were created based on the parameters established for AHB, EHB, and DHB since no reference standards were available and mass fragmentation was comparable (see Fig. 3). Plausibility of retention times was checked by comparing retention times of AHB, EHB, and DHB with the peaks attributed to the additionally hydroxylated species as these should elute right before the respective major metabolite. Finally, the generated MRMs were optimized with respect to the potentials and energies by means of flow injection analysis (FIA).
Statistical analyses
All statistical analyses were performed with Prism (GraphPad, Version 8.0.1, La Jolla, CA, USA) software package. Values below LOQ were set to LOQ/2 for calculations. The non-parametric Mann-Whitney U test was applied for the determination of statistical differences between subgroups. For the calculation of non-parametric coefficients of correlation between urinary levels of DHHB metabolites, Spearman’s correlations were used. Non-parametric tests were chosen to avoid distorted statistics caused by extreme values. p values of < 0.05 were regarded as statistically significant.
Results and discussion
Performance of the analytical method
The newly developed and optimized method described above enables the simultaneous, sensitive, and precise determination of three major metabolites of DHHB. The metabolic dealkylation of DHHB to AHB and EHB was postulated based on the biotransformation of similar compounds like the UV filter EDP (2-ethylhexyl 4-(N,N-dimethylamino)benzoate) whose dimethylated aniline function is demethylated either once or twice at the aniline group resulting in a secondary or primary amine derivative [7, 8]). The same metabolic pathway was proven for the diethylated aniline function of DHHB which can be deethylated once (EHB) or twice (AHB). Additionally, the hydrolysis of the ester moiety to the respective carboxylic acid is also a pathway observed for EDP [8] or drugs like the ACE inhibitors trandolapril or quinapril [10].
DHHB itself could not be detected in any sample of the metabolism study showing that DHHB is not excreted in human urine. Therefore, DHHB was not taken into account for the final analytical method. DHB is the only metabolite of DHHB which has already been reported as a metabolite of DHHB in in vivo experiments in rats [11]. All three of the suggested metabolites of DHHB could be confirmed within a human metabolism study with oral and dermal application of DHHB to five participants (manuscript in preparation).
Furthermore, the study revealed the need of an enzymatic hydrolysis step before the sample preparation procedure, since all analytes were partly present in their conjugated form as glucuronides. As DHHB itself possesses an ester moiety, an enzyme extract without esterase activity was used in order to avoid formation of DHB from DHHB during the sample preparation which may lead to an overestimation of urinary DHB concentrations. The enzyme extract β-glucuronidase/arylsulfatase from Helix pomatia was previously reported to contain lipase and esterase activities besides the glucuronidase activity [12]. Hence, this enzyme mixture was not suited for the purpose of this method. Alternatively, β-glucuronidase from E. coli without esterase activity was used. As a consequence the sum of unconjugated and glucuronidated metabolites is captured in this method while possibly occurring sulfated metabolites are not detectable. However, a comparison between the two enzyme extracts did not show different concentrations of the metabolites suggesting that sulfated conjugates were only present to a very low extent, if any. A phosphate buffer (1 M) was used in order to maintain a stable pH value of 6.4 for all samples, which is the optimal working pH for the β-glucuronidase from E. coli (5.0 and 7.5 according to the manufacturer).
In addition, chemical hydrolysis with 6 N HCl was tested as an alternative approach yielding lower analyte concentrations, presumably due to analyte degradation during acidic incubation. The kinetics of the enzymatic hydrolysis were evaluated by processing highly concentrated samples derived from a metabolism study (manuscript in preparation) with different incubation times. Figure 2 shows the dependency of the enzymatic hydrolysis on the incubation time. After 15 min, hydrolysis of the glucuronidated analytes appears to be complete. To assure reproducible results and a complete hydrolysis, incubation time was set to 1.5 h. Additional experiments revealed a decrease of analyte concentrations in samples after more than 6 h of incubation (data not shown). Deconjugation efficiency was monitored by spiking all samples with 500 ng of MUG in order to monitor the enzymatic hydrolysis [13]. In case no MUG was detectable in the UPLC-MS/MS analysis the enzymatic hydrolysis was complete.
Due to matrix suppression and interferences in the chromatography an extraction step was necessary. Liquid-liquid extraction (LLE) was tested with dichloromethane, chloroform, ethyl acetate, diethyl ether, methyl tert-butyl ether (MTBE), and hexane. Best recoveries were obtained by extracting with ethyl acetate and MTBE, respectively. Pipetting steps were executed more conveniently using ethyl acetate because of its lower vapor pressure. Hence, ethyl acetate was used in the final method instead of MTBE. As all analytes possess both a basic amine group and an acidic carboxyl moiety, pH adjustment in proximity of the isoelectric point (pH 4.6 ± 0.2) was crucial to achieve satisfactory yields during LLE. Moderate to satisfying recoveries between 40 and 80% were achieved after optimization of the LLE regarding pH and solvent. Apparently, the presence of zwitterions with a positive charge at the amine group and a negative charge at the carboxylic group may influence the yield during extraction. To increase recovery rates solid-phase extraction (SPE) was also tested with MAX, MCX, and HLB cartridges from Waters and with Chromabond C18 columns from Macherey-Nagel (Düren, Germany). This approach leads to higher recovery rates, but signal intensities decreased due to a stronger matrix suppression. Thus, LLE was superior in terms of overall sensitivity.
Efficient ionization of the compounds was achieved with positive electrospray ionization (ESI+). Applied mass transitions and MS/MS parameters are shown in Table 1. Mass transitions used as quantifiers were chosen considering intensity and selectivity of the transition. Figure 3 a shows structure suggestions for the most prominent fragments from AHB, EHB, and DHB. Cleavage of the molecules at the ketone bridge seems to be very likely, because both fragments resulting from this cleavage were detectable. Furthermore, the elimination of the carboxyl group as observed in DHB is thinkable. Similar fragmentation patterns are observed for the analogous hydroxylated species (see Fig. 3b).
For internal standards (IS), the corresponding mass transitions were evaluated. MRM chromatograms for all analytes and their internal standards are shown in Fig. 4. IS concentrations were set in the middle of the calibration range to 10 μg/L and the applied transitions (corresponding to the quantifier mass transition) were found to be selective with sufficient sensitivity.
Method performance data are shown in Table 2. Parameters reported were evaluated according to FDA guidelines on bioanalytical method validation [9]. LOQs were set to 0.1 μg/L for AHB and EHB and to 0.05 μg/L for DHB being the lowest measurable concentrations with intra- and inter-day precisions below 20% CV. Intra- and inter-day accuracies at LOQ were in the acceptable range of 80–120%. The LOD was calculated from the LOQ by dividing the LOQ through 3. Calibration was found to be linear in a range from 0.1 to 200 μg/L for AHB and EHB and in a range from 0.05 to 100 μg/L for DHB, respectively (correlation coefficients R2 > 0.995).
Samples above ULOQ can be diluted up to 100-fold with water still yielding in accurate results (85–115% accuracy).
Intra- and inter-day precisions for all analytes at all concentration levels tested had CVs between 0.4 and 18.8% fulfilling the acceptance criteria. Intra- and inter-day accuracies ranged between 86.2 and 107.0%. Recovery values including losses during sample extraction were in a range between 42 and 79%. AHB signals in prepared urine matrix were suppressed by the matrix yielding in 25–42% signal area compared with an aqueous standard solution. DHB signals were also suppressed by the matrix, but to a lower extent (49–87% signal area compared with an aqueous standard). In contrast, a moderate signal enhancement of 110–116% could be observed for EHB.
Reagent blank samples were injected together with tested samples, no blank values >LLOQ for any analyte could be determined. Despite optimization of the washing method for the UPLC-MS/MS system between runs, small carryover effects in the range of the LOQs were observed after five injections of the highest calibrator for all analytes. However, high values close to the ULOQ have not been measured in the urine samples of non-occupationally exposed individuals so far. Therefore, in the case of a sample with high concentrations close to ULOQ, the following sample shall be reinjected to overcome false positive results.
In-house and custom synthesized standards were checked for impurities with 1H-NMR and HPLC-UV. No impurities were detectable and therefore, a purity of > 95% was assumed. Experiments addressing the stability of the standards will follow in the near future, since there is only a limited amount of each standard available.
All analytes were found to be stable in urine at room temperature (22 °C) for at least 18 h. Prepared samples can be stored at − 20 °C for at least 3 weeks and for a maximum of five freeze-thaw cycles. Post-preparative stability was proven for at least 1 week stored in the autosampler at 10 °C. Long-term stability studies over longer storage periods are currently in progress.
A few methods haven been published on the determination of DHHB, e.g., in sunscreens [14, 15], chlorinated water [16], and porcine skin samples [15]; however, to the best of our knowledge, the current method describes for the first time the simultaneous analysis of the three major DHHB metabolites in one method.
Urinary excretion of DHHB metabolites in a small group of the general population
To evaluate potential exposure due to the usage of DHHB containing consumer products in everyday life, 58 spot urine samples were collected from volunteers who were not exposed occupationally with DHHB and analyzed with the validated method. The subjects were not intentionally exposed to DHHB-containing consumer products but were advised to behave as usual. There were no restrictions with regard to selection of the volunteers and the time of urine collection. In total, 27 females and 31 males aged between 18 and 83 took part in this study.
The results are shown in Table 3. Urinary concentration levels of DHHB metabolites are shown as concentrations (μg/L). Additionally, values were normalized for the creatinine level as micrograms per gram creatinine taking into account the varying dilutions of the spot urine samples.
The metabolite determined with the highest concentrations was EHB with an average of 1.39 μg/g creatinine, followed by AHB (0.65 μg/g creatinine) and DHB (0.38 μg/g creatinine) with the lowest concentrations. AHB was found above LOQ in 19 samples (33%), EHB in 20 samples (36%), and DHB in 14 samples (26%). Thus, in approximately two-thirds of the study samples, no DHHB metabolites were detectable indicating a low exposure to DHHB in non-occupationally exposed subjects.
All three compounds were found to be strongly correlated (Spearman r = 0.81–0.92) showing that AHB, EHB, and DHB are specific metabolites to monitor DHHB exposure.
Levels of DHHB metabolites were compared for different subgroups and statistical significance was evaluated by means of the Mann-Whitney U test. Subgroups were divided as follows for comparison: gender (male/female), age (18–35/50–83), daytime of sample collection (4 am–2 pm/2:30 pm–1 am), seasons of sample collection (winter, spring/summer), age of the spot urine (> 1 year/< 1 year). No difference in DHHB metabolite levels could be observed for any subgroup comparison.
It has to be noted that only a small number of samples from the Munich area was analyzed not being necessarily representative for the exposure of the general population. To assess the exposure to DHHB in the general population, human biomonitoring studies with a larger sample size in a representative environment (rural/urban, locations throughout the country/region of interest) will be necessary.
Determination of additionally hydroxylated metabolites using a UPLC-QTrap system
Following the procedure described in the “UPLC-QTRAP experiments for the identification of minor metabolites of DHHB” section, EMS experiments lead to four plausible molecule masses: m/z = 273 for AHB-OH, m/z = 289 for AHB-(OH)2, m/z = 301 for EHB-OH, and m/z = 329 for DHB-OH. Subsequently conducted EPI experiments lead to multiple product ions which were translated into MRM transitions. Furthermore, retention times of the evaluated peaks were reviewed and regarded as valid if the additionally hydroxylated species eluted immediately before their analogue possessing just one hydroxyl group. At this point the occurrence of AHB-(OH)2 was rejected, because retention times of the peaks observed in EPI experiments were found to be 0.2 min after those of AHB and no MRM transitions could be generated from the found product ions. Plausible mass fragments for additionally hydroxylated metabolites of DHHB are shown in Fig. 3b. They are fragmented in analogy to AHB, EHB, and DHB (Fig. 3a). Cleavage of the molecules again occurred at their ketone functions leading to mainly two fragments. The fragment with m/z 149 was observed for every additionally hydroxylated metabolite as well as for AHB, EHB, and DHB. The second fragments of the additionally hydroxylated metabolites differ in the degree of alkylation of the amine function, leading to m/z = 126 for AHB-OH, 154 for EHB-OH, and 182 for DHB-OH. Those mass fragments correspond to the mass fragments of AHB (110), EHB (138), and DHB (166) which are lacking of the additional hydroxyl group (− 16). Therefore, the fragmentation patterns observed suggest that the additional hydroxyl moieties are exclusively located in the aniline ring. The exact positions of the hydroxyl functions could not be clarified without authentic reference material.
Eight plausible MRM transitions for the three compounds AHB-OH, EHB-OH, and DHB-OH were determined without the use of a standard by applying suitable EMS, EPI, and FIA experiments as summarized in Table 4. As cell exit potentials (CXPs) could only be adjusted to a maximum of 15 V in FIA experiments, CXPs obtained from the manual tuning of AHB, EHB, and DHB, respectively, were implemented in the final MRM transitions. Concentration levels of AHB-OH, EHB-OH, and DHB-OH were estimated to be 100-fold lower compared with the major metabolites AHB, EHB, and DHB based on the comparison of the area ratios. In conclusion, it was shown that the additionally hydroxylated species were found in negligible concentrations in urine, and hence, these metabolites are not suited as biomarkers of exposure to DHHB.
Conclusion
We developed and validated an UPLC-MS/MS method with sufficient sensitivity for the simultaneous determination of the three major and specific DHHB metabolites AHB, EHB, and DHB in urine of non-occupationally exposed subjects. All three major metabolites were detectable in approx. one-third of the urine samples from a pilot study with 58 healthy adult volunteers. Further minor metabolites with an additional hydroxyl function in the aniline ring were detected based on EMS experiments. However, these minor metabolites were not included in the final method due to their very low abundance. In conclusion, the new method proved its suitability to determine the three major metabolites AHB, EHB, and DHB in HBM studies to assess the DHHB exposure in the general population. This method will help to evaluate the exposure to DHHB in future HBM studies in larger populations.
References
Vielhaber G, Grether-Beck S, Koch O, Johncock W, Krutmann J. Sunscreens with an absorption maximum of > or =360 nm provide optimal protection against UVA1-induced expression of matrix metalloproteinase-1, interleukin-1, and interleukin-6 in human dermal fibroblasts. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2006;5(3):275–82.
Uter W, Goncalo M, Yazar K, Kratz EM, Mildau G, Liden C. Coupled exposure to ingredients of cosmetic products: III. Ultraviolet filters. Contact Dermatitis 2014;71(3):162–169.
Mikkelsen SH, Lassen C, Warming M, Hansen E, Brinch A, Brooke D, et al. Survey and health assessment of UV filters. Denmark: The Danish Environmental Protection Agency; 2015. 286 p.
European Commission. Commission Directive 2005/9/EC of 28 January 2005 amending Council Directive 76/768/EEC, concerning cosmetic products, for the purposes of adapting Annex VII thereto to technical progressText with EEA relevance. 2005.
European Chemicals Agency. hexyl 2-(1-(diethylaminohydroxyphenyl)methanoyl) benzoate 2019 [cited 2019 07.03.2019]. Available from: https://echa.europa.eu/de/substance-information/-/substanceinfo/100.103.916.
Verband der chemischen Industrie e.V. Überblick zur Kooperation von BMUB und VCI beim Thema Human-Biomonitoring 2016 [Available from: https://www.vci.de/themen/chemikaliensicherheit/human-biomonitoring/ueberblick-kooperation-vci-bmub-human-biomonitoring-gesundheit-produktverantwortung.jsp.
Chisvert A, León-González Z, Tarazona I, Salvador A, Giokas D. An overview of the analytical methods for the determination of organic ultraviolet filters in biological fluids and tissues. Anal Chim Acta. 2012;752(Supplement C):11–29.
León Z, de Vlieger J, Chisvert A, Salvador A, Lingeman H, Irth H, et al. Identification of the biotransformation products of 2-ethylhexyl 4-(N,N-dimethylamino)benzoate. Chromatographia. 2010;71(1):55–63.
U.S. Food and Drug Administration (FDA). Bioanalytical method validation - guidance for industry. USA2018.
Hajnal K, Gabriel H, Aura R, Erzsébet V, Blanka SS. Prodrug strategy in drug development. Acta Medica Marisiensis. 2016;62(3):356.
BASF. Fact sheet für Stoffauswahl zum BMU/VCI-Projekt Humanbiomonitoring: Uvinul A plus. unpublished.
Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, et al. Quantitative detection of eight phthalate metabolites in human urine using HPLC−APCI-MS/MS. Anal Chem. 2000;72(17):4127–34.
Grignon C, Dupuis A, Albouy-Llaty M, Condylis M, Barrier L, Carato P, et al. Validation of a probe for assessing deconjugation of glucuronide and sulfate phase II metabolites assayed through LC-MS/MS in biological matrices. J chromatogr B, Analytical technologies in the biomedical and life sciences. 2017;1061–1062:72–8.
De Orsi D, Giannini G, Gagliardi L, Porrà R, Berri S, Bolasco A, et al. Simple extraction and HPLC determination of UV-A and UV-B filters in sunscreen products. Chromatographia. 2006;64(9):509–15.
Souza C, Maia Campos PMBG. Development of a HPLC method for determination of four UV filters in sunscreen and its application to skin penetration studies. Biomed Chromatogr. 2017:e4029-n/a.
Grbović G, Trebše P, Dolenc D, Lebedev A, Sarakha M. LC/MS study of the UV filter hexyl 2-[4-(diethylamino)-2-hydroxybenzoyl]-benzoate (DHHB) aquatic chlorination with sodium hypochlorite. J Mass Spectrom. 2013;48(11):1232–40.
Funding
The method development for the determination of urinary DHHB metabolites (including the synthesis of part of the reference compounds) was funded by the Chemie Wirtschaftsförderungsgesellschaft, Frankfurt/Main, Germany. The method was developed as part of a 10-year human biomonitoring initiative between the Federal Ministry for Environment, Nature Conservation and Nuclear Safety (BMU) and the Verband der chemischen Industrie e.V. (German Chemical Industry Association (VCI)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Studies with human subjects were approved by the Ethic Committee of the Bayerische Landesärztekammer, Munich, Germany. All subjects gave their informed consent.
Conflict of interest
The authors MS, NP, FB, GS, and MS declare that they have no conflict of interest. EL is employed by a company manufacturing DHHB.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 329 kb)
Rights and permissions
About this article
Cite this article
Stoeckelhuber, M., Pluym, N., Bracher, F. et al. A validated UPLC-MS/MS method for the determination of urinary metabolites of Uvinul® A plus. Anal Bioanal Chem 411, 8143–8152 (2019). https://doi.org/10.1007/s00216-019-02201-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02201-6