Abstract
Molecularly imprinted polymers (MIPs) have long been studied for applications in biomolecule recognition and binding; compared with natural antibodies, they may offer advantages in cost and stability. We report on the development of MIPs that “self-report” concentrations of bound analytes via fluorescence changes in embedded quantum dots (QDots). Composite QDot/MIPs were prepared using phase inversion of poly(ethylene-co-vinyl alcohol) (EVAL) solutions with various ethylene mole ratios in the presence of salivary target molecules (e.g. amylase, lipase, and lysozyme). These major protein components of saliva have been implicated as possible biomarkers for pancreatic cancer. The optimum (highest imprinting effectiveness) ethylene mole ratios of the commercially available EVALs were found to be 32, 38, and 44 mol% for the imprinting of amylase, lipase, and lysozyme, respectively. QD fluorescence quenching was observed on binding of analytes to composite MIPs in a concentration-dependent manner, and was used to construct calibration curves. Finally, the composite MIP particles were used for the quantitative detection of amylase, lipase, and lysozyme in real samples (saliva) and compared with a commercial Architect ci 8200 chemical analysis system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saliva is a multifunctional biofluid with anti-bacterial, digestive, lubricative, buffering, and tissue-coating attributes. Three major protein constituents of saliva are α-amylase, lipase, and lysozyme, which are involved in digestion. α-Amylase and lipase have also been found to be possible biomarkers of pancreatic cancer, when found in the blood. Salivary and pancreatic α-amylase shares a homology of 97% and, therefore, an established method for α-amylase biosensing may be effective for screening without major modification [1]. Pancreatic cancer, liver cancer, and ovarian cancer are occasionally referred to as silent killers owing to the very late onset of symptoms. Remission from pancreatic cancer is extremely rare, and fewer than 5% of diagnosed patients live longer than five years. Thus early detection of this disease by use of chemical markers is critical.
Digestive functions of proteins in biological fluids are vital to maintaining human health. Moreover, salivary α-amylase has been used as an index of endogenous adrenergic activity and as a non-invasive biomarker for the sympathetic nervous system [2]. A recent study used total protein of whole saliva as a biomarker of anaerobic thresholds [3]. Rohleder and Nater [4] have reviewed the methods and medical uses of α-amylase detection, focusing on enzyme-kinetic detection methods. Other methods, e.g., enzyme-linked immunosorbent assays (ELISAs) and radio immuno assays (RIAs), have also been used, despite the fact that they are more time-consuming [4].
The use of synthetic materials as substitutes for biological antibodies and receptors [7] have applications in bioseparation, biosensing, and delivery of bioactive molecules. Molecularly imprinted polymers (MIPs) have the advantage of higher stability than natural antibodies, and an acceptable recognition capability could make them candidates for prescreening for cancer using a home-care system. Unlike the imprinting of smaller molecules, protein imprinting is slow, and many issues, for example the size of binding cavities, complementary bonds, the flexibility of the structure, and the solubility of the target proteins [8, 9] must be considered. Target proteins that have been adopted as targets for imprinting include albumin [10–22], lysozyme [23–30], ribonuclease [29, 31–33], hemoglobin [17, 34–38], and myoglobin [29, 35, 39, 40]. Surface imprinting on a thin film (2D) and bulk imprinting in a membrane (3D) are generally favorable for biosensing and separation applications. Surface imprinting on microparticles may be used for packing columns, and imprinted nanoparticles can be used to sense and actively remove proteins from biological mixtures when magnetic nanoparticles are incorporated via a microemulsion or suspension polymerization. Another important issue is the price of template proteins; as purified proteins tend to be quite costly, the development of protein imprinting has been slow.
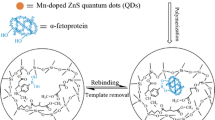
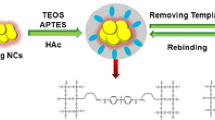
In detecting and quantifying biomarkers for disease, specific binding is only half the challenge – after the analyte is bound, it must be detected by an optical or enzymatic process. The use of quantum dots in this detection process is an attractive approach. Quantum dots [5] can now be found in computing, biological/biomedical, photovoltaic, and light-emitting device applications. For biological applications, quantum dots are attractive as fluorescent markers for imaging and sensing owing to their lower photobleaching rate compared with conventional fluorescent organic compounds [6]. Given the high stability of their photoluminescence, quantum dots are promising for use in biosensing applications. Furthermore, there is increasing evidence that QDs respond to the local environment, and that direct interactions between QDs and various proteins can perturb (typically quench) their fluorescence [43]. Thus, integrating QDs into MIP particles or films could result in materials that change fluorescence intensity as a direct readout of target binding.
In this study amylase, lipase, and lysozyme were imprinted into a biocompatible polymer, poly(ethylene-co-ethylene alcohol). The imprinted and washed particles were characterized for their ability to rebind target molecules and the rebinding was compared with that of non-imprinted control particles. Initial studies used particles without QDs, in order to determine optimum compositions (ethylene mol%) for binding. Particles were then made using these optimum compositions, but with QDs embedded in the particles during synthesis and/or imprinting. We found that the QD composite MIPs did, in fact, show significant fluorescence changes (quenching) on binding targets. Moreover, the imprinted targets were much more effective at causing QD quenching than unimprinted (irrelevant) targets; in other words, the cross-reactivity of the QD/MIP composite particles was low. This low cross-reactivity suggests that, with different color QDs in different MIPs, fluorescence detection of analytes is possible. Finally, using a calibration curve, the concentrations of salivary proteins in real samples were measured and compared with results from a commercial analytical chemistry unit.
Experimental
Reagents
α-Amylase (EC 3.2.1.1, Cat. No. 10080, 43.6 U mg−1) and lipase (EC 3.1.1.3, Cat. No. 62300, powder, 15–35 U mg−1) both from hog pancreas and lysozyme (EC 3.2.1.17) from hen egg white were purchased from Fluka Biochemika (Buchs, Switzerland). Poly(ethylene-co-vinyl alcohol), EVAL, with ethylene 27, 32, 38, and 44 mol% (product nos 414077, 414093, 414085, and 414107) were from Sigma–Aldrich (St Louis, MO, USA). Dimethyl sulfoxide (DMSO, product #161954) was purchased from Panreac (Barcelona, Spain) and used as the solvent to dissolve EVAL polymer particles at a concentration of 1% w/w. Absolute ethyl alcohol (ACS-grade) was from J.T. Baker (NJ, USA). Sodium dodecyl sulfate (SDS) was purchased from Sigma–Aldrich and used for the removal of target molecules. Quantum dot kits (QDot 545, 605, 655 IKT) were purchased from Invitrogen (Carlsbad, CA, USA). All chemicals were used as received unless otherwise mentioned.
Formation of salivary protein-imprinted poly(ethylene-co-ethylene alcohol)/quantum dot composite nanoparticles
Details of the synthesis of salivary protein-imprinted (amylase-imprinted, lipase-imprinted, and lysozyme-imprinted) and non-imprinted EVAL nanoparticles can be found elsewhere [41]; three steps were involved:
-
1.
dissolving proteins at various imprinting concentrations in DMSO and adding granular EVAL to the protein–DMSO solution to form clear EVAL solution (0.1% w/w EVAL in DMSO);
-
2.
before dispersion of the EVAL solution in 10 mL non-solvent solution [42] (e.g. deionized water–isopropanol 2:3 w/w) for EVAL, 10 μL stock QDots solution (1 μmol L−1) was added for each milliliter of the final solution; and then
-
3.
removal of the template molecule by dialysis with 150 mL 1 wt% SDS solution for 15 min, twice, and with deionized water for 15 min, six times.
All nanoparticles were equilibrated with deionized water overnight before use. The non-imprinted polymers (NIPs) were prepared identically, except that the template protein was omitted.
A UV–visible spectrometer (Lambda 40; Perkin–Elmer, Wellesley, MA, USA) was then used to confirm that leaching and/or washout of the template molecules was complete, as determined by the absorbencies of the target molecules (amylase: 265 nm; lipase: 260 nm; and lysozyme: 280 nm). The preliminary binding measurements of target molecules to the imprinted or non-imprinted EVAL thin films were performed with 5 mL 0.1 mg mL−1 target molecules (unless otherwise stated) for 30 min by UV–visible spectrometry. Finally, samples were freeze-dried (DI-series, Panchun Scientific, Taiwan) to obtain amylase, lipase, or lysozyme-imprinted or non-imprinted polymer nanoparticles.
Surface morphology examination by scanning electron microscopy
Amylase, lipase, and lysozyme-imprinted, and non-imprinted polymers were freeze dried before examination by use of a scanning electron microscope (S4700; Hitachi High-Technologies, Tokyo, Japan). Particles were attached to the sample holder by transfer from forceps on to double-sided cellophane tape. The particle sizes of each sample were then visually estimated.
Size distribution of protein-imprinted and non-imprinted EVAL/QDots composite nanoparticles
Amylase, lipase, and lysozyme-imprinted polymer particles with different imprinting template concentrations, and non-imprinted polymer particles were monitored by use of a particle sizer (90Plus; Brookhaven Instruments, New York, USA). Measurement of particle-size distribution was based on dynamic light scattering (DLS) at 25 °C with 3 min duration data collection at the 90° detection angle. The average count rate of the background was 15 kcps and that of each measurement was between 20 and 500 kcps. The scattering laser power of this instrument is the standard 35 mW. The Contin algorithm was used to analyze data.
Fluorescence measurement of the adsorption of target proteins on the protein-imprinted EVAL/QDots composite nanoparticles
The amylase, lipase, and lysozyme-imprinted, and non-imprinted polymer/QDots composite nanoparticles were monitored with a fluorescence spectrophotometer (F-7000; Hitachi, Japan) with excitation wavelengths 430, 385, and 325 and emission wavelengths 655, 605, and 545 nm, respectively (as shown in Scheme 1). Various concentrations of amylase, lipase, and lysozyme were then added to a suspension of 50 μL stock composite EVAL MIP nanoparticles in 1.95 mL PBS to plot the calibration curves. Saliva samples were secreted by our colleagues 10 min after rinsing the mouth and 4 h before the test. In general, 5–10 mL saliva was collected and centrifuged at 6,000 rpm for 10 min. Seven hundred microliters of the salivary supernatant sample was also stored in an Eppendorf microcentrifuge tube at 4 °C and analyzed, at E-Da Hospital, with an Architect ci 8200 system (Abbott Laboratories. Abbott Park, Illinois, USA) which combines immunoassay and clinical chemistry on one integrated platform and runs up to 200 immunoassay tests and up to 1200 clinical chemistry tests an hour. Each nanoparticle solution contained 10 μL stock saliva supernatant sample in a 3 mL cuvette, 50 μL MIP/QDots composite nanoparticles, and 1.9 mL PBS mixture. The fluorescence of the MIP/QDots composites was then measured at 545 nm when incubated with saliva for 300 s to measure the lysozyme concentration; measurement was then repeated at 655 and 605 nm to detect the concentrations of amylase and lipase, respectively.
Results and discussion
This study began with an assessment of the optimum EVAL composition (ethylene mol%) for imprinting each of the target proteins (Fig. 1) [19, 29]. Absorption of target molecules from a 0.1 mg mL−1 solution was determined by use of UV–visible spectroscopy on the supernatant; results are reported as the amount of absorbed target per gram of MIPs. Measurements of binding to non-imprinted control particles (NIPs) are also shown. Interestingly, a different ethylene mol% was optimum for each of the three target proteins (where optimum binding was defined as the highest ratio of MIP binding to NIP binding.) The optimum ethylene mol% is not the composition that maximized the general “adhesiveness” of the EVAL for the target, because binding of amylase and lysozyme to optimally composed NIPs is not maximum. Rather, the optimum ethylene mol% is likely to reflect several factors, including the flexibility and packing of the EVAL matrix and its ability to conform to the specific targets. A quantitative summary of the composition-dependent binding to MIPs is shown in Table 1.
The effect of the concentration of the template protein in the precipitation (phase inversion) bath on the resulting sizes of EVAL MIPs was also examined. Sizes of these particles were determined by dynamic light scattering, as they contained no QDs to interfere with the scattering signal. As shown in Fig. 2, a higher imprinting concentration of a protein generates a smaller mean size of EVAL composite particles before template removal. However, the particle sizes decrease following removal of the template proteins, and the ultimate particle sizes increase continuously with higher imprinting protein concentrations. Figure 2(a) shows the sizes of the EVAL composite particles as the imprinting concentration of amylase was varied from 0.003 to 0.1 mg mL−1; above this range the mean particle size (pre-wash) decreased from 732 to around 444 nm, whereas the post-wash size increased from 212 to 283 nm. The lipase-imprinted EVAL composite particles are 200 nm smaller (pre-wash) than amylase-imprinted EVAL composite particles at target concentrations of 3.0 μg mL−1 (Fig. 2(b)); however, the post-wash sizes are very similar—within 20 nm. In contrast, although the mean size of lysozyme-imprinted EVAL composite particles before washing decreased from around 476 to 378 nm for the same range of imprinting concentrations, the mean particle size after washing substantially increased—from 60 to 319 nm.
The unusual trends in the particle sizes warrant some discussion. Clearly, in the limit of zero imprinting concentration, the “before washing” size and the “after washing” size must converge, because there is nothing to wash out. We suspect that the DLS-reported particle sizes for unwashed particles are affected by MIP aggregation, making this size estimate large. High imprinting concentrations, before washing, would lead to better particle dispersion through surface adsorption and consequent steric and electrostatic barriers to particle aggregation. Particle sizes after washing are unlikely to show aggregation, in large part owing to inclusion of the surfactant SDS in the wash buffer.
For QD/MIP composite particles, DLS is likely to be unreliable, owing to optical artifacts introduced by the fluorescent QDs. For this reason, scanning electron microscopy was used to examine the sizes of composite QD/MIP particles (after washing). Figure 3 shows results for amylase-imprinted composite QD/MIPs, prepared with different template concentrations. These sizes are quite comparable with those found for the QD-free MIPs using DLS, demonstrating that incorporation of QDs does not significantly alter the particle sizes. Similar results were obtained for the two other template proteins.
Figure 4 shows fluorescence changes in composite QD/MIP particles on binding targets. The fluorescence intensities decrease when target molecules are added to the particle suspension; each step in the descending staircase represents a discrete addition of an aliquot of target. The decrease in QD fluorescence must be caused by a local change in the QD environment associated with protein binding, but the mechanism of quenching is unknown. Environmental factors have been shown to affect QD fluorescence (usually by quenching [43]). In the experiments shown in Fig. 4, spectrally distinguishable QDs were used in the preparation of MIPs for each target: QD545 for lysozyme, QD605 for lipase, and QD655 for amylase. Figure 4(a) shows that non-imprinted QD-containing particles (NIPs) show only a very tiny fluorescence decrease (black line), ca. a tenth of the response of the amylase MIPs. This is actually a little better than expected from the preliminary experiments, in which NIPs bound about one-fourth as much amylase as MIPs. Figure 4(c) shows the lack of cross-reactivity of the lysozyme MIPs when titrated with lipase or amylase.
Fluorescence of QD/MIP composite particles, measured at peak emission wavelengths for each QD. (a) Amylase QD655/MIPs, titrated with amylase. Also shown are QD655/NIPs, which show a small quenching effect with amylase. Control MIPs have no added proteins. (b) Lipase QD605/MIPs, titrated with lipase. (c) Lysozyme QD545/MIPs, titrated with lysozyme, or with the non-target proteins lipase or amylase
The fluorescence changes in Fig. 4 were replotted in Fig. 5 as a function of the concentration of the target protein. As a phenomenological approach, the quenching was fit to a standard binding isotherm (with a single dissociation constant); fit curves are shown in the figure. These curves should properly be taken as guides to the eye, because:
-
1.
template concentrations were not high enough to saturate the fluorescence quenching; and
-
2.
it is unlikely that a single-affinity binding-site model is appropriate for the heterogeneous binding sites present in MIPs.
The concentrations studied are appropriate for biomedical applications, however, because the biological concentrations of amylase and lipase are around 0.1–3 mg mL−1 and 0.1–10 μg mL−1, respectively. With irrelevant, non-target proteins, some evidence of fluorescence quenching was observed (amylase with either lipase MIPs or lysozyme MIPs), but only at much higher concentrations than those of the targets. To demonstrate the possibility of one-pot fluorescence detection, a mixture of the three kinds of QD/MIPS (each recognizing a different target, and each having embedded QDs with distinct emission wavelengths) was prepared. When this mixture of QD/MIPs was titrated with the different target proteins, the appropriate fluorescence emission wavelength showed a decrease, as shown in Fig. 6.
Theoretically, the proposed nanoprobes have the same advantage as ELISA in terms of specifically determining the concentration of the amylase protein, instead of indirectly deriving the concentration from its biological activity [4]. However, routine measurements of amylase and lipase are normally done via enzymatic activity. Figure 7 displays the calibration curves of the activities of amylase and lipase measured by use of a commercial Architect ci 8200 system in the hospital, when using different concentrations of amylase and lipase. By using those calibration curves, enzymatic activities are converted to the concentration of salivary protein.
These MIP/QDots nanoparticles were then used for saliva sample measurements. Tables 2 and 3 summarize the results from analyses of five saliva samples from the authors and their colleagues by use of the Architect ci 8200 system and which fell in the ranges: amylase 14,570–81,520 U L−1 and lipase 3,860–5,274 U L−1, converted with the calibration curves in Fig. 7. The reference concentrations of amylase and lipase are 1.01–2.75 mg mL−1 and 0.55–0.67 mg mL−1, respectively. For the salivary lysozyme measurements, the reference concentration is around 1 μg mL−1 [44]. According to Table 4, all five salivary samples indicate that the lysozyme concentration is around 1.0–2.3 μg mL−1. The average accuracies for amylase and lipase-imprinted EVAL/QDots composite nanoparticles are 96.21 ± 2.44 and 96.15 ± 1.10%. The detection limit and linear range of the optical signal may be enhanced by longer monitoring duration and using higher concentrations of those nanoprobes. In this work, the detection limits were around 0.1, 0.1, and 0.013 μg mL−1 and the linear ranges were 1–1500, 1–1500 and 0.25–8.0 μg mL−1 for amylase, lipase, and lysozyme stock solution, respectively. Moreover, synthesis of the composite is routinely performed in our laboratory; we have found that the composite MIPs can be stored for at least six months [41]. The nanoparticles used can be repeatedly washed and used for adsorption at least three times, as shown in Fig. 8.
Conclusions
Replacing natural antibodies with molecularly imprinted polymers is a highly promising route for preparing tailor-made receptors for target molecules. Quantum dots offer a signaling capability for analytical and bioanalytical applications. In this work, the optimum (highest imprinting effectiveness) ethylene mole ratios of the commercially available EVALs were determined to be 32, 38, and 44 mol% for imprinting of amylase, lipase, and lysozyme, respectively. Mean particle sizes of the washed, protein-imprinted MIP particles increased with increasing template concentration; this trend was borne out with composite EVAL/QDots nanoparticles studied by electron microscopy. The non-invasive analysis of digestive proteins has important medical potential for use in detecting oral and pancreatic cancers. The incorporation of QD sensors into the MIP matrix offers a straightforward route to combining binding and readout, based on the apparent quenching of QDs by their contact with the target proteins.
References
Lorentz K (2005) Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes part 9. IFCC method for α-Amylase (1, 4-α-D-Glucan 4-Glucanohydrolase, EC 3.2.1.1). Clin Chem Lab Med 36:185–203
Nater UM, Rohleder N (2009) Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34:486–496
Bortolini MJS, De Agostini GG, Reis IT, Lamounier RPMS, Blumberg JB, Espindola FS (2009) Total protein of whole saliva as a biomarker of anaerobic threshold. Res Q Exerc Sport 80:604–610
Rohleder N, Nater UM (2009) Determinants of salivary [alpha]-amylase in humans and methodological considerations. Psychoneuroendocrinology 34:469–485
Rossetti R, Nakahara S, Brus LE (1983) Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J Chem Phys 79:1086–1088
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933–937
Ye L, Mosbach K (2008) Molecular imprinting: synthetic materials as substitutes for biological antibodies and receptors. Chem Mater 20:859–868
Bossi A, Bonini F, Turner APF, Piletsky SA (2007) Molecularly imprinted polymers for the recognition of proteins: the state of the art. Biosens Bioelectron 22:1131–1137
Turner NW, Jeans CW, Brain KR, Allender CJ, Hlady V, Britt DW (2006) From 3D to 2D: a review of the molecular imprinting of proteins. Biotechnol Prog 22:1474–1489
Bonini F, Piletsky S, Turner APF, Speghini A, Bossi A (2007) Surface imprinted beads for the recognition of human serum albumin. Biosens Bioelectron 22:2322–2328
Zhou X, He X-W, Chen L-X, Li W-Y, Zhang Y-K (2009) Optimum conditions of separation selectivity based on molecularly imprinted polymers of bovine serum albumin formed on surface of Aminosilica. Chinese J Anal Chem 37:174–180
Zhao K, JWei u, Cheng G, Yang C, Chen L (2009) Preparation of bovine serum albumin-imprinted calcium polyacrylate alginate hybrid microspheres via Ca2+ crosslinking. J Appl Polym Sci 113:1133–1140
Zdyrko B, Hoy O, Luzinov I (2009) Toward protein imprinting with polymer brushes. Biointerphases 4:FA17–FA21
Wang J, Hua ZD, Chen ZY, Li YZ, Zhao MP (2009) Molecular imprinting of protein by coordinate interaction. Chin Chem Lett 20:747–750
Wang H, Li W, He X, Chen L, Zhang Y (2008) m-Aminophenylboronic acid as a functional monomer for fabricating molecularly imprinted polymer for the recognition of bovine serum albumin. React Funct Polym 68:1291–1296
Wang H, He Y, He X, Li W, Chen L, Zhang Y (2009) BSA-imprinted synthetic receptor for reversible template recognition. J Sep Sci 32:1981–1986
Lu Y, Yan C-L, Gao S-Y (2009) Preparation and recognition of surface molecularly imprinted core-shell microbeads for protein in aqueous solutions. Appl Surf Sci 255:6061–6066
Long Y, Sun Y, Wang Y, Xing X, Zhao Z, Wang C, Fan Y, Mi H (2008) Molecular imprinted polymer with positively charged assistant recognition polymer chains for adsorption/enrichment of low content target protein. Chin Sci Bull 53:2617–2623
Lee M-H, Thomas JL, Tasi S-B, Liu B-D, Lin H-Y (2009) Formation and Recognition Characteristics of Albumin-Imprinted Poly(Ethylene-co-Vinyl-Alcohol) Membranes. J Nanosci Nanotechnol 9:3469–3477
Hu C-H, Chou T-C (2009) Albumin molecularly imprinted polymer prepared with a semi-rigid crosslinker in mixed organic/aqueous media. Microchim Acta 165:399–405
Hu C-H, Chou T-C (2009) Albumin molecularly imprinted polymer with high template affinity – Prepared by systematic optimization in mixed organic/aqueous media. Microchem J 91:53–58
Ghasemzadeh N, Nyberg F, Hjertén S (2008) Highly selective artificial gel antibodies for detection and quantification of biomarkers in clinical samples. II. Albumin in body fluids of patients with neurological disorders. J Sep Sci 31:3954–3958
Brown ME, Puleo DA (2008) Protein binding to peptide-imprinted porous silica scaffolds. Chem Eng J 137:97–101
Zhang W, Qin L, He X-W, Li W-Y, Zhang Y-K (2009) Novel surface modified molecularly imprinted polymer using acryloyl-[beta]-cyclodextrin and acrylamide as monomers for selective recognition of lysozyme in aqueous solution. J Chromatogr 1216:4560–4567
Qin L, He X-W, Zhang W, Li W-Y, Zhang Y-K (2009) Surface-modified polystyrene beads as photografting imprinted polymer matrix for chromatographic separation of proteins. J Chromatogr 1216:807–814
Huang C-Y, Tsai T-C, Thomas JL, Lee M-H, Liu B-D, Lin H-Y (2009) Urinalysis with molecularly imprinted poly(ethylene-co-vinyl alcohol) potentiostat sensors. Biosens Bioelectron 24:2611–2617
Bergmann NM, Peppas NA (2008) Configurational biomimetic imprinting for protein recognition: structural characteristics of recognitive hydrogels. Ind Eng Chem Res 47:9099–9107
Bereli N, Me A, Baydemir G, Say R, Galaev IY, Denizli A (2008) Protein recognition via ion-coordinated molecularly imprinted supermacroporous cryogels. J Chromatogr 1190:18–26
Lin H-Y, Hsu C-Y, Thomas JL, Wang S-E, Chen H-C, Chou T-C (2006) The microcontact imprinting of proteins: The effect of cross-linking monomers for lysozyme, ribonuclease A and myoglobin. Biosens Bioelectron 22:534–543
Hirayama K, Sakai Y, Kameoka K (2001) Synthesis of polymer particles with specific lysozyme recognition sites by a molecular imprinting technique. J Appl Polym Sci 81:3378–3387
Tan CJ, Tong YW (2006) Preparation of superparamagnetic ribonuclease a surface-imprinted submicrometer particles for protein recognition in Aqueous Media. Anal Chem 79:299–306
Hsu C-Y, Lin H-Y, Thomas JL, Wu B-T, Chou T-C (2006) Incorporation of styrene enhances recognition of ribonuclease A by molecularly imprinted polymers. Biosens Bioelectron 22:355–363
Hsu C-Y, Lin H-Y, Thomas JL, Chou T-C (2006) Synthesis of and recognition by ribonuclease A imprinted polymers. Nanotechnology 17:S77–S83
Xia Y-Q, Guo T-Y, Zhao H-L, Song M-D, Zhang B-H, Zhang B-L (2009) Protein recognition onto silica particles using chitosan as intermedium substrate. J Biomed Materi Res 90A:326–332
Wang Y, Zhou Y, Sokolov J, Rigas B, Levon K, Rafailovich M (2008) A potentiometric protein sensor built with surface molecular imprinting method. Biosens Bioelectron 24:162–166
Uysal A, Demirel G, Turan E, Çaykara T (2008) Hemoglobin recognition of molecularly imprinted hydrogels prepared at different pHs. Anal Chim Acta 625:110–115
Gai Q, Liu Q, Li W, He X, Chen L, Zhang Y (2008) Preparation of bovine hemoglobin-imprinted polymer beads via the photografting surface-modified method. Front Chem Chin 3:370–377
Li L, He X, Chen L, Zhang Y (2009) Preparation of core-shell magnetic molecularly imprinted polymer nanoparticles for recognition of bovine hemoglobin. Chem Asian J 4:286–293
Linares AV, Vandevelde F, Pantigny J, Falcimaigne-Cordin A, Haupt K (2009) Polymer films composed of surface-bound nanofilaments with a high aspect ratio, molecularly imprinted with small molecules and proteins. Adv Funct Mater 19:1299–1303
Turan E, Özçetin G, Caykara T (2009) Dependence of protein recognition of temperature-sensitive imprinted hydrogels on preparation temperature. Macromol Biosci 9:421–428
Lin H-Y, Ho M-S, Lee M-H (2009) Instant formation of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles and their use in one-pot urinalysis. Biosens Bioelectron 25:579–586
Young TH, Cheng LP, Hsieh CC, Chen LW (1998) Phase behavior of EVAL polymers in water-2-Propanol Cosolvent. Macromolecules 31:1229–1235
Gerhards C, Schulz-Drost C, Sgobba V, Guldi DM (2008) Conjugating Luminescent CdTe Quantum Dots with Biomolecules†. J Phys Chem B 112:14482–14491
Virella G, Goudswaard J (1978) Measurement of salivary lysozyme. J Dent Res 57:326–328
Acknowledgements
We appreciate financial support from National Science Council of ROC under Contract No. NSC 98-2220-E-390-002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, MH., Chen, YC., Ho, MH. et al. Optical recognition of salivary proteins by use of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles. Anal Bioanal Chem 397, 1457–1466 (2010). https://doi.org/10.1007/s00216-010-3631-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3631-x