Abstract
The principle and applications of stable isotope dilution assays (SIDAs) in mycotoxin analysis are critically reviewed. The general section includes historical aspects of SIDAs, the prerequisites and limitations of the use of stable isotopically labelled internal standards, and possible calibration procedures. In the application section actual SIDAs for the analysis of trichothecenes, zearalenone, fumonisins, patulin, and ochratoxin A are presented. The syntheses and availability of labelled mycotoxins for use as internal standards is reviewed and specific advances in food analysis and toxicology are demonstrated. The review indicates that LC–MS applications, in particular, require the use of stable isotopically labelled standards to compensate for losses during clean-up and for discrimination due to ion suppression. As the commercial availability of these compounds continues to increase, SIDAs can be expected to find expanding use in mycotoxin analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Principle of stable isotope dilution assays
The roots of stable isotope dilution assays (SIDAs) date back to the beginning of the 20th century when Soddy [1] discovered the existence of isotopes and George Hevesy used radioactive isotopes to determine the content of lead in rocks and the solubility of lead salts in water [2]. Most elements have a natural distribution of both stable and radioactive isotopes. For example, natural carbon consists of C-12 (98%), of C-13 (1.1%), and radioactive C-14. For organic molecules of known empirical formula the natural isotopic distribution of the entire compound can be calculated from the relative frequency of the individual elements.
Although very similar in their properties, isotopes can be enriched or depleted due to their different masses leading to alteration of the natural values. If an element or compound showing a natural isotopic distribution is mixed with such an isotopically different material (Fig. 1), the naturally abundant isotopes are diluted in the resulting material; this is the origin of the term “dilution” in SIDA. The principle of SIDA is comprehensively sketched in Fig. 2. After addition of the labelled standard and its equilibration with the analyte, the ratio of the isotopologues is stable because of their nearly identical chemical and physical properties. Final mass spectrometry allows differentiation between the isotopologues and, with the known amount of the internal standard, the content of the analyte can be calculated. In contrast to this, a structurally different internal standard may be discriminated against and, therefore, cause systematic errors and imprecision. In other words, losses of the analyte are completely compensated for by identical losses of the isotopologue whereas a structurally different IS is likely to show different losses and is, therefore, less suited.
Principle of a stable isotope dilution assay. After addition of an isotopologic standard to the analyte and equilibration the isotopic ratio remains stable until final mass spectral analysis. For a structurally different internal standard, however, the ratio between standard and analyte can alter during sample preparation resulting in systematic errors
Benefits and limitations of using an isotopologic internal standard
As shown above, the ideal compensation for losses renders SIDA a perfect tool for a series of analytical applications. Generally, trace compounds are a typical target for SIDAs. Trace analyses often require tedious clean-up procedures due to matrix interferences, which usually causes losses of the analyte. When structurally different internal standards are used, additional recovery and spiking experiments are necessary, which further increase the work-load. But often recoveries are not reproducible, which is an additional cause of imprecision. In all these cases, SIDA offers significant benefits.
Apart from rendering quantitative results accurately by compensation for losses, the use of an isotopologic standard enhances the specificity of the determination. In addition to the specific mass spectrometric information on the analyte the coeluting internal standard shows analogous signals shifted by the introduced mass increment. Therefore, the chromatogram of a SIDA showing the coeluting peaks in the respective mass traces ensures the correct assignment of the analyte (Fig. 3).
The major prerequisites for analyzing a given mycotoxin by SIDA are the availability of a GC–MS or LC–MS combination and of the labelled internal standard. The former are basic equipment of a laboratory nowadays; the latter, however, imposes the biggest obstacle to wider use of SIDA. Of all mycotoxins discovered to date, about 25 have been synthesized as labelled analogues, but only 11 of these are commercially available. The consequence is that the compound of interest often has to be synthesized. A major psychological hindrance that prevents analysts from synthesizing labelled mycotoxins is the price of labelled educts. However, this is not a convincing argument as can be explained by the following example: one gram of a labelled educt may cost around €1000 and the yield of the synthesis may be 10%, both of which are realistic figures. The price for 100 mg of the labelled product is therefore €1000. Because for a SIDA only 1 to 10 μg of the labelled standard is required, 100 mg of the standard enables at least 10000 analyses to be performed. Hence the material cost of using a labelled standard would be approximately €0.10 per sample, which is negligible compared to the cost of labour and equipment.
Prerequisites for isotopologic standards
As mentioned above, the basic principle of SIDA is to transfer the concentration of the analyte into an isotopologue ratio, which has to be stable during successive analytical steps. Stable labelling is, therefore, the first prerequisite for an internal standard. As carbon–carbon and carbon–nitrogen bonds are very unlikely to be cleaved, labels consisting of [13C] or [15N] are considered to be very stable. In contrast, losses of [18O] or [2H] can occur if these labels are at labile positions. On the one hand, [18O] in carboxyl moieties can be exchanged in acid or basic solutions. This was shown by Boni et al. [4], who observed loss of the [18O] label in tryptophan heated in aqueous solutions. On the other hand, deuterium is susceptible to so-called protium–deuterium exchange if it is activated by adjacent carbonyl groups or aromatic systems. Consequently, mycotoxins containing only labile hydrogen atoms, such as patulin, are not suitable as internal standards when carrying deuterium labels.
Another labile labelling position is the trimethylsilyl (TMS) group, as demonstrated by Price [5], who added [2H9]-TMS-patulin as an internal standard for patulin to the extract of apple juice. During the derivatization procedure the [2H9]-TMS groups of the internal standard and the [1H9]-TMS groups of the silylating agent exchanged and, therefore, made exact quantification impossible.
Another difficulty in maintaining a stable isotopologue ratio arises from small differences in physical or chemical properties of the isotopologues, commonly referred to as isotope effects (IE). IE arise because of different energy contents caused by the mass differences of the isotopes. IE are mainly observable for hydrogen, as the mass difference between [1H] and [2H] is proportionally much higher than that between [13C] and [12C] or [15N] and [14N]. The well-known and frequently occurring IE affect chromatographic behaviour and result in different retention times of isotopologues in general.
In order to prevent chromatographic separations of the isotopologues during clean-up and thus changes in the isotopologue ratio, isotope effects have to be minimized either by choosing labelling with [13C] or [15N] or by introducing only the necessary number of [2H].
As quantification by mass spectrometry is intended, the standard has to be unequivocally distinguishable from the analyte. This requires the presence of signals either of the molecular ion or of fragments showing the mass increment introduced by the labels. Loss of the label from the detected signals must therefore be avoided.
Another problem in isotopologue differentiation arises from spectral overlap between standard and analyte. In case of the analyte the natural abundance of isotopes, in particular of [13C], [18O], or [34S], results in isotope clusters of each fragment showing not only the nominal mass mA, but also to a lesser extent mA + 1, mA + 2, or even higher masses. In particular, [13C] in compounds containing a large number of carbon atoms causes a significant abundance of m + 1 and m + 2 due to relatively high natural abundance. To avoid overlap of the signals of those natural isotopologues with mS of the standard, the mass difference must therefore be sufficient. For mycotoxins, which have carbon numbers usually ranging from 10 to 20, a mass increment of at least three units generally meets this requirement. However, the number of labels, especially of deuterium, should not be too high, in order to minimize the mentioned chromatographic isotope effect.
In contrast , signals of the labelled material falling on the signals of the analyte may also result in spectral overlap. This occurs if the labelling is not complete because of the low isotopic purity of labelled educts or because of incomplete labelling in synthetic steps, e.g. during deuterations of aromatic systems.
If spectral overlap cannot be avoided, calculation procedures have been developed to enable quantification after all. However, these procedures are the more complicated the more pronounced the overlap is.
Another important prerequisite for accurate quantification is the need for equilibration between analyte and standard in the sample to be extracted. As the labelled standard is added to the sample as a solution either in the extracting solvent or directly to the matrix itself, it will probably be recovered to a large extent during the extraction procedure. For the analyte, this might not be true, as it can be trapped in compartments of the matrix and be less extractable by the solvent. Therefore, a suitable time has to be chosen to enable equilibration of standard and analyte in all parts of the sample and to ensure that analyte and standard show the same concentration ratio in all compartments as far as possible.
Calibration procedures
The amount of analyte present in the sample appears as the isotopologue ratio, which can be calculated from the intensity ratio of suitable ions measured by mass spectrometry. The relationship between isotopologue ratio and intensity ratio has to be established by a series of calibration experiments that consist of analysing the intensity ratios of defined standard analyte mixtures. If there is no spectral overlap, the calibration function is supposed to be linear. However, usually there are still unlabelled analytes in the labelled material and small intensities of natural isotopologues of the analyte falling at the signals of the standard. Therefore, the calibration function is linear only in a restricted region, which is outlined in Fig. 4. Under the supposition that the standard contains 2% unlabelled material und natural isotopologues contribute 5% intensity to the signal of the standard, the calibration function can only be assumed to be a straight line for molar ratios of analyte to standard ranging between 0.2 and 5. When excess of the standard or analyte is present the function is governed by the unlabelled residues in the standard or by the natural isotopologues of the analyte, respectively.
Occasionally, however, spectral overlap cannot be avoided and, therefore, suitable procedures for calculation are required. In general, there are several ways to solve this problem: for example, hyperbolic or polynomial models have been elaborated [6, 7] which approximate the real calibration relationship by use of a mathematic function. As these procedures are rather complicated, several authors have proposed linearization methods which transform the non-linear function into a linear one [8]. An isotopologic mycotoxin with high spectral overlap recently has been introduced by Bretz et al. [9], who demonstrated that the overlap in case of [2H1]-deoxynivalenol can be reduced by monitoring a specific MS fragmentation.
Applications of stable isotope dilution assays to mycotoxins
Fusarium toxins
The anamorphic genus Fusarium is a large group of moulds consisting of about 145 different species [10] which are a hazard to food safety because many of these fungi are plant pathogens and infect crops used for human nutrition. Their capability of producing mycotoxins, especially trichothecenes, zearalenones, and fumonisins, has been associated with severe food-borne and feed-borne intoxication or poisoning in the past. In general, Fusarium toxins are basically produced in the field during growth of the plant, but unsuitable storage conditions, including high moisture content, can also lead to severe mycotoxin accumulation [11].
Trichothecenes
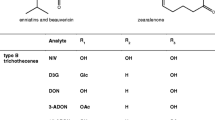
Trichothecenes are a group of tetracyclic sesquiterpenoids that, in general, share an epoxide functionality at C-12 and C-13 and a double bond at C-9 and C-10. The great diversity of the trichothecene family is due to both the different numbers and different positions of hydroxyl moieties on the basic trichothecene nucleus. As esterification of the hydroxyl groups is also possible with a number of different corresponding acids, over 170 trichothecenes have so far been isolated from fungal cultures. To keep track of this large group of compounds the trichothecenes are classified into four groups, A–D [12]. Whereas type C and the macrocyclic type D are of minor importance in food commodities, type A and type B trichothecenes are regularly found in cereal products [13]. The most common members of these two groups are shown in Fig. 5.
Type A trichothecenes
As early as the late 1980s 3-[2H3]-acetyl-T2-toxin and 15-[2H3]-HT2-toxin were prepared chemically and used as internal standards for the determination of both toxins in human blood and animal urine samples [14]. Extracts were derivatized with trifluoroacetic acid anhydride to enable gas chromatographic separation before tandem mass spectrometric detection. Recovery of both components from blood samples was about 90% and the limit of detection (LOD) about 0.5 μg kg−1. Though the latter authors mentioned that the method using stable isotope labelled standards is superior to that with external calibration, no further use of isotopically labelled internal standards has been reported for type A trichothecenes either in physiological studies or in food analysis for a long time.
Because gas chromatographic analysis of trichothecenes is hampered by the inevitable derivatisation step and severe matrix interferences, alternative methods were needed for trace analysis and, as a consequence, liquid chromatography–mass spectrometry increasingly comes to the fore. One of the first methods for the simultaneous quantification of type A trichothecenes with single stage LC–MS [15] used deuterated T2-toxin, which was commercially available at that time, as internal standard. Recovery of T2-Toxin was only 77.4%, but was 90% for HT2-toxin, 86% for diacetoxyscirpenol, and 95% for monoacetoxyscirpenol. Limits of quantification (LOQ) were 50 μg kg−1 for T2-toxin and diacetoxyscirpenol, 60 μg kg−1 for monoacetoxyscirpenol and 85 μg kg−1 for HT2-toxin.
Recently these recoveries were further improved by using carbon-13 labelled standards for T2-toxin, HT2-toxin, diacetoxyscirpenol, and monoacetoxyscirpenol, and LC with tandem MS detection [16]. With these standards recoveries of about 112–127% for T2-toxin, 93–107% for HT2-toxin, 90–91% for diacetoxyscirpenol, and 109–112% for monoacetoxyscirpenol could be achieved, depending on the spiking level. LODs and LOQs were determined using a statistical approach including addition of the toxins to suitable matrices [17]. These studies resulted in LODs of 3 μg kg−1 (T2-toxin), 30 μg kg−1 (HT2-toxin), 1 μg kg−1 (diacetoxyscirpenol), and 30 μg kg−1 (monoacetoxyscirpenol) and in LOQs of 8 μg kg−1 (T2-toxin), 80 μg kg−1 (HT2-toxin), 4 μg kg−1 (diacetoxyscirpenol), and 80 μg kg−1 (monoacetoxyscirpenol).
Type B trichothecenes
Successful development of a stable isotope dilution assay for the most prevalent type B trichothecene, deoxynivalenol, was hindered for a long time by the rather complicated molecular structure of this mycotoxin, which resisted chemical attempts to synthesize isotopically labelled derivatives. Only recently [2H1]-deoxynivalenol was synthesized and used in stable isotope dilution assays [9], together with [2H3]-3-acetyldeoxynivalenol that had previously been synthesized by the same group [18]. With this method recovery of deoxynivalenol was between 99 and 103%, and between 87 and 96% for 3-acetyldeoxynivalenol using LC–MS–MS. LOQs and LODs were not determined. But the use of an internal standard which differs by only one mass unit from the analyte leads to spectral overlap because of the natural isotopic pattern. As already stated in the introduction, special requirements are necessary both in terms of instrumental equipment and mathematical calculations for use of this stable isotope-labelled standard.
Broader application found a commercial fully carbon-13-labelled internal standard of deoxynivalenol. Without clean-up recovery of deoxynivalenol was 95 ± 3% for wheat and 99 ± 3 for maize, with a lower LOQ of 8 μg L−1 [19]. When clean-up with Mycosep 225 columns was performed, recovery of 101 ± 2.4% was achieved for maize whereas external calibration only results in 76 ± 1.9% [20]. Both studies were performed using LC–MS–MS.
The commercial carbon-13-labelled standard for deoxynivalenol was also used in the first comprehensive stable isotope dilution LC–MS–MS assay for type B trichothecenes, with carbon-13-labelled 3-acetyldeoxynivalenol, 15-acetyldeoxinvalenol, and 4-acetylnivalenol (fusarenon X), all of which were synthesized by the authors of this review [21]. Recovery was determined at different contamination levels and ranged between 97 and 100% for deoxynivalenol, between 95 and 105% for 3-acetyldeoxynivalenol, between 100 and 115% for 15-acetyldeoxynivalenol, and between 94 and 101% for 4-acetylnivalenol. The LODs were 2 μg kg−1 for deoxynivalenol, 5 μg kg−1 for 3-acetyldeoxynivalenol, 4 μg kg−1 for 15-acetyldeoxynivalenol, and 1 μg kg−1 for 4-acetylnivalenol. The LOQs were 6 μg kg−1 for deoxynivalenol, 16 μg kg−1 for 3-acetyldeoxynivalenol, 11 μg kg−1 for 15-acetyldeoxynivalenol, and 4 μg kg−1 for 4-acetylnivalenol. A summary of performance data for the before mentioned SIDAs is presented in Table 1.
Zearalenone
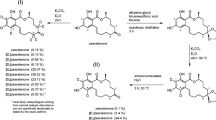
Some Fusarium species, for example F. graminearum and F. culmorum, produce a series of macrocyclic resorcylic acid lactones (Fig. 6), of which only zearalenone is regularly found in naturally contaminated cereal grain [13]. All members of this group have characteristic estrogenic effects that lead to severe problems in stock farming, especially infertility of swine when fed over a long period [22]. The possibility of labelling zearalenone by proton–deuterium exchange is well known, but at first was only used for NMR and conformation studies [23] and for research on the metabolic pathways of zearalenone in animals [24]. Labelled zearalanone synthesized by catalytic deuteration of zearalenone was used as internal standard for detection of zearalenone metabolites in several feeding trials [25, 26], as also were other labelled commercially available deuterated zearalenone metabolites [27].
The first stable isotope dilution assay (SIDA) for the determination of zearalenon and its metabolites in river water was recently reported [28]. As proton–deuterium exchange is very readily performed with zearalenone the stability of the label under varying and difficult conditions in food extracts may be critical and so far no stable isotope dilution assay for food commodities has been developed.
Instead, a new technique called alternate isotope-coded derivatization assay (AIDA) has been reported for determination of zearalenone as a possible alternative to SIDA, if no labelled standards are available [29]. In this approach both labelled and unlabelled derivatized internal standards were prepared from stock solutions using a labelled and an unlabelled derivatization reagent, respectively. Samples were split into two parts. One part was spiked with the labelled derivatized internal standard, extracted, and derivatized with the unlabelled derivatization reagent before LC–MS analysis. The other part was spiked with the unlabelled derivatized internal standard and subsequently derivatized with the labelled derivatization reagent. Thereby, two independent quantitations are performed in which labelled and unlabelled derivatized analytes are used as alternative internal standards. In this way, both quantitations can be used to confirm one another. Using the described AIDA for the quantitation of zearalenone in maize flour the LOD was 5 μg kg−1 and the LOQ 10 μg kg−1. Analysis of certified reference material resulted in an average recovery of 110%. Hence, this technique seems to have promise, although it can only be used with analytes that are readily derivatized. Furthermore losses during sample preparation prior to derivatization are not compensated with these internal standards.
Fumonisins
Few Fusarium species, especially F. verticillioides (formerly known as F. moniliforme) and F. proliferatum, are able to produce fumonisins, a series of long-chained polyhydroxylamines structurally related to sphingosine (Fig. 7). Discovery of fumonisins B1 and B2 resulted from intensive research on fungal isolates from mouldy corn after severe intoxication of farm animals in South Africa [30]. Concerning human health, fumonisins were reported to disrupt sphingolipid metabolism and to putatively enhance the risk of oesophageal cancer [31].
It soon was noticed that feeding liquid cultures of F. verticillioides with [2H3]-methyl l-methionine resulted in highly efficient incorporation of the label into fumonisin B1. As the label was found to occur only in the methyl groups at C-12 and C-16 a mixture of [2H6]-FB1 (90%) and [2H3]-FB1 (9%) was obtained [32]. SIDAs were subsequently developed to determine FB1 in corn and corn products based on GC–MS and FAB–MS measurement. Using hydrolysis and TFA derivatisation to enable gas chromatography, a limit of detection of 10 μg kg−1 for FB1 was achieved. In contrast with this, FAB–MS after strong anion-exchange (SAX) clean-up could be applied at contamination levels greater than 100 μg kg−1. Recovery was variable and incomplete when no internal standard was used, but 100% when [2H6]-FB1 was applied [40]. The following years saw the development of LC–MS and LC–MS–MS methods for determination of FB1 and FB2 in corn and corn products [33, 34], FB1 and hydrolyzed FB1 in corn products [35], N-(carboxymethyl)-FB1 in corn products [36], and FB1 in asparagus spears and garlic bulbs [37, 38]. All these methods were based on use of [2H6]-FB1. Detailed information about recovery and LODs and LOQs can be found in Table 2.
Patulin
Patulin (4-hydroxy-4H-furo[3, 2]pyran-2(6H)-one; PAT) is generated by different species of Penicillium, Aspergillus, and Byssochlamys. The most widespread among these fungi, Penicillium expansum, is known to invade fruits, berries, vegetables, bread, and meat products. Because PAT is reputed to be acutely toxic, teratogenic [39], and possibly cancerogenic [40] mould on fruit and bread is a serious problem for the whole food chain.
Standard methods in patulin analysis
The method most frequently used to quantify PAT is high-performance liquid chromatography (HPLC) coupled to UV-detection [41]. Because of its low specificity, this method is highly susceptible to matrix interferences. Therefore, LC coupled to MS is becoming increasingly popular in PAT analysis. Three LC–MS applications including external calibration have been reported and have been used to quantify PAT in apple products [42–44]. These methods used detection of PAT in negative-ion mode, either with atmospheric pressure chemical ionization (APCI) or atmospheric pressure photo ionization (APPI). However, the authors admitted that isotopically labelled standards would improve the accuracy of their analytical methods.
Gas chromatographic (GC) procedures that determined PAT as the acetate [45], trimethylsilyl(TMS)ether [46], or its heptafluorobutanoic ester [47] did not attract much interest, because of incomplete derivatization and the lack of a suitable internal standard.
Stable isotope dilution analysis
To overcome the aforementioned constraints, [13C2]-PAT as the first isotopologic IS was synthesized in a six-step preparation [48] starting from l-(+)-arabinose. For a SIDA based on GC–MS detection, the volatility of PAT was increased by trimethylsilylation. The mass spectra of TMS-[13C2]-PAT and TMS-PAT obtained in electron-impact (EI) mode enabled differentiation of the isotopomers by monitoring the respective molecular ions [49].
For the SIDA based on LC–MS, extraction and clean-up procedures were similar to those in the GC–MS method without the need for additional clean-up and derivatization. The ratio of PAT to its isotopologue [13C2]-PAT was determined by detecting the deprotonated molecules at m/z 153 and m/z 155, respectively; these ions were obtained in the negative electrospray ionization mode.
A second SIDA for PAT was developed using [13C3]-PAT and detection by LC–MS in negative ESI mode [50]. However, the synthesis of the standard has not been described. LOD for apple juice was reported to be 1 μg kg−1, which was low enough to monitor the PAT content of fruit products. In contrast to the first SIDA, PAT was extracted from the juice by SPE and desorbed from the solid phase with methanol.
In view of the increasing need for labelled PAT, a new isotopologue of PAT with an isotopic distribution for [13C7], [13C6], [13C5], and [13C0] of 29%, 3%, 68%, and 0%, respectively, has been generated and commercialized [51]. This IS is particularly useful for LC–MS and its application being increasingly used for PAT analysis compared with LC–MS methods without IS.
Patulin concentration in different food commodities
Application of the GC–MS stable isotope dilution assay to commercial and home-made fruit products gave results ranging from below 0.02 to 26 μg kg−1 [52]. The SIDA reported by Ito et al. was applied only to apple juices and showed contents to range from below 1 μg kg−1 to 45.6 mg kg−1 [50].
Besides fruit, many other foods are ideal substrates for Penicillium moulds, e.g. baked products. These kind of sample require more sophisticated clean-up procedures and, therefore, the use of a labelled IS offers specific advantages. In this respect, [13C2]-PAT was used to quantify PAT in wheat bread that either became spontaneously mouldy or was infected with pure cultures of Penicillium expansum. Both types of sample revealed PAT contents of up to 21 mg kg−1 [49].
The new SIDA was also applied in research into possible PAT contamination in mould-ripened products such as sausages and cheese. Four samples of camembert-like cheese and five samples of mould-ripened sausages were analyzed, but PAT could not be detected.
Application of SIDA in toxicological studies
Although PAT was discovered over 60 years ago, its toxicology is not yet fully understood. In feeding trials its acute and chronic toxicity, teratogenicity, and immunotoxicity has been proven. It has, moreover, been shown to be a potent carcinogen after subcutaneous application, whereas orally given PAT produced no tumours [52]. To date, a final risk evaluation lacks data on resorption, metabolism, and detoxification, which were not available because of the lack of analytical methods for quantification of PAT in clinical samples. To open new inroads to a better understanding, the newly developed SIDA was used for this purpose.
To study the gastric absorption of PAT, commercial apple juice enriched with different amounts of the mycotoxin was applied to the model of the perfused rat stomach [53]. The PAT concentrations in juices, perfusate fractions and tissues were quantified by SIDA.
After application of PAT, the mycotoxin appeared almost instantly in the vascular fractions suggesting that penetration of PAT occurs by passive diffusion. Moreover, a balance of the applied doses points to the existence of a degrading mechanism which is very effective for lower doses of the mycotoxin.
In agreement with the ex-vivo study already mentioned, a human physiological study revealed no detectable PAT in a volunteer’s serum after consumption of an apple juice (1 L) adjusted to a PAT content of 49.9 μg L−1 [54]. A possible explanation of this finding is that PAT was degraded when it encountered blood.
To study the degradation kinetics in whole blood, a small amount (7 ng) of PAT was added to freshly drawn blood. Even after a short reaction time of 2 min, however, no PAT was detectable in the blood by GC–HRMS. Only after adding a much larger amount (100 μg) of PAT the mycotoxin could be detected by GC–MS, revealing a short half-life of 21.2 s [54].
As PAT bears an α, β, γ, δ-unsaturated carbonyl moiety, it is likely to react with cellular nucleophiles. One of the most abundant nucleophile is the tripeptide glutathione (GSH), which has been shown to form adducts with PAT spontaneously [55].
When the mycotoxin was reacted with freshly drawn blood, the respective PAT-GSH adducts were detected by LC–MS and tentatively identified by LC–MS–MS and from their UV-spectra [56].
Ochratoxin A
The mycotoxin ochratoxin A (7-carboxy-5-chloro-8-hydroxy-3,4-dihydro-3R-methylisocoumarin-l-β-phenylalanine; OTA) is produced by several Penicillium (e.g. veridicatum) and Aspergillus (e.g. ochraceus) species. In particular cereals, coffee, grape products, and liquorice products are frequently contaminated with OTA because of inappropriate conditions during growth, storage, and manufacture of the raw material and subsequent invasion by these moulds.
As OTA is a potent hepatotoxin and nephrotoxin [57] and is clearly associated with a kidney disease referred to as Balkan endemic nephropathy [58], the European Union has set OTA limits for cereal products, cereals, and raisins of 3 μg kg−1, 5 μg kg−1, and 10 μg kg−1, respectively [59].
The methods most frequently used to analyze OTA in foods are HPLC with fluorescence detection (LC–FD) and enzyme-linked immunosorbent assays (ELISA) [60]. Whereas the latter are mainly suitable for screening purposes, validation studies revealed that LC–FD suffers from several constraints, for example widely differing recoveries.
For these reasons, LC–MS–MS is becoming increasingly popular in OTA analysis. The last years have seen a multitude of applications for meat, milk, grapes, wine, beer, spices, feedstuffs, and cereals [61–63]. However, the uses of labelled standards are scarce. A first study was reported by Jorgensen and Vahl [64], who used OTA [2H3]-methyl ester as the standard and included derivatization of OTA to its methyl ester. However, this assay was not a “true” SIDA because the standard was added after methylation, which included the risk of incomplete derivatization.
Application of a SIDA for OTA
A first synthetic approach to stable isotopically labelled OTA was reported by Lindenmeier et al. [65]. The route to [2H5]-OTA included hydrolyzing unlabelled OTA and subsequent coupling of the resulting isocoumarin derivative ochratoxin α (OTα) to labelled l-phenylalanine. After hydrolysis of OTA, OTα was activated by addition of thionyl chloride, which converted it into OTα chloride, which was then reacted with [2H5]-phenylalanine methyl ester.
In contrast to this, a fully [13C]-labelled OTA has been made commercially available [66].
LC–MS–MS
Separation of OTA from main interferences in food samples can be achieved on an RP-18 column. The isotopologues could be distinguished in the positive ESI mode by monitoring, by tandem MS, the ions resulting from loss of formic acid from the respective protonated molecules [65].
Sample purification
Generally, immunoaffinity clean-up is applied in OTA analysis and the respective extracts are devoid of interferences and display a well shaped and clearly separated OTA peak (Fig. 8). However, clean-up on silica cartridges in combination with LC–fluorescence detection without use of labelled standards may achieve good results if a correction for recovery is included. This was shown for raisins, wine, and nutmeg (Table 3; unpublished data).
Validation studies of the SIDA
Addition experiments revealed an LOD of 0.5 μg kg−1 and an LOQ of 1.4 μg kg−1 for OTA. These data proved the SIDAs to be sensitive enough to quantify OTA contents even below the EU limits for foods. SIDA of the reference materials revealed the OTA content of CRM 472 to be 8.0 ± 0.3 μg kg−1. Thus, the bias from the certified reference value of CRM 472 was as low as 2.1%.
Quantification of OTA in food
Quantitation of OTA using SIDA was performed for food samples in which OTA is most likely to occur, for example wheat flour, coffee, liquorice, beer, wine, and spices. Higher concentrations were found in mulled wine (n.d.–3.3 μg kg−1), nutmeg powder (1.8 μg kg−1), and raisins (n.d.–29.8 μg kg−1). Within the latter products, eight out of nine samples contained detectable contents of the mycotoxin; of these, three samples (CV of each sample less than 3.2%) exceeded the legal limit of 10 μg kg−1 [65].
Discussion of perspectives
Recent years have seen increasing use of LC–MS applications in mycotoxin analyses including multimethods that were able to detect as many as 39 different toxins within one run [67]. However, the quantitative value of these assays has to be called in question, because for each matrix separate validations including studies on precision, recovery, and trueness would have to be performed. Therefore, the use of labelled IS is becoming increasingly popular. This development is mirrored by the growing supply of commercially available labelled mycotoxins, starting with patulin and followed by trichothecenes and ochratoxin A. However, many of these standards are quite expensive, in particular the [13C]-isotopologues. In chemical syntheses of the isotopologues the effort and expense are quite manageable as detailed in the introduction section, but the analytical chemist in a routine laboratory often lacks the additional time and personnel for this work. Therefore, another objective of current research is to prepare standards in high yields to enable cheap offers. These mainly include [2H]-labels, which have to be carefully judged because loss of the label and isotope effects may pose additional problems.
As the prices for isotopologic standards still can be expected to remain quite high in the near future, multiple SIDAs will not become widely-used, because mycotoxins occur at very different concentrations and, therefore, would require high standard additions for the most abundant mycotoxins. Thus, selectively combined SIDAs rather than multiple SIDAs will become more important. In the course of this development, further objectives include not only combining the detection in one run but also developing combined extraction and clean-up procedures to enable sensitive quantification of the mycotoxins under study. To select the compounds to be quantified, exact quantification by SIDA must be preceded by effective screening methods such as already existing multimethods based on LC–MS–MS or immunochemical assays for multiple analytes. Many groups involved in mycotoxin research are concentrating their efforts on these objectives and the next few years will certainly show significant progress in this respect.
References
Soddy F (1913) Nature 92:399–400
Hevesy G, Paneth F (1913) Z Anorg Chem 82:323–328
Aston FW (1919) Nature 104:334
Boni RL, Simpson JT, Naritsin DB, Saito K, Markey SP (1994) Biol Mass Spectrom 23:27–32
Price KR (1979) Biomed Mass Spectrom 6:573–574
Jonckheere JA, Leenheer APD (1983) Anal Chem 55:153–155
Sabot J-F, Pinatel H (1993) Analyst 118:831–834
Fay LB, Metairon S, Baumgartner M (2001) Flavour Fragr J 16:164–168
Bretz M, Beyer M, Cramer B, Humpf H-U (2006) Mol Nutr Food Res 50:251–260
Moss MO, Thrane U (2004) Toxicol Lett 153:23–28
Schrodter R (2004) Toxicol Lett 153:47–49
Ueno Y, Nakajima M, Sakai K, Ishii K, Sato N, Shimada N (1973) J Biochem 74:285–296
Schollenberger M, Muller H-M, Rufle M, Suchy S, Planck S, Drochner W (2005) Int J Food Microbiol 97:317–326
Pawlosky RJ, Mirocha CJ, Wen Y, Abbas HK (1989) J Assoc Off Anal Chem 72:807–812
Razzazi-Fazeli E, Rabus B, Cecon B, Bohm J (2002) J Chromatogr A 968:129–142
Asam S, Rychlik M (2006) J Agric Food Chem 54:6535–6546
Hädrich J, Vogelgesang J (1999) Dtsch Lebensm Rundsch 95:428–436
Bretz M, Beyer M, Cramer B, Humpf H-U (2005) Mol Nutr Food Res 49:1151–1153
Häubl G, Berthiller F, Rechthaler J, Jaunecker G, Binder EM, Krska R, Schuhmacher R (2006) Food Addit Contam 23:1187–1193
Häubl G, Berthiller F, Krska R, Schuhmacher R (2006) Anal Bioanal Chem 384:692–696
Asam S, Rychlik M (2006) Eur Food Res Technol 224:769–783
Shier WT, Shier AC, Xie W, Mirocha CJ (2001) Toxicon 39:1435–1438
Munoz L, Riguera R (1990) Magn Reson Chem 28:665–667
Miles CO, Erasmuson AF, Wilkins AL, Towers NR, Smith BL, Garthwaite I, Scahill BG, Hansen RP (1996) J Agric Food Chem 44:3244–3250
Zoellner P, Jodlbauer J, Kleinova M, Kahlbacher H, Kuhn T, Hochsteiner W, Lindner W (2002) J Agric Food Chem 50:2494–2501
Songsermsakul P, Sontag G, Cichna-Markl M, Zentek J, Razzazi-Fazeli E (2006) J Chromatogr B 843:252–261
Launay FM, Young PB, Sterk SS, Blokland MH, Kennedy DG (2004) Food Addit Contam 21:52–62
Hartmann N, Erbs M, Wettstein FE, Schwarzenbach RP, Bucheli TD (2007) J Chromatogr A 1138:132–140
Sforza S, Dall'Asta C, Moseriti A, Galaverna G, Dossena A, Marchelli R (2005) Angew Chem Int Ed 44:5126–5130
Marasas WFO (2001) Environ Health Perspect Suppl 109:239–243
Marasas WFO, Riley RT, Hendricks KA, Stevens VL, Sadler TW, Gelineau-van Waes J, Missmer SA, Cabrera J, Torres O, Gelderblom WCA, Allegood J, Martinez C, Maddox J, Miller JD, Starr L, Sullards MC, Roman AV, Voss KA, Wang E, Merrill AHJ (2004) J Nutr 134:711–716
Plattner RD, Shackelford DD (1992) Mycopathologia 117:17–22
Plattner RD, Branham BE (1994) JAOAC Int 77:525–532
Lukacs Z, Schaper S, Herderich M, Schreier P, Humpf H-U (1996) Chromatographia 43:124–128
Hartl M, Herderich M, Humpf HU (1999) Eur Food Res Technol 209:348–351
Hartl M, Humpf HU (1999) J Agric Food Chem 47:5078–5083
Seefelder W, Hartl M, Humpf H-U (2001) J Agric Food Chem 49:2146–2151
Seefelder W, Gossmann M, Humpf H-U (2002) J Agric Food Chem 50:2778–2781
Becci PJ, Hess FG, Johnson WD, Gallo MA, Babish JG, Dailey RE, Parent RA (1981) J Appl Toxicol 1:256–261
Dickens F, Jones HEH (1961) Br J Cancer 15:85–100
AOAC (1997) Official Methods of Analysis of AOAC International 16th edn. AOAC International; Gaithersburg, Maryland, Method 995.10
Sewram V, Nair JJ, Nieuwoudt TW, Leggott NL, Shephard GS (2000) J Chromatogr A 897:365–374
Takino M, Daishima S, Nakahara T (2003) Rapid Commun Mass Spectrom 17:1965–1972
Roach JAG, White KD, Trucksess MW, Thomas FS (2000) J AOAC Int 83:104–112
Ralls JW, Lane RM (1977) J Food Sci 42:1117–1119
Suzuki T, Fujimoto Y, Hoshino Y, Tanaka A (1974) Agric Biol Chem 38:1259–1260
Tarter EJ, Scott PM (1991) J Chromatography 538:441–446
Rychlik M, Schieberle P (1998) J Agric Food Chem 46:5163–5169
Rychlik M, Schieberle P (1999) J Agric Food Chem 47:3749–3755
Ito R, Yamazaki H, Inoue K, Yoshimura Y, Kawaguchi M, Nakazawa H (2004) J Agric Food Chem 52:7464–7468
Coring systems, http:www.coring.de
Osswald H, Frank HK, Komitowski D, Winter H (1978) Food Cosmet Toxicol 16:243–247
Rychlik M, Kircher F, Schusdziarra V, Lippl F (2004) Food Chem Toxicol 42:729–735
Rychlik M (2003) Food Addit Contam 20:829–837
Fliege R, Metzler M (2000) Chem Res Toxicol 13:373–381
Rychlik M (2005) Ernaehrung 29:61–68
Kuiper-Goodman T, Scott PM (1989) Biomed Environ Sci 2:179–248
Hult K, Plestina R, Habazin-Novak V, Radic B, Ceovic S (1982) Arch Toxicol 51:313–321
EC directive (2006) 1881/2006
Märtlbauer E, Terplan G (1988) Arch Lebensm Hyg 39:133–156
Losito I, Monaci L, Palmisano F, Tantillo G (2004) Rap Commun Mass Spectrom 18:1965–1971
Goryacheva IY, De Saeger S, Lobeau M, Eremin SA, Barna-Vetro I, Van Peteghem C (2006) Anal Chim Acta 577:38–45
Reinsch M, Toepfer A, Lehmann A, Nehls I, Panne U (2006) Food Chem 100:312–317
Jorgensen K, Vahl M (1999) Food Addit Contam 16:451–456
Lindenmeier M, Schieberle P, Rychlik M (2004) J Chromatogr A 1023:57–66
Biopure reference compounds, http:www.biopure.at
Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Rap Commun Mass Spectrom 20:2649–2659
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rychlik, M., Asam, S. Stable isotope dilution assays in mycotoxin analysis. Anal Bioanal Chem 390, 617–628 (2008). https://doi.org/10.1007/s00216-007-1717-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1717-x