Abstract
A multi-mycotoxin stable isotope dilution LC-MS/MS method was developed for 14 Fusarium toxins including modified mycotoxins in cereals (deoxynivalenol, 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, HT2-toxin, T2-toxin, enniatin B, enniatin B1, enniatin A1, enniatin A, beauvericin, fusarenone X, nivalenol, deoxynivalenol-3-glucoside, and zearalenone). The chromatographic separation of the toxins with particular focus on deoxynivalenol and deoxynivalenol-3-glucoside was achieved using a C18-hydrosphere column. An expedient sample preparation method was developed that uses solid-phase extraction for the purification of trichothecenes combined with zearalenone, enniatins, and beauvericin and provides excellent validation data. Linearity, intra-day precision, inter-day precision, and recoveries were ≥0.9982, 1–6 %, 5–12 %, and 79–117 %, respectively. Method accuracy was verified by analyzing certified reference materials for deoxynivalenol, HT2-toxin, and T2-toxin with deviations below 7 %. The results of this method found barley malt samples from 2012, 2013, and 2014 frequently contaminated with high concentrations of enniatin B, deoxynivalenol, and its modified mycotoxin deoxynivalenol-3-glucoside. Samples from 2012 were especially contaminated. Fusarenone X was not detected in any of the analyzed samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fusarium head blight is considered as a devastating fungal disease and has recently gained importance. The infection of barley causes enormous losses concerning yield, grade, and end-use quality, as with other small-grain crops. Heavy infestation of brewing barley with Fusarium species can induce severe mycotoxin contamination and can impair solving as well as malting and brewing properties. Nevertheless, food and feedstuff infected by fungi can affect human and animal health, resulting in headaches, vomiting, visual disturbances, multiple hemorrhages, sepsis, feed refusal by animals, etc. [1]. The wide range of toxins produced by various Fusarium species causes diseases. Fumonisins, trichothecenes, zearalenones, and their modified forms constitute the major classes of Fusarium toxins.
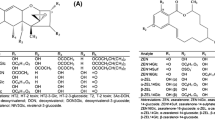
Zearalenone (ZEA) (Fig. 1), a resorcyclic acid lactone and metabolite of inter alia Fusarium graminearum, is not acutely toxic but is well known for its estrogenic activity [2]. Enniatins (ENNs) and beauvericin (BEA) are cyclic depsipeptides of three d-α-hydroxyisovaleric acids alternating with three differing N-methylamino acids (Fig. 1) often produced by Fusarium avenaceum and Fusarium tricinctum [2]. They have general antibiotic and phytotoxic effects and are toxic to insects [2]. Trichothecenes can be classified into four groups: types A, B, C, and D. Types A and B (Fig. 1) play the most important roles in food and feed. Modified mycotoxins such as deoxynivalenol-3-glucoside (D3G) can be formed by plant metabolism [3]. D3G can again be cleaved in human and animal gastrointestinal tracts in glucose and deoxynivalenol (DON) that presents a potential food safety and health risk. However, modified mycotoxins are not detectable using standard approaches.
Moreover, type A trichothecenes and enniatins show little UV absorption. Therefore, LC-MS/MS methods are mainly used in multi-mycotoxin analysis [4–6]. Internal or isotope-labeled standards are highly recommended to compensate for matrix effects [7]. Sufficient removal of disruptive matrix components is another possibility for minimizing matrix interferences such as ion suppression or enhancement. In this regard, cereal-based products are often purified by solid-phase extraction to analyze trichothecenes [5, 8].
However, there is no solid-phase extraction method available to date for trichothecenes combined with zearalenone, beauvericin, and enniatins. Therefore, this study intended to develop a multi-mycotoxin stable isotope dilution LC-MS/MS method using solid-phase extraction for sample purification.
Materials and methods
Chemicals and reagents
Acetonitrile, methanol, water (analytical grade), and potato starch were purchased from VWR (Ismaning, Germany). Formic acid (>95 %) was bought from Sigma-Aldrich (Steinheim, Germany) and the reference material (DON in maize flour) was acquired from Biopure (Romer Labs, Tulln, Austria). The unlabeled reference compounds (nivalenol (NIV), D3G, DON, fusarenone X (FUSX), 3-acetyldeoxynivalenol (3-ADON), 15-acetyldeoxynivalenol (15-ADON), HT2-toxin (HT2), T2-toxin (T2), and ZEA) and some labeled standards ([13C]15-DON and [13C]22-HT2) were bought from Coring System Diagnostix (Gernsheim, Germany); BEA was obtained from AnaSpec (San Jose, USA), ENN B from Bioaustralis (New South Wales, Australia), and the other enniatins, B1, A1, and A, from Enzo Life Sciences (Lörrach, Germany). The labeled internal standards ([13C]2-3-ADON, [13C]4-T2-toxin, [15N]3-ENN B, [15N]3-ENN B1, [15N]3-ENN A1, [15N]3-ENN A, and [15N]3-BEA) were synthesized in our laboratory as reported previously [9–11]. Certified reference material BRM 003001 (naturally contaminated corn flour) was obtained from Biopure Referenzsubstanzen GmbH (Tulln, Austria).
Preparation of standard solutions
Stock solutions of labeled and unlabeled toxins were prepared in concentrations of 10–100 μg/mL in acetonitrile (trichothecenes, ZEA) or methanol (ENNs) and further diluted to final concentrations of 1 and 0.1 μg/mL. All solutions were stored at 4 °C in the dark.
LC-MS/MS
LC-MS/MS was performed on a Shimadzu LC-20A Prominence system (Shimadzu, Kyoto, Japan) using a Hydrosphere RP-C18 column (150 × 3.0 mm2, S-3 μm, 12 nm, YMC Europe GmbH, Dinslaken, Germany) with a C18-guard column (Phenomenex, Aschaffenburg, Germany) as stationary phase that was kept at 40 °C. The binary gradient system (Electronic Supplementary Material Table S-1 and Table S-2) consisted of (A) 0.1 % formic acid and (B) methanol with 0.1 % formic acid at a flow rate of 0.2 mL/min. The gradient for the negative electrospray ionization (ESI) mode was started and held at 10 % B for 2 min, raised linearly from 10 to 99 % B during the next 6 min, and then maintained at 99 % B for 7.5 min. Next, the mobile phase returned to 10 % B within 2 min and the system was equilibrated for 9.5 min before the next run. The gradient for the positive ESI mode was started and held at 10 % B for 2 min, raised linearly from 10 to 87 % B during the next 6 min, held at 87 % for 7 min, raised to 100 % B during the next 5 min, and then maintained at 100 % B for 3.5 min. Next, the mobile phase returned to 10 % B within 2 min and the system was equilibrated for 9.5 min before the next run. The injection volume was 10 μL.
The LC was interfaced with a hybrid triple quadrupole/linear ion trap mass spectrometer (API 4000; Applied Biosystems Inc., Foster City, CA, USA). It operated in the negative ESI mode for the analytes NIV, D3G, and ZEA and in the positive ESI mode for the analytes DON; 3-ADON; 15-ADON; FUSX; HT2; T2; ENN B, B1, A1, and A; and BEA. The ion source parameters (Electronic Supplementary Material Table S-3) for the negative mode were set as follows: curtain gas 20 psi, collision activated dissociation (CAD) gas pressure medium, ion spray voltage −4500 eV, spray gas 50 psi, dry gas 65 psi, and temperature 525 °C. The ion source parameters (Electronic Supplementary Material Table S-3) for the positive mode were set as follows: curtain gas 20 psi, CAD gas pressure high, ion spray voltage 4500 eV, spray gas 80 psi, dry gas 75 psi, and temperature 450 °C. MS parameters were optimized by direct infusion of each standard solution (50 ng/mL to 1 μg/mL) into the source. The mass spectrometer was operated in the scheduled multiple reaction monitoring (MRM) mode for MS/MS measurements at the conditions detailed in Electronic Supplementary Material Table S-4. A waste valve diverted the column effluent to the mass spectrometer from 7 to 16 min in the negative mode and from 7 to 24 min in the positive mode. The remainder of the run was diverted to the waste. Data acquisition was performed with Analyst 1.6.2 software (Applied Biosystems Inc., Foster City, CA, USA).
Calibration and quantitation
For the response curves, constant amounts of internal standard (S) were mixed with varying amounts of analyte (A) in molar ratios between 0.02 and 50 (1:50, 1:20, 1:10, 1:5, 1:2, 1:1; 2:1, 5:1, 10:1, 20:1, and 50:1). All analytes applying stable isotope dilution assay (SIDA) had [13C]- or [15N]-labeled isotopologues, and [13C]2-3-ADON was internal standard for 15-ADON. The response curves were calculated from molar ratios [n(S)/n(A)] versus peak area ratios [A(S)/A(A)] after LC-MS/MS measurement. Response functions were obtained using linear regression. The response factors were between 0.75 and 1.30.
Six matrix calibration points were prepared for the matrix calibration curve. The matrix calibration curves were calculated from concentrations of the analyte [c(A)] versus peak area [A(A)] after LC-MS/MS measurement.
The contents of the analytes in cereal samples were either calculated by the respective response function (DON; 3-ADON; 15-ADON; HT2; T2; ENN B, B1, A1, or A; or BEA) or by the respective matrix calibration function (NIV, D3G, ZEA, or FUSX).
Sample preparation
Finely ground solid samples, e.g., barley, malt, oat, wheat, or maize (1 g), had 10 mL of acetonitrile/water (84/16, v/v) added and were shaken in a laboratory shaker at 225 rpm for 2 h at room temperature. The resulting slurry was filtered through a folded filter (595 1/2, 70 mm diameter, Schleicher & Schuell, Dassel, Germany). Then, 4 mL of the filtrate was spiked with the internal standards (50 μL of [13C]15-DON (1 μg/mL), 100 μL of [13C]2-3-ADON (1 μg/mL), 30 μL of [13C]22-HT2 (1 μg/mL), 30 μL of [13C]4-T2 (1 μg/mL), and 80 μL of [15N]3-ENN B, [15N]3-ENN B1, [15N]3-ENN A1, [15N]3-ENN A, and [15N]3-BEA, respectively (0.1 μg/mL)). The filtrate volume was reduced when necessary to fall into the linear range of the response curves and to minimize standard consumption, but it was restocked to 4 mL with acetonitrile/water (84/16, v/v) before purification. The mixture was vortexed for 20 s and was completely applied on a Bond Elut Mycotoxin cartridge (Agilent Technologies, Santa Clara, CA, USA). The liquid was passed through the cartridge by vacuum suction with an elution rate of 2 drops per second, and then the eluate was collected and evaporated until dry. The sample was reconstituted with 200 μL methanol/water (1/1, v/v) and membrane filtered (0.45 μm). The LC-MS/MS analysis was performed next.
Starch (1 g) was chosen as the blank matrix for the matrix-matched calibration and was spiked with six different amounts of analytes (70–500 μg/kg for NIV, 20–500 μg/kg for D3G, 1–300 μg/kg for ZEA, and 15–200 μg/kg for FUSX). After evaporation of the solvent, the same preparation as for samples described above was performed.
Method validation
LODs and LOQs
Potato starch free of the monitored mycotoxins was chosen as the blank matrix and used to determine limits of detection (LODs) and limits of quantitation (LOQs) as suggested by Vogelgesang and Hädrich [12]. Therefore, the blank matrix was spiked with the unlabeled analytes at four different amounts (1.5, 5, 10, and 15 μg/kg for DON; 3, 10, 20, and 30 μg/kg for 3-ADON; 5, 15, 30, and 50 μg/kg for 15-ADON; 0.5, 1, 3, and 5 μg/kg for HT2 and T2; and 0.4, 1, 2, and 4 for the ENNs and BEA), each in triplicate. The LODs and LOQs were determined by signal to noise ratio for those analytes quantified by matrix-matched calibration (NIV, D3G, ZEA, and FUSX).
Precision
Naturally contaminated barley malt with contents of D3G at 340 μg/kg, DON at 100 μg/kg, HT2 at 6 μg/kg, T2 at 4 μg/kg, ENN A1 at 11 μg/kg, ENN A at 10 μg/kg, BEA at 2 μg/kg, and ZEA at 3 μg/kg was spiked with FUSX (20 μg/kg), NIV (350 μg/kg), 3-ADON (50 μg/kg), and 15-ADON (100 μg/kg). This barley malt and wheat flour naturally contaminated with ENN B (70 μg/kg) and ENN B1 (7 μg/kg) was used for intra-day (n = 3) and inter-day (n = 3, every week within 3 weeks) precision measurements.
Recovery of SIDA and matrix calibration
Blank samples (potato starch) were spiked in triplicate with different amounts of analytes (50, 100, and 300 μg/kg for DON; 50, 100, and 150 μg/kg for 3-ADON and 15-ADON; 50, 100, and 300 μg/kg for HT2 and T2; 20, 50, and 100 for the ENNs; 50, 100, and 250 μg/kg for BEA; 50, 100, and 150 μg/kg for FUSX; 350, 400, and 450 μg/kg for NIV; 50, 100, and 300 μg/kg for D3G; and 50, 100, and 250 μg/kg for ZEA) and analyzed as described previously. Recoveries were calculated as the ratio of detected and spiked contents.
Accuracy
Reference materials (474 ± 30 μg/kg certified content of DON in maize flour and consensus values of 9.57 ± 1.04, 26.5 ± 3.4, 8.46 ± 1.02, 21.1 ± 3.4, and 15.7 ± 2.6 μg/kg HT2 and 4.21 ± 2.17, 7.92 ± 2.25, 3.40 ± 2.18, 6.09 ± 2.25, and 4.58 ± 2.20 μg/kg T2 in five different oat samples from an inter-laboratory ring test performed under the supervision of the German Federal Office of Consumer Protection and Food Safety, BVL) were analyzed as described previously.
Results and discussion
Method development
LC-MS/MS
DON, FUSX, 3-ADON, 15-ADON, HT2, T2, ENNs, and BEA were measured in the positive ESI mode. The protonated molecules were used as precursor ions for type B trichothecenes, ENNs, and BEA. In contrast, sodium adducts of HT2 and T2 gave more intensive and reproducible dissociation patterns. The labeled standards had fragmentation patterns similar to those of the respective unlabeled compounds. Detailed explanations of MS/MS fragmentation of type A trichothecenes, type B trichothecenes, ENNS, BEA, and their respective labeled analogs have been described elsewhere [9–11]. NIV, D3G, and ZEA were analyzed in the negative ESI mode, and the abundant [M−H]− ions were used as precursor ions. To enable optimal MS conditions for all analytes, two chromatographic runs in the positive as well as negative ESI modes were performed for each sample.
D3G had the same fragmentation pattern as DON (Electronic Supplementary Material Fig. S-1) due to in-source fragmentation and loss of glucose from D3G in the positive ESI mode. A chromatographic separation of these analytes was necessary to allow quantification of DON and not the sum of D3G and DON in the positive ESI mode. This was achieved using a Hydrosphere RP-C18 column (YMC Europe GmbH, Dinslaken, Germany) as a stationary phase. We were able to assure baseline separation of all other analytes except for BEA coeluting with ENN B1 and 3-ADON coeluting with 15-ADON. The use of a Gemini C6-phenyl or a Synergi Polar RP column would have avoided a coelution of BEA with ENNs [11, 13] and of acetylated DONs [10]. However, there are several reasons these coelutions on the Hydrosphere RP-C18 column were not critical. First, the potentially increased sensitivity for ENN B1 and BEA is negligible when considering their different fragmentation patterns and the very low LODs and LOQs of our method. Furthermore, the position isomers 3-ADON and 15-ADON are differentiated by their partially different product ions resulting from their common precursor [M+H]+ ion (m/z 339.1) and especially by the intensities of the product ions (Fig. 2). The three most important fragments of 3-ADON are m/z 213.1, m/z 231.1, and m/z 137.1 compared with those of 15-ADON, which are m/z 261.1, m/z 137.1, and m/z 231.1. The respective product ions were chosen as quantifiers that were not produced from the other isomer, m/z 213.1 for 3-ADON and m/z 261.1 for 15-ADON. Both ADONs revealed the same qualifiers (3-ADON m/z 231.1 and 15-ADON m/z 137.1) but in completely different intensities. In accordance with Berger et al. [14], Berthiller et al. [5], and our own validation data, quantification of 3-ADON and 15-ADON was possible without chromatographic separation.
Sample purification
Sample purification using solid-phase extraction was designed to reduce labeled internal standard consumption, to maximize analyte sensitivity, and to achieve satisfactory validation results. Moreover, a cleanup step is important to extend the HPLC column lifetime and to maintain the performance of the ESI-MS source.
Multifunctional SPE cartridges usually containing activated carbon have been used for sample purification, especially for trichothecene analysis, as reported in the literature [5, 8, 15]. In contrast, dilute-and-shoot methods [4] are often applied for ENNs and some modified mycotoxins. Therefore, this study presents the first multi-mycotoxin stable isotope dilution method for trichothecenes combined with ZEA, ENNs, BEA, and the modified mycotoxin D3G using solid-phase extraction for sample purification. The use of a Bond Elut Mycotoxin cartridge (Agilent Technologies, Santa Clara, CA, USA) without activated carbon was mandatory to achieve good recoveries and to prevent adsorption of the non-polar toxins (ZEA, ENNs, and BEA).
Calibration and quantitation
Response functions were obtained using linear regression. The response factors ranged between 0.75 (3-ADON) and 1.30 (ENN B1, A1, and A). The coefficients of determination exceed 0.9982, and, as confirmed by Mandel test, the calibration curves show linearity for DON and 15-ADON within the molar ratios 0.1–10; 3-ADON and HT2 within 0.02–20; T2 within 0.02–50; ENN B within 0.05–10; ENN B1, A1, and A within 0.05–20; and BEA within 0.05–5.
A wide range of linearity for the matrix-matched calibration results for NIV between 70 and 500 μg/kg, for D3G between 20 and 500 μg/kg, for ZEA between 1.5 and 300 μg/kg, and for FUSX between 15 and 150 μg/kg.
Method validation
LODs and LOQs
The LODs and LOQs for the SIDAs were calculated according to Vogelgesang and Hädrich [12]. The LODs and LOQs for the matrix-matched calibration were calculated from the signal (S) to noise (N) ratio (LOD = 3 × S/N, LOQ = 10 × S/N). The blank matrix potato starch free of the monitored mycotoxins was used. The LODs range between 0.1 and 5 μg/kg and the LOQs range between 0.2 and 15 μg/kg—except for NIV and D3G, whose LODs and LOQs are 70 and 200 μg/kg and 10 and 30 μg/kg, respectively (Table 1). The results for ZEA and types B and A trichothecenes are comparable with recently reported results [4, 10, 16, 17]. The high LOD and LOQ of NIV with 70 and 200 μg/kg, respectively, are due to the low MS/MS sensitivity and are comparable with the limits reported by Njumbe Ediage et al. [18]. The LODs and LOQs of the ENNs and BEA using the method presented here reveal 2–100 times higher sensitivity than those previously reported [11, 13, 19]. However, Tolosa et al. [20] published a method ten times more sensitive for ENNS, which is clearly attributed to the fivefold sample weight applied.
Precision
The intra-day (n = 3) and inter-day (n = 3) coefficients of variation are shown in Table 1. The intra-day precision ranges between 1 and 6 % and the inter-day precision ranges between 5 and 12 %. Thus, the precision data for the stable isotope dilution assays presented here are similar to those reported for ENNs by Hu et al. [11] and for trichothecenes by Asam et al. [9, 10]. The matrix-matched calibration results are comparable with those of the SIDAs mentioned and more precise than the methods described by Biselli et al. [21] and Njumbe Ediage et al. [18]. However, when comparing precision, one has to be aware that homogeneity of the samples is a crucial aspect and the precision of our method from 1 g of sample was only achievable from thoroughly homogenized samples.
Recovery of SIDA and matrix-matched calibration
The recoveries of the SIDAs and matrix-matched calibration were determined at three different spiking levels for each mycotoxin. The recoveries range between 86 and 109 % for the SIDAs with relative standard deviations (RSDs) below 7 % (Table 1) and between 79 and 117 % for the matrix calibration with maximal RSD of 17 % (Table 1). These SIDA and matrix-matched calibration recoveries both correspond to the expected levels of stable isotope dilution assays. Figures 3 and 4 present the LC-MS/MS chromatograms of spiked barley malt samples in the negative and positive ESI modes, respectively.
Accuracy
The accuracy of this method was confirmed by analyzing commercially available reference material (CRM) and samples from inter-laboratory method studies. The CRM consisted of maize flour and has a certified content of 474 ± 30 μg/kg DON. The SIDA of DON revealed a content of 468 ± 10 μg/kg (n = 2) which differs from the assigned value by 1.27 % (Table 2). Five different oat samples from inter-laboratory method studies were contaminated with HT2 between 9.57 ± 1.04 and 26.5 ± 3.4 μg/kg and T2 between 3.40 ± 2.18 and 7.92 ± 2.25 μg/kg. Results and differences between detected and assigned values of HT2 and T2 are provided in Table 2 and vary between 0.60 and 4.45 % and 2.14 and 6.73 %, respectively. The maximum difference to the respective certified reference values of 1.27 % for DON, 4.45 % for HT2, and 6.73 % for T2 indicates the accuracy for different matrixes using this multi-method.
Analysis of (modified) mycotoxins in cereals
Barley malt samples collected in various German federal states over a period of 3 years from 2012, 2013, and 2014 were analyzed with the multi-mycotoxin method presented. The results are summarized in Table 3. High contamination frequencies and concentrations of DON, D3G, and ENN B were detected, especially in 2012.
Over 93 % of the analyzed barley malt samples are contaminated with ENN B and over 83 % with ENN B1. The average ENN B concentration of the 3 years is 3 mg/kg. The maximum ENN B level of 60 mg/kg is from 2012, which is almost comparable with artificially inoculated barley malt [22]. The contents of ENN A1 and ENN A are lower and at maximal one fifth of those ENN B reached. ENNs consistently occur in decreasing order by incidence and concentration: ENN B, ENN B1, ENN A1, and ENN A. These results are consistent with other studies [11, 23, 24]. BEA was detected in four samples with low contents below 48 μg/kg over all 3 years. In contrast, Mediterranean countries like Morocco or Spain had BEA and ENN levels of wheat, barley, rice flour, and other cereal products that ranged between 1 and 800 mg/kg [19, 25].
The samples have little contamination with ZEA, except for one of barley malt with 250 μg/kg.
No analyzed samples contain type A trichothecenes from 2012. The other years are not heavily loaded with HT2 and T2. The highest amounts of HT2 and T2 are found in 2014 at 45 and 40 μg/kg, respectively.
NIV was found in two barley malt samples. One from 2012 has a high content of 464 μg/kg. The contents of 15-ADON are lower than those of 3-ADON, which occur most frequently at a 30 % rate and content range between 33 and 436 μg/kg in 2012. FUSX was not detected in any of the analyzed samples.
The other type B trichothecenes, DON, and its modified metabolite, D3G, were found in barley malt at frequencies up to 73 and 53 %, respectively. The amount of DON ranges between 31 μg/kg and 10 mg/kg and the amount of D3G between 28 μg/kg and 19 mg/kg. During malting, and particularly during the germination process, DON is exposed to glucosylation and can become D3G [26]. The content of D3G in 68 % of analyzed malts exceeds the content of DON up to tenfold, while the other 32 % of samples are below LOD or LOQ. Earlier studies reported a wide range between 1 μg/kg and 5 mg/kg of DON and D3G in barley, wheat, and brewing malt [17, 27, 28]. Moreover, most of the reported malt samples had higher D3G levels than DON levels [28, 29].
Conclusions
In the EU, the maximum regulatory permitted content of DON is 1250 μg/kg in unprocessed cereals, which is also usually applicable to brewing malt [30]. Two of the analyzed malt samples should not have been placed on the market even if the D3G content is ignored and the official regulatory limits for DON are considered. This mycotoxin contamination is likely transferred into beer as brewing barley malt is not intended for direct human consumption. To which extent the toxins under study are transferred into beer will be the focus of further investigations, and adaptation of this method to beer and liquid samples is under way.
Abbreviations
- 15-ADON:
-
15-Acetyldeoxynivalenol
- 3-ADON:
-
3-Acetyldeoxynivalenol
- BEA:
-
Beauvericin
- D3G:
-
Deoxynivalenol-3-glucoside
- DON:
-
Deoxynivalenol
- ENN:
-
Enniatin
- ESI:
-
Electrospray ionization
- FUSX:
-
Fusarenone X
- HT2:
-
HT2-toxin
- NIV:
-
Nivalenol
- SPE:
-
Solid-phase extraction
- T2:
-
T2-toxin
- ZEA:
-
Zearalenone
References
Rodricks JV, Hesseltine CW, Mehlman MA (1977) Mycotoxins in human and animal health. Proceedings of a conference on mycotoxins in human and animal health, convened at University of Maryland University College, Center of Adult Education, October 4-8, 1976. Pathotox, Park Forest South, Ill
Desjardins AE (2006) Fusarium mycotoxins. Chemistry, genetics and biology. APS Press, St. Paul
Rychlik M, Humpf H, Marko D, Dänicke S, Mally A, Berthiller F, Klaffke H, Lorenz N (2014) Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res 30(4):197–205
Sulyok M, Berthiller F, Krska R, Schuhmacher R (2006) Development and validation of a liquid chromatography/tandem mass spectrometric method for the determination of 39 mycotoxins in wheat and maize. Rapid Commun Mass Spectrom 20(18):2649–2659
Berthiller F, Schuhmacher R, Buttinger G, Krska R (2005) Rapid simultaneous determination of major type A- and B-trichothecenes as well as zearalenone in maize by high performance liquid chromatography–tandem mass spectrometry. J Chromatogr A 1062(2):209–216
Berthiller F, Sulyok M, Krska R, Schuhmacher R (2007) Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int J Food Microbiol 119(1–2):33–37
Klötzel M, Gutsche B, Lauber U, Humpf H (2005) Determination of 12 type A and B trichothecenes in cereals by liquid chromatography-electrospray ionization tandem mass spectrometry. J Agric Food Chem 53(23):8904–8910
Klötzel M, Lauber U, Humpf H (2006) A new solid phase extraction clean-up method for the determination of 12 type A and B trichothecenes in cereals and cereal-based food by LC-MS/MS. Mol Nutr Food Res 50(3):261–269
Asam S, Rychlik M (2006) Synthesis of four carbon-13-labeled type a trichothecene mycotoxins and their application as internal standards in stable isotope dilution assays. J Agric Food Chem 54(18):6535–6546
Asam S, Rychlik M (2007) Quantitation of type B-trichothecene mycotoxins in foods and feeds by a multiple stable isotope dilution assay. Eur Food Res Technol 224(6):769–783
Hu L, Rychlik M (2012) Biosynthesis of 15 N 3-labeled enniatins and beauvericin and their application to stable isotope dilution assays. J Agric Food Chem 60(29):7129–7136
Vogelgesang J, Hädrich J (1998) Limits of detection, identification and determination: a statistical approach for practitioners. Accred Qual Assur 3:242–255
Sørensen JL, Nielsen KF, Rasmussen PH, Thrane U (2008) Development of a LC-MS/MS method for the analysis of enniatins and beauvericin in whole fresh and ensiled maize. J Agric Food Chem 56(21):10439–10443
Berger U, Oehme M, Kuhn F (1999) Quantitative determination and structure elucidation of type A- and B-trichothecenes by HPLC/ion trap multiple mass spectrometry. J Agric Food Chem 47(10):4240–4245
Asam S, Rychlik M (2007) Studies on accuracy of trichothecene multitoxin analysis using stable isotope dilution assays. Mycotoxin Res 23(4):191–198
Wilcox J, Donnelly C, Leeman D, Marley E (2015) The use of immunoaffinity columns connected in tandem for selective and cost-effective mycotoxin clean-up prior to multi-mycotoxin liquid chromatographic-tandem mass spectrometric analysis in food matrices. J Chromatogr A 1400:91–97
Nathanail AV, Syvähuoko J, Malachová A, Jestoi M, Varga E, Michlmayr H, Adam G, Sieviläinen E, Berthiller F, Peltonen K (2015) Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal Bioanal Chem 407(16):4745–4755
Njumbe Ediage E, van Poucke C, de Saeger S (2015) A multi-analyte LC-MS/MS method for the analysis of 23 mycotoxins in different sorghum varieties: the forgotten sample matrix. Food Chem 177:397–404
Mahnine N, Meca G, Elabidi A, Fekhaoui M, Saoiabi A, Font G, Mañes J, Zinedine A (2011) Further data on the levels of emerging Fusarium mycotoxins enniatins (A, A1, B, B1), beauvericin and fusaproliferin in breakfast and infant cereals from Morocco. Food Chem 124(2):481–485
Tolosa J, Font G, Mañes J, Ferrer E (2014) Natural occurrence of emerging Fusarium mycotoxins in feed and fish from aquaculture. J Agric Food Chem 62(51):12462–12470
Biselli S, Hummert C (2005) Development of a multicomponent method for Fusarium toxins using LC-MS/MS and its application during a survey for the content of T-2 toxin and deoxynivalenol in various feed and food samples. Food Addit Contam 22(8):752–760
Hu L, Gastl M, Linkmeyer A, Hess M, Rychlik M Fate of enniatins and beauvericin during the brewing process determined by stable isotope dilution assays. Accepted Manuscript
Uhlig S, Torp M, Heier BT (2006) Beauvericin and enniatins A, A1, B and B1 in Norwegian grain: a survey. Food Chem 94(2):193–201
Vaclavikova M, Malachova A, Veprikova Z, Dzuman Z, Zachariasova M, Hajslova J (2013) ‘Emerging’ mycotoxins in cereals processing chains: changes of enniatins during beer and bread making. Food Chem 136(2):750–757
Meca G, Zinedine A, Blesa J, Font G, Mañes J (2010) Further data on the presence of Fusarium emerging mycotoxins enniatins, fusaproliferin and beauvericin in cereals available on the Spanish markets. Food Chem Toxicol 48(5):1412–1416
Maul R, Müller C, Rieß S, Koch M, Methner F, Nehls I (2012) Germination induces the glucosylation of the Fusarium mycotoxin deoxynivalenol in various grains. Food Chem 131(1):274–279
Berthiller F, Dall’Asta C, Schuhmacher R, Lemmens M, Adam G, Krska R (2005) Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography–tandem mass spectrometry. J Agric Food Chem 53(9):3421–3425
Kostelanska M, Zachariasova M, Lacina O, Fenclova M, Kollos A, Hajslova J (2011) The study of deoxynivalenol and its masked metabolites fate during the brewing process realised by UPLC–TOFMS method. Food Chem 126(4):1870–1876
Kostelanska M, Hajslova J, Zachariasova M, Malachova A, Kalachova K, Poustka J, Fiala J, Scott PM, Berthiller F, Krska R (2009) Occurrence of deoxynivalenol and its major conjugate, deoxynivalenol-3-glucoside, in beer and some brewing intermediates. J Agric Food Chem 57(8):3187–3194
Die Kommission der Europäischen Gemeinschaften (2006) Verordnung (EG) Nr. 1881/2006 zur Festsetzung der Höchstgehalte für bestimmte Kontaminanten in Lebensmitteln
Acknowledgments
We thank Cajetan Geißinger, Chair of Brewing and Beverage Technology, TU München, and Katharina Hofer, Chair of Phytopathology, TU München, for providing wheat, barley, and malt samples. This project was supported by the Forschungskreis der Ernährungsindustrie e.V. (FEI, Bonn), the AiF, the German Federal Ministry of Economic Affairs and Energy (AiF-Project No.: 17221 N), and the Wissenschaftsförderung der Deutschen Brauwirtschaft e.V. Moreover, the authors gratefully acknowledge the support by the Faculty Graduate Center Weihenstephan of TUM Graduate School at Technische Universität München, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 117 kb)
Rights and permissions
About this article
Cite this article
Habler, K., Rychlik, M. Multi-mycotoxin stable isotope dilution LC-MS/MS method for Fusarium toxins in cereals. Anal Bioanal Chem 408, 307–317 (2016). https://doi.org/10.1007/s00216-015-9110-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9110-7