Abstract
A rapid and reliable method was developed and applied for the simultaneous determination of 17 organochlorine pesticides (OCPs) in propolis. After extraction with hexane and acetone (1:1, v/v), four sorbents (florisil, silica, graphitized carbon, and tandem graphitized carbon plus florisil) were assayed for the clean-up step. The elution solvents hexane and ethyl acetate (1:1, v/v), hexane and dichloromethane (3:7, v/v), and ethyl acetate and hexane (2:8, v/v) were studied. The results showed that the combination of the tandem graphitized carbon and florisil cartridge with the elution solvent of 6mL of ethyl acetate and hexane (2:8, v/v), which was capable of eliminating matrix interference and providing colorless eluates, was the most efficient clean-up procedure for propolis extracts when testing for OCPs. The analytical technique employed was gas chromatography with electron capture detection (GC–ECD). The correlation coefficients from linear regression for the analyzed concentrations (5∼100 μg/kg) were >0.9961. The limits of detection (LODs) varied between 0.8 μg/kg for 4,4′-DDE and 11.4 μg/kg for endosulfan II, and the limits of quantitation (LOQs) ranged from 2.6 to 38.1 μg/kg. The average recoveries varied between 62.6 and 109.6%. Relative standard deviations (RSD%) ranged from 0.8 to 9.4%. Sample analysis indicated that 4,4′-DDE was detected more often in propolis than other pesticides, such as β-HCH, δ-HCH and heptachlor.

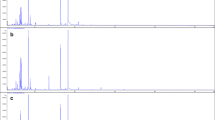
GC-ECD chromatogram of a standard solution with 0.1 mg/L of OCPs
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organochlorine pesticides (OCPs) are widely used in most countries to protect crops from insects and to increase crop yields, as well as for forestry purposes, cosmetic use and human safety considerations (e.g., to prevent viruses/diseases spread by insects) [1]. Although some of the compounds belonging to OCPs, like aldrin, dieldrin, heptachlor, DDT, HCH, etc. (which are also important constituents of the toxic group known as persistent organic pollutants, POPs), have been banned or restricted [2], they are still detected in the environment, even in arctic samples [3, 4], a region in which they have of course never been used. This is because OCPs can be carried by the wind, and are deposited through wet or dry deposition processes in remote areas, or they undergo atmospheric degradation. They can revolatilize repeatedly [5], and, depending on their persistence in the environment, can travel tens, hundreds or thousands of kilometers [6]. The OCPs, due to their highly persistent nature (resulting from their chemical stability and lipophilic character), accumulate in different environmental compartments and in the food chain, thus causing elevated contamination in the human body. Previous studies show that different organs of the plant exhibit different accumulation patterns of pesticides, with the following sequence of contamination levels: leaves > stalks > roots [7]. Propolis is a brownish resinous material collected by worker bees from the leaf buds of numerous tree species like birch, poplar, pine, alder, willow and palm [8]. The quality of propolis depends on its botanical and geographical origin [9]. Bees are insects that are greatly affected by insecticides as well as other pesticides in general, as they can work anywhere that they can fly to. They collect the contaminated leaf buds and transport them back to the swarm, allowing pesticides to enter the bee’s body, which ultimately results in the contamination of apiarian products consumed by human beings. Thus, it presents a severe threat to human health and food safety.
Different national regulations have established maximum residual levels (MRLs) of pesticide in foodstuffs in order to answer issues relating to food quality and trade disputes. Council regulations have established MRLs for various product groups, including meat, milk, vegetable, fruit, cereals, tea and others, of OCPs (namely HCH, lindane, DDT, dieldrin, endosulfan, endrin, heptachlor and methoxychlor, with values of 0.007∼0.3, 0.001∼0.7, 0.04∼1, 0.006∼0.2, 0.004∼0.1, 0.0008∼0.05, 0.004∼0.2 and 0.01 mg kg−1, respectively [10]). According to European Union (EU) regulations, since honey is a natural product it must be free of chemicals [11]. Japan has also established the Positive List System for Agricultural Chemical Residues in Foods, including honey, beef, and fish, etc. There are some reports of determinations of OCP residues in honey and beeswax [12, 13], but rarely in propolis[14], which makes it difficult to regulate its quality. Therefore, the development of a method for performing pesticide residue analysis in propolis is urgently required.
At present, analytical methods that are used to monitor pesticide residues generally require the extraction and isolation–concentration of pesticides from the studied matrix and a final determination using chromatographic procedures [15]. Propolis is a very complex matrix with a composition that depends on its botanical origin. It typically consists of waxes, resins, water, inorganics, phenolics and essential oils [16]. Impurities in complex matrices may interfere with the analytical signals of interest. Thus, sample preparation is a key element in pesticide residue analysis in propolis. Conventional methods are time-consuming and tedious, and they generally use large amounts of glassware and organic solvents. In addition, there is growing concern about the amount of hazardous waste produced and solvents used in laboratories [17]. Solid-phase extraction (SPE) on different adsorbents has gained interest in recent years because its good reproducibility, the fact that it eliminates the need for solvents, as well as its rapidity, versatility, economy, and automation. Different types of sorbents, in particular florisil, alumina and silica [18, 19], as well as tandem alumina/silica [20, 21], have been employed to separate OCPs from all kinds of matrices. In particular, florisil has often been recommended for the purification of vegetable foods, but it is not always adequate for this task [22]. Recently, increased attention has been devoted to carbon systems [23–25].
The goal of the present work was to develop and apply a rapid and reliable multiresidue method. Sample preconditioning was performed by optimizing several parameters, including the SPE absorbent used (florisil, silica and carbon), the elution solvent and the volume of elution. The OCPs were determined using GC–ECD.
Material and method
Materials and chemicals
OCP standards [aldrin; HCH (α-, β-, γ-, δ-); 4,4′-DDD; 4,4′-DDE; 4,4′-DDT; aldrin, dieldrin; endosulfan (I, II), endosulfan sulfate; endrin; endrin aldehyde; heptachlor; heptachlor epoxide; methoxychlor] were purchased from ULTRA Scientific, Inc. (North Kingstown, RI, USA). Acetone, n-hexane 95%, dichloromethane, benzene, acetonitrile, methanol and ethyl acetate were obtained from JT Baker SOLUSORE® (Phillipsburg, NJ, USA).
Stock solutions of OCPs were prepared in hexane at concentrations of 1 and 10 mg/L and stored at 4 °C in a refrigerator. A working standard solution was created by adding analytes to a pesticide-free propolis sample to achieve concentrations of 0.005∼0.1 mg/L for each pesticide.
Instruments
A Hewlett Packard (Palo Alto, CA, USA) 6890 GC equipped with an HP7683 autosampler, an ECD system and 30 m × 0.25 mm × 0.25 μm (DM-5ms) capillary columns from Dikma (Richmond Hill, NY, USA) were used. The SPE study was performed in an off-line mode using various solid-phase extraction cartridges, including Cleanert florisil SPE tubes 6 mL (1 g), Cleanert silica SPE tubes 6mL (1 g), and Cleanert PestiCarb SPE tubes 6 mL (1 g), supplied by Agela Technologies (Newark, DE, USA).
Sample preparation
Four propolis samples were collected at random from different beekeepers. The propolis was broken into pieces, successively ground into powder, and then kept at 4 °C in a refrigerator. The voucher specimens were stored in the herbarium at the Bee Product Quality Supervision and Testing Center, Bee Research Institute, Chinese Academy of Agricultural Sciences.
Extraction and clean-up
Propolis samples (1 g) were weighed into an extraction vessel and extracted with 30 mL of hexane and acetone (1:1, v/v) by vortexer for about 5 min. The nearly dissolved organic combination was centrifuged at 5000 rpm for 5 min, and then 10 mL of the clear supernatant were transferred into a conical flask and concentrated to 0.5∼1.0 mL on a rotary evaporator below 50 °C.
Three kinds of SPE cartridges (1 g, 6 mL), including florisil, silica gel and graphitized carbon, were investigated in our study. The florisil cartridge was coupled behind a graphitized carbon cartridge with an adaptor. The single or double columns were successively rinsed with 6 ml of elution solvent and 6 mL of acetone and hexane (1:1, v/v). Hexane and ethyl acetate (1:1, v/v), hexane and dichloromethane (3:7, v/v), and ethyl acetate and hexane (2:8, v/v) were tested as elution solvents in our research. Then the cartridges were loaded with concentrated extract and the pesticides were eluted with 6 mL of elution solvent. Finally, the eluates were collected in tubes and dried under a gentle nitrogen stream. Once they had been redissolved in hexane, the solutions were filtered through a syringe 0.45 μm PTFE filter and determined by GC-ECD.
Analysis and statistics
Nitrogen was the carrier gas, flowing at 1.0 mL/min through the column, and 20 mL/min makeup gas (99.999%). The temperature of the injector, operating in splitless mode (volume injected 1 μL), was held at 260 °C, and the electron capture detector temperature was 290 °C. The oven temperature was programmed from 100 °C (held for 1 min) to 230 °C (held for 5 min) at a rate of 8 °C/min, and 230 to 290 °C (held for 10 min) at a rate of 10 °C/min. Figure 1 shows a chromatogram of a standard solution containing of 0.1 mg/L of each pesticide injected under these chromatographic conditions.
GC-ECD chromatogram of a standard solution with 0.1 mg/L of OCPs. Target compounds are numbered as follows: (1) α-HCH; (2) β-HCH; (3) γ-HCH; (4) δ-HCH; (5) heptachlor; (6) aldrin; (7) heptachlor exo-epoxide; (8) endosulfan I; (9) 4,4′-DDE; (10) dieldrin; (11) endrin; (12) endosulfan II; (13) 4,4′-DDD; (14) endrin aldehyde; (15) endosulfan sulfate; (16) 4,4′-DDT; (17) methoxychlor
The linearity of the calibration curve was determined by linear regression analysis of the concentration versus response curve. Recovery experiments were performed by spiking propolis samples with all the pesticide standards at four concentration levels to derive the efficiency of extraction and investigate the analytical procedure. LODs and LOQs were determined as the analyte concentrations in the spiked propolis that produced a chromatographic peak with a height equal to three times and ten times the noise of a blank propolis.
Results and discussion
Method development
In multiresidue methods, the extracting solvent has to be suitable for the extraction of compounds covering a wide polarity range, and it should be able to thoroughly disintegrate the matrix in a high-speed homogenizer. The most widely used solvents are acetone and acetonitrile. Consequently, a 1:1 mixture of acetone and hexane was chosen for use in our study, as employed in other scientific works [26]. Since propolis has a complex, SPE is frequently recommended as a clean-up step to diminish the levels of interferents and also to avoid damaging the capillary column. The solvent and sorbent are optimized, which can affect the retention and elution. Preliminary experiments were carried out using a single solvent as elution solvent, but the results were unsatisfactory. Most adsorbent columns provide good clean-up only when they are eluted with solvent mixtures of low polarity, thus eluting less polar residues and leaving more polar coextractives in the column [15]. Therefore, we chose to investigate various binary mixtures in our research, based on those used in previous literature [27–29], including hexane and ethyl acetate (1:1, v/v), hexane and dichloromethane (3:7, v/v), and ethyl acetate and hexane (2:8, v/v). Although good recoveries were obtained with all three mixtures, the first two eluted many endogenous substances from the extraction. This may be because the higher the solvent polarity, the more interferents there are and the lower the clean-up efficiency [30]. Therefore, the mixture of ethyl acetate and hexane (2:8, v/v) was chosen as the elution solvent, in accordance with the results of previous studies [31].
Most OCP residues in plant materials include a clean-up step using adsorption columns, in particular those with florisil, silica and graphitized carbon [32, 33], and we also investigated these sorbents for preconditioning purposes. The absorption and elution capacities of different adsorbents were tested by spiking with 1 mL of standard solution containing 0.1 mg/L of each pesticide, and the elution system and analysis were applied as described in the previous section. The results obtained for each adsorbent are shown in Table 1, which shows the mean recoveries and relative standard deviations (n = 4). Satisfactory recoveries and relative standard deviations were obtained for all procedures. All of the adsorbents were also used to clean up the propolis samples. We found that no sorbent completely eliminated matrix interference, but each had respective advantages in relation to suppressing different kinds of interfering compounds. Moreover, florisil and silica gave colored eluates. Thereby, we attempted to use tandem carbon and florisil cartridges to purify the matrix based on the above research. Figure 2 shows GC–ECD chromatograms corresponding to blank propolis extracts purified with the adsorbents considered. It can be seen that the clean-up efficiencies of the absorbents can be ranked as follows: silica≈ florisil < graphitized carbon < tandem graphitized carbon and florisil. Furthermore, the graphitized carbon sorbent, which has a strong affinity for planar molecules, can remove pigments (e.g., chlorophyll and carotenoids) and sterols [34]. To investigate the volume of elution solvent required, five fractions of 2 mL of elution solvent were collected individually. The assay data indicated that 6 mL are enough to quantitatively elute the analytes. Thus, the graphitized carbon cartridge coupled with the florisil cartridge was selected as the purification method, along with 6 mL of ethyl acetate and hexane (2:8, v/v) as elution solvent.
The results obtained using the proposed method for determining OCPs in propolis by GC–ECD improve upon those given by a previously published method for the analysis of pesticides in plant material and bee products by chromatography [35, 36]. Figure 3 shows GC–ECD chromatograms of propolis extracts for an unspiked sample and a spiked sample.
Remarks about the method
When standards were prepared by spiking blank propolis extracts with known amounts of pesticides, higher peak areas were obtained for the same pesticide concentrations. Large differences were also observed in the detector response between the calibration graphs obtained with standard solutions of the OCPs and those obtained with spiked propolis extract [37, 38]. A similar matrix effect in the determination of pesticides has been previously reported for honey and other foodstuffs. Therefore, the linearity of the chromatographic method was determined using propolis extracts spiked at six different levels. The response for all pesticides was linear, with correlation coefficients in the range 0.9961∼0.9997. Table 2 summarizes the calibration data, the LODs and the LOQs for the studied pesticides. LODs varied between 0.8 μg/kg for 4,4′-DDE and 11.4 μg/kg for endosulfan II. LOQs ranged from 2.6 to 38.1 μg/kg.
Precision and recovery results obtained at four concentrations are shown in Table 3. Recoveries at the LODs ranged from 71.5% to 109.6%. The recovery decreases as the fortified concentration increases. Average recoveries for the other concentrations varied between 62.6% and 102.9%. The uncertainties of the recoveries, reported as RSD% (precision), varied between 0.8% and 9.4%.
Four propolis samples were analyzed in duplicate and the mean results recorded. Three samples were found to be contaminated with different OCP residues. Sample A was polluted with dieldrin at the level of 18.8 μg/kg. The residues of sample B contained 3.92 μg/kg of β-HCH and 5.05 μg/kg of 4, 4′-DDE. There were no OCPs in sample C, but three kinds of pesticides were detected in sample D, including δ-HCH, heptachlor and 4,4′-DDE, at concentrations of 17.4, 2.7 and 31.0 μg/kg, respectively.
Conclusion
A procedure for the analysis of OCPs in propolis samples based on double column series SPE was developed. A tandem florisil and carbon cartridge along with 6 mL of ethyl acetate and hexane (2:8, v/v) as the eluting solvent, which was capable of suppressing the matrix interference and providing colorless eluates, was found to be the most efficient preconditioning system for determining OCPs from propolis extracts. Recoveries obtained were satisfactory at four concentrations (LOD and three spike concentrations) in the propolis extracts. The LODs and LOQs of the method were 0.8∼11.4 μg/kg and 2.6∼38.1 μg/kg, respectively, which were also in accordance with the results of current studies. Analysis showed that real propolis samples were possibly polluted by OCPs to some degree.
References
Ludovic T, Tom H, Pierrette B, Li YF (2006) Atmos Environ 40:1563–1578
Subir KN, Mukesh KR (2008) Bull Environ Contam Toxicol l80:5–9
Garbarino JR, Snyder CE, Leiker TJ, Hoffman GL (2002) Water Air Soil Pollut 139:183–214
Hung H, Halsall CJ, Blanchard P, Li HH, Fellin P, Stern G, Rosenberg B (2002) Environ Sci Technol 36:862–868
Gouin T, Mackay D, Jones KC, Harner T, Meijer SN (2004) Environ Pollut 128:139–148
Shen L, Wania F, Lei YD, Muir DCG, Bidleman TF (2005) Environ Sci Technol 39:409–420
Muniategui LS, Barriada PM, Gonzalez CMJ, Lopez MP, Prada RD, Fernandez FE (2002) In: Euroanalysis Conf 12, Dortmund, Germany, 8–13 Sept 2002
Sibel S, Semiramis K (2005) J Ethnopharmacol 99:69–73
Herrera A, Perez AC, Conchello P, Bayarri S, Lazaro R, Yague C, Arino A (2005) Anal Bioanal Chem 381:695–701
EC (1998) Informal coordination of MRLs established in Directives 76/895/EEC, 86/362/EEC, 86/363/EEC, and 90/642/EEC (5058VI98). European Commission, Brussels
EC (1974) Directive 74/409/EEC of 22 July 1974 on the harmonization of the laws of the Member States relating to honey. European Commission, Brussels
Korta E, Bakkali A, Berrueta LA, Gallo B, Vicente F, Bogdanov S (2003) Anal Chim Acta 475:97–103
Lenicek J, Sekyra M, Rychtecka Novotna A, Vasova E, Titera D, Vesely V (2006) Anal Chim Acta 571:40–44
Santana dos Santos TF, Aquino A, Dorea HS, Navickiene S (2008) Anal Bioanal Chem 390:1425–1430
Tekel J, Hatrik S (1996) J Chromatogr A 754:397–410
Atac U, Kadriye S, Ozant O, Dilsah C, Omur G, Bekir S (2005) Microbiol Res 160:89–195
Pico Y, Fernandez M, Ruiz MJ, Font G (2007) J Biochem Biophys Methods 70:117–131
Tuinstra L, Roos AH, Griepink B, Maier E, Fresenius A (1997) J Anal Chem 357:1035–1041
Easton MDL, Luszniak D, Vonder GE (2002) Chemosphere 46:1053–1074
Jacobs MN, Covaci A, Schepens P (2002) Environ Sci Technol 36:2797–2805
Rodil R, Carro AM, Lorenzo RA, Cela Torrijos R (2005) Anal Chem 77:2259–2265
Schenck FJ, Calderon L, Saudarg DE (1996) J AOAC Int 79:1454–1458
Sheridan RS, Meola JR (1999) J AOAC Int 82:982–990
Adou K, Bontoyan WR, Sweeney PJ (2001) J Agric Food Chem 49:4153–4160
Stajnbaher D, Zupancic KL (2003) J Chromatogr A 1015:185–198
Sandra RR, Mario SG, Fatima RNK, Bernhard MA (2004) J Chromatogr A 1048:153–159
Beatriz A, Consuelo SB, Josea LT (2004) J Agric Food Chem 52:5828–5835
Jimenez JJ, Bernal JL, Nozal MJ, Alonso C (2004) J Chromatogr A 1048:89–97
Serpil YK (2006) Anal Chim Acta 571:298–307
Barriada PM, Concha GE, Gonzalez CMJ, Muniategui LS, Lopez MP, Prada RD, Fernandez FE (2004) J Chromatogr A 1061:133–139
Barriada PM, Concha GE, González CMJ, Muniategui LS, Lopez MP, Prada RD, Fernandez FE (2003) J Chromatogr A 1008:115–122
Zawiyah S, Yaakob BCM, Nazimah SAH, Chin CK (2006) J Chromatogr A 1127:254–261
Jansson C (2000) J AOAC Int 83:714–719
Iglesias GI, Barriada PM, Gonzalez CMJ, Muniategui LS, Lopez MP, Prada RD (2008) Anal Bioanal Chem 391:745–752
Blascob C, Linoa CM, Picob Y, Penaa A, Fontb G, Silveiraa MIN (2004) J Chromatogr A 1049:155–160
Beceiro GE, Concha GE, Guimaraes A, Goncalves C, Lorenzo SM, Alpendurada MF (2007) J Chromatogr A 1141:165–173
Jones A, McCoy C (1997) J Agric Food Chem 45:2143–2147
Fillion J, Sauve F, Selwyn J (2000) J AOAC Int 83:698–713
Acknowledgements
This work was financially supported by Science and Technology Department’s 11th Five-Year State Plan for Science and Technology Support, sub-projects (2006BAD06B04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, F., Chen, L., Wang, Q. et al. Determination of organochlorine pesticides in propolis by gas chromatography–electron capture detection using double column series solid-phase extraction. Anal Bioanal Chem 393, 1073–1079 (2009). https://doi.org/10.1007/s00216-008-2474-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2474-1