Abstract
Rationale
(R)-Ketamine produced beneficial effects in a variety of models of inflammatory diseases, including low dose of bacterial lipopolysaccharide (LPS) (0.5–1.0 mg/kg)-induced endotoxemia. LPS-treated mice have been used as animal model of delirium.
Objectives
We investigated the effects of (R)-ketamine in neuroinflammation and cognitive impairment in rodents after administration of high dose of LPS.
Methods
LPS (5 mg/kg) or saline was administered intraperitoneally (i.p.) to mice. (R)-Ketamine (10 mg/kg) was administrated i.p. 24 h before and/or 10 min after LPS injection.
Results
LPS (5.0 mg/kg) caused a remarkable splenomegaly and increased plasma levels of pro-inflammatory cytokines [i.e., interleukin (IL-6), IL-17A, and interferon (IFN)-γ]. There were positive correlations between spleen weight and plasma cytokines levels. Furthermore, LPS led to increased levels of pro-inflammatory cytokines in the prefrontal cortex (PFC) and hippocampus. Moreover, LPS impaired the natural and learned behaviors, as demonstrated by a decrease in the number of mice’s entries and duration in the novel arm in the Y maze test and an increase in the latency of mice to eat the food in the buried food test. Interestingly, the treatment with (R)-ketamine (twice 24 h before and 10 min after LPS injection) significantly attenuated LPS-induced splenomegaly, central and systemic inflammation, and cognitive impairment.
Conclusion
Our results highlighted the importance of combined prophylactic and therapeutic use of (R)-ketamine in the attenuation of LPS-induced systemic inflammation, neuroinflammation, and cognitive impairment in mice. It is likely that (R)-ketamine could be a prophylactic drug for delirium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic infection and inflammation could trigger a spectrum of sickness behavior (i.e., fatigue, lethargy, appetite loss, sleep disorder, locomotor activity decrease, neuropsychiatric symptoms) and cognitive impairment in humans and rodents (Draper et al. 2018; Kealy et al. 2020; Savage et al. 2019; Schedlowski et al. 2014; Sultan et al. 2021; Yamanashi et al. 2021). Delirium is shown to be a maladaptive sickness behavior response (Cunningham and Maclullich 2013), which is associated with multiple adverse consequences (i.e., extended length of hospital stay, decreased chance of discharge to home, and long-term cognitive impairment) and significant financial burden (Inouye et al. 2014; Leslie et al. 2008; McCusker et al. 2003; Pandharipande et al. 2013). However, little is known of the pathophysiology of delirium, and no effective prophylactic and therapeutic measures have been identified. Clinical observations and experimental studies have suggested a close relationship between systemic inflammation and delirium-related cognitive impairment (Annane and Sharshar 2015; Biesmans et al. 2013; D’Esposito and Postle 2015; Jackson et al. 2009; Oliveira-Lima et al. 2019; Schmidt et al. 2002; Sultan et al. 2021; Yamanashi et al. 2021). Systemic administration of bacterial lipopolysaccharides (LPS) plays an important role in the triggering of innate immune response and leads to delirium in rodents (Biesmans et al. 2013; D’Esposito and Postle 2015; Oliveira-Lima et al. 2019; Sultan et al. 2021; Yamanashi et al. 2021). LPS-induced animal model to mimic Gram-negative bacterial infection is widely used to investigate the psychiatric and cognitive consequences of bacterial infection and systemic inflammation (Biesmans et al. 2013; D’Esposito and Postle 2015; Oliveira-Lima et al. 2019; Zhang et al. 2014b, 2020).
Neuroinflammation after systemic administration of LPS has been suggested to play an essential role in the pathophysiology of endotoxemia-derived cognitive impairment. Microglial cells, which are the resident immune cells of the central nervous system (CNS), contribute to neuroinflammation, and microglia are morphologically activated with increased cell numbers in the cortex, hippocampus, and thalamus of mice 24 and 48 h after intraperitoneal injection of high dose of LPS (5 mg/kg) (Hoogland et al. 2018; Murray et al. 2012; Oliveira-Lima et al. 2019; Walker et al. 2019). The expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and monocyte chemoattractant protein 1 in the brain was also markedly increased after systemic LPS (5 mg/kg) administration (Hoogland et al. 2018; Murray et al. 2012; Walker et al. 2019; Yang et al. 2020). It is reported that LPS-induced acute cognitive impairment can be mimicked by systemic stimulation with IL-1β (Griffin et al. 2013) or TNF-α (Hennessy et al. 2017), and that LPS-induced cognitive impairment was attenuated by systemic administration of IL-1 receptor antagonist (Cunningham and Sanderson 2008; Skelly et al. 2019). Furthermore, systemic administration of LPS is reported to increase IL-17A expression in the plasma and hippocampus, and IL-17A is involved in LPS-induced neuroinflammation and cognitive impairment in aged rats via microglial activation (Sun et al. 2015). Moreover, systemic LPS administration could rapidly increase the protein expression of interferon-γ (IFN-γ) in the plasma, spleen, and hippocampus, resulting in rodent models of neurological disorders (Curtin et al. 2009; Jaehne et al. 2015; Lestage et al. 2002; Seemann and Lupp 2016). Collectively, it is likely that systemic administration of high dose (i.e., 5 mg/kg) of LPS causes systemic inflammation and neuroinflammation in the brain, resulting in cognitive impairment in rodents.
It has been well-established that (R,S)-ketamine, an N-methyl-d-aspartate receptor (NMDAR) antagonist, possesses potent anti-inflammatory properties (Alqahtani et al. 2020; Chang et al. 2018a, b; De Kock et al. 2013; Lu et al. 2020; Mastrodonato et al. 2020; Ward et al. 2011). (R,S)-Ketamine could reverse LPS-mediated upregulation of pro-inflammatory cytokines and enzyme expression in microglia cells in vitro (Lu et al. 2020). Pretreatment with (R,S)-ketamine attenuated systemic inflammation in rats after LPS administration, in a dose-dependent manner (Lu et al. 2020), suggesting prophylactic effect of (R,S)-ketamine. (R,S)-Ketamine reduces the number of microglia and macrophages in the hypothalamus and hippocampus 24 h after transient hypoxia in fetal sheep (Chang et al. 2018a). Interestingly, LPS-induced depression-like behaviors in mice were improved after subsequent administration of (R,S)-ketamine, suggesting therapeutic effect of (R,S)-ketamine (Zhang and Hashimoto 2019). In addition, a single intravenous infusion of (R,S)-ketamine significantly decreased serum levels of IL-6 in treatment-resistant patients with depression (Yang et al. 2015). Collectively, it seems that (R,S)-ketamine has potent anti-inflammatory actions (Hashimoto 2015).
(R,S)-Ketamine is an equal mixture of (R)-ketamine and (S)-ketamine. Although (R)-ketamine is less potent at NMDAR than (S)-ketamine (Hashimoto 2019, 2020), our group reported that (R)-ketamine exerts more potent neuroprotective effects than (S)-ketamine in a variety of models of inflammatory diseases, including low-dose LPS (0.5 mg/kg)- or chronic social defeat stress-induced depression-like behaviors (Chang et al. 2018b; Yang et al. 2017), ischemic stroke (Xiong et al. 2020), Parkinson’s disease (Fujita et al. 2020), osteoporosis (Fujita and Hashimoto 2020), and ulcerative colitis (Fujita et al. 2021). Collectively, it is likely that (R)-ketamine would be a novel therapeutic drug without side effects of ketamine (Hashimoto 2019, 2020; Wei et al. 2020, 2021; Yang et al. 2019). However, it remains unclear whether (R)-ketamine could exert anti-inflammatory effects on systemic inflammation and neuroinflammation and thus improve cognitive impairment in rodents after endotoxemia induced by systemic administration of high dose of LPS (5 mg/kg).
The aim of the present study was to evaluate the prophylactic and/or therapeutic effects of (R)-ketamine on systemic inflammation, neuroinflammation, and delirium-related cognitive impairment produced by LPS (5 mg/kg)-induced endotoxemia.
Materials and method
Animals
Adult male C57BL/6 mice (aged 8 weeks) were purchased from Japan SLC Inc. (Hamamatsu, Shizuoka, Japan). Mice were housed under controlled conditions for temperature and humidity, 12-h light/dark cycles (lights on 07:00–19:00), and allowed free access to food (CE-2; CLEA Japan, Inc., Tokyo, Japan) and water. All experiments with the mice were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA, and approved by the Chiba University Institutional Animal Care and Use Committee (permission number: 2–308). Animals were deeply anesthetized with inhaled isoflurane and rapidly killed by cervical dislocation. All efforts were made to ameliorate animal suffering.
Administration and behavioral tests
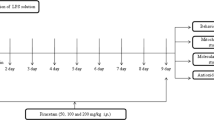
The mice were randomly divided to three groups (n = 10/group). Saline (10 ml/kg) or lipopolysaccharides (LPS; 5 mg/kg, Sigma-Aldrich, Tokyo, Japan) was given intraperitoneally (i.p.) to mice (Fig. 1a). (R)-Ketamine hydrochloride was prepared by recrystallization of (R,S)-ketamine (Ketalar®, ketamine hydrochloride, Daiichi Sankyo Pharmaceutical Ltd., Tokyo, Japan) and d-( −)-tartaric acid, as described previously (Zhang et al. 2014a). The dose (10 mg/kg as hydrochloride) of (R)-ketamine dissolved in the 0.9% saline was used as previously reported (Fujita et al. 2020; Zhang et al. 2014a, b, 2018, 2019). Saline (10 ml/kg) or (R)-ketamine (10 mg/kg) was administrated to mice in three different protocols as follows: (1) a single administration of (R)-ketamine 24 h prior to LPS injection; (2) a single administration of (R)-ketamine 10 min after LPS injection; and (3) (R)-ketamine was injected twice 24 h before and 10 min after LPS injection.
Effects of combined prophylactic and therapeutic use of (R)-ketamine on spleen weight after LPS treatment. a Treatment schedule. Adult mice were intraperitoneally injected with lipopolysaccharides (LPS, 5 mg/kg) or saline (10 ml/kg). (R)-Ketamine (10 mg/kg) or saline (10 ml/kg) were intraperitoneally injected to mice at 24 h before and 10 min after LPS injection. Spleen was collected 24 h after injection of LPS or saline. b Body weight loss in mice treated with (R)-ketamine or saline 24 h after LPS injection (unpaired Student’s t test: t = 2.385, P = 0.0283). c Representative picture of spleen and spleen weight (one-way ANOVA: F2,27 = 20.51, P < 0.0001). d The ratio of spleen weight/body weight (one-way ANOVA: F2,27 = 29.06, P < 0.0001). Data are shown as mean ± SEM (n = 10/group). *P < 0.05, ***P < 0.0001. LPS, lipopolysaccharides; R-KT, (R)-ketamine
The mice were deeply anesthetized with inhaled isoflurane (5%) 24 h after injection of saline or LPS. Blood was collected via cardiac puncture, placed into tubes containing ethylenediaminetetraacetic acid (EDTA), and immediately centrifuged at 3000 g for 5 min at 4 °C to get plasma and then stored at − 80 °C until bioanalysis. The bilateral prefrontal cortex (PFC) and hippocampus were collected rapidly and stored at − 80 °C until bioanalysis. The weights of spleens were recorded immediately after spleen removal.
Behavioral tests
The Y maze test and buried food test have been shown to be capable of assessing delirium (Peng et al. 2016). The Y maze test was performed 26 h before and 22 h after a single injection of saline or LPS to assess the spatial recognition memory (learned behavior). The buried food test was performed 24 h before and 24 h after a single injection of saline or LPS to assess the natural behavior.
The Y maze test was performed as described previously with a litter modification (Peng et al. 2016). Each maze consisted of three equal arms (30 cm length × 8 cm width × 15 cm height) termed novel, start, and other, which were at a 120° angle from each other. The first training trial was 10 min in duration; the novel arm was blocked but the mouse could explore the other two open arms. After 1 h, the second retention trial (retention) was conducted. The mouse was placed back in the same start arm with free access to all 3 arms for 5 min. A video camera was installed 60 cm above the chamber to record the number of entries and the time spent in the novel arm. Each of the arms of the Y maze was cleaned with 75% ethanol solution between trials.
The buried food test was conducted as described previously with slight modifications (Peng et al. 2016). Two days prior to the test, each mouse was given several pieces of sweetened cereal. On the day of the test, the test cage was prepared with clean bedding with a height of 3 cm, and 1 sweetened cereal pellet was buried 0.5 cm below the surface of bedding in one corner of the test cage. The mouse was then placed in the diagonal corner to explore for 5 min, and the latency of finding the buried cereal pellet was recorded. Latency was defined as the time between when the mouse was placed in the test cage and when the mouse grasped the cereal pellet in its forepaws or teeth. If the mouse could not find the buried cereal pellet within 5 min, the latency was recorded as 5 min. The bedding from the test cage was changed and the test cage was cleaned with 75% ethanol solution after each test.
Enzyme-linked immunosorbent assay (ELISA)
Plasma levels of IL-6 (Cat number: 88–7064, Invitrogen, Camarillo, CA, USA), IL-17A (Cat number: RK00039, ABclonal, Inc., Tokyo, Japan), and IFN-γ (Cat number: RK00019, ABclonal, Inc., Tokyo, Japan) were measured using commercial ELISA kits according to the manufacturer’s instructions.
Western blot
Tissue samples from the PFC and hippocampus were homogenized in ice-cold Laemmli lysis buffer and then centrifuged at 3000 g for 10 min at 4 °C to get the supernatants. The protein concentrations were quantified using a bicinchoninic acid (BCA) protein assay kit (Bio-Rad, Hercules, CA). The samples were then mixed with an equal volume of loading buffer (125 mM Tris/HCl, pH 6.8, 20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol, and 4% sodium dodecyl sulfate) and boiled for 10 min at 95 °C. Proteins were separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Mini-PROTEAN® TGX™ Precast Gel; Bio-Rad) and then transferred onto polyvinylidene difluoride (PVDF) membranes using a Trans Blot Mini Cell (Bio-Rad). The membranes were blocked with 5% skim milk in TBS with 0.1% Tween 20 (TBST) for 1 h at room temperature and then incubated with the following primary antibodies: rabbit polyclonal anti-ionized calcium-binding adapter molecule 1 (Iba-1; 1:1,000, Cat number: 016–20,001, Wako Pure Chemical Industries, Ltd., Tokyo, Japan), rabbit monoclonal anti-IL-6 (1:1,000, Cat number: 12912S, Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit monoclonal anti-IL-17A (1:1,000, Cat number: A0688, ABclonal, Inc., Tokyo, Japan), rabbit monoclonal anti-IFN-γ (1:1,000, Cat number: A12450, ABclonal, Inc., Tokyo, Japan), rabbit monoclonal anti-inducible nitric oxide synthase (iNOS; 1:1,000, Cat number: ab3523, Abcam, Cambridge, MA, USA), and mouse monoclonal anti-β-actin (1:10,000, Sigma-Aldrich Co., Ltd., St Louis, MO, USA) overnight at 4 °C. After three washes with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibody (1:5,000) for 1 h at room temperature. After being washed three washes with TBST, the bands were visualized using enhanced chemiluminescence (ECL) plus the Western Blot Detection system (GE Healthcare Bioscience) and captured by ChemiDoc™ Touch Imaging System (170–01,401; Bio-Rad Laboratories, Hercules, CA). The images were subjected to grayscale analysis using Image Lab™ 3.0 software (Bio-Rad Laboratories).
Statistical analysis
Data were expressed as the mean ± standard error of the mean (S.E.M). Data were analyzed using one-way analysis of variance (ANOVA) followed by post-hoc Newman-Keuls’s multiple comparison tests. Correlation was analyzed by Pearson correlation. P < 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS version 20.0 software (SPSS, Tokyo, Japan).
Results
Effects of (R)-ketamine on spleen weight and plasma levels of inflammatory cytokines after LPS treatment
First, we examined the effects of (R)-ketamine (10 mg/kg) in three different protocols on the spleen weight and plasma levels of inflammatory cytokines in mice after LPS treatment (Fig. 1a). Treatment with (R)-ketamine (twice, 24 h before and 10 min after LPS injection) significantly decreased the body weight loss (Fig. 1b) and attenuated LPS-induced increase in the spleen weight (Fig. 1c) and the ratio of spleen weight/body weight at 24 h after LPS injection (Fig. 1d).
Previously, we reported increased spleen weight and inflammatory cytokines in blood after administration of a low dose of LPS (0.5 mg/kg) (Zhang et al. 2020). Here, we examined whether (R)-ketamine (10 mg/kg) attenuates increases in plasma levels of cytokines and spleen weight in mice after LPS (5 mg/kg) administration (Fig. 2a). Treatment with (R)-ketamine (twice, 24 h before and 10 min after LPS injection) significantly attenuated the increase in the plasma levels of IL-6, IL-17A, and IFN-γ after LPS (5 mg/kg) administration (Fig. 2b–d). Interestingly, there were positive correlations between the spleen weight and plasma levels of inflammatory cytokines (IL-6, IL-17A, or IFN-γ) in the three groups (Fig. 2e–g), indicating a close association between inflammation in the periphery and increased spleen weight. The data are consistent with our previous report (Zhang et al. 2020).
Effects of combined prophylactic and therapeutic use of (R)-ketamine on plasma inflammatory cytokines after LPS treatment. a Treatment schedule. Adult mice were intraperitoneally injected with lipopolysaccharides (LPS, 5 mg/kg) or saline (10 ml/kg). (R)-Ketamine (10 mg/kg) or saline (10 ml/kg) were intraperitoneally injected to mice at 24 h before and 10 min after LPS injection. Plasma was collected 24 h after injection of LPS or saline. b Plasma levels of IL-6 (one-way ANOVA: F2,27 = 27.14, P < 0.0001). c Plasma levels of IL-17A (one-way ANOVA: F2,27 = 10.26, P = 0.0005). d Plasma levels of IFN-γ (one-way ANOVA: F2,27 = 3.519, P = 0.0439). e There was a positive correlation (r = 0.599, P = 0.001) between spleen weight and plasma IL-6. f There was a positive correlation (r = 0.451, P = 0.012) between spleen weight and plasma IL-17A. g There was a positive correlation (r = 0.641, P < 0.001) between spleen weight and plasma IFN-γ. Data are shown as mean ± SEM (n = 10/group). *P < 0.05, **P < 0.01, ***P < 0.0001. IFN-γ, interferon; IL, interleukin; LPS, lipopolysaccharides; R-KT, (R)-ketamine
In contrast, pretreatment with (R)-ketamine (24 h before LPS injection) did not alter the spleen weight and the ratio of spleen weight/body weight in mice after LPS administration (Supplemental Fig. 1a–d). In addition, a single administration of (R)-ketamine (10 min after LPS injection) did not affect the spleen weight, the ratio of spleen weight/body weight, and the plasma levels of inflammatory cytokines (IL-6, IL-17A, and IFN-γ) after LPS administration (Supplemental Fig. 2a–g).
Effects of (R)-ketamine on the neuroinflammation and delirium-related cognitive impairment after LPS administration
Western blot analysis showed increased expression of Iba-1, IL-6, IL-17A, IFN-γ, and iNOS in both the PFC and hippocampus after LPS (5 mg/kg) administration. Increased protein levels of Iba-1, IL-6, IL-17A, IFN-γ, and iNOS in both the PFC and hippocampus by LPS were significantly attenuated by the combined prophylactic and therapeutic use of (R)-ketamine (Fig. 3b–k).
Effects of combined prophylactic and therapeutic use of (R)-ketamine on the neuroinflammation and cognitive function after LPS treatment. a Treatment schedule. Adult mice were intraperitoneally injected with lipopolysaccharides (LPS, 5 mg/kg) or saline (10 ml/kg). (R)-Ketamine (R-KT; 10 mg/kg) or saline (10 ml/kg) were intraperitoneally injected to mice at 24 h before and 10 min after LPS injection. The Y maze test was performed 26 h before and 22 h after injection of LPS or saline. The buried food test was performed 24 h before and 24 h after injection of LPS or saline. The prefrontal cortex (PFC) and hippocampus were collected immediately after the buried food test. b, c Western blot analysis of ionized calcium-binding adapter molecule 1 (Iba-1) and β-actin in the PFC (one-way ANOVA: F2,27 = 7.494, P = 0.0026) and hippocampus (one-way ANOVA: F2,27 = 5.805, P = 0.0080). d, e Western blot analysis of IL-6 and β-actin in the PFC (one-way ANOVA: F2,27 = 4.811, P = 0.0163) and hippocampus (one-way ANOVA: F2,27 = 3.822, P = 0.0345). f, g Western blot analysis of IL-17A and β-actin in the PFC (one-way ANOVA: F2,27 = 6.467, P = 0.0072) and hippocampus (one-way ANOVA: F2,27 = 6.701, P = 0.0043). h, i Western blot analysis of IFN-γ and β-actin in the PFC (one-way ANOVA: F2,27 = 5.897, P = 0.0075) and hippocampus (one-way ANOVA: F2,27 = 5.999, P = 0.0070). j, k Western blot analysis of iNOS and β-actin in the PFC (one-way ANOVA: F2,27 = 10.45, P = 0.0004) and hippocampus (one-way ANOVA: F2,27 = 7.673, P = 0.0023). l, m Entries in the novel arm (one way ANOVA: F2,27 = 16.11, P < 0.0001) and duration in the novel arm (one way ANOVA: F2,27 = 6.941, P = 0.0037) in the Y maze test. n The latency to eat food in the buried food test (one-way ANOVA: F2,27 = 10.75, P = 0.0004). Data are shown as mean ± SEM (n = 10/group). *P < 0.05, **P < 0.01, ***P < 0.0001. IFN-γ, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase
In the Y maze test, LPS (5 mg/kg) significantly reduced the number of mice’s entries and duration in the novel arm as compared to the saline control (Fig. 3l, m), suggesting cognitive impairment. LPS-induced reduction of entries and duration in the novel arm was significantly attenuated by the combined prophylactic and therapeutic use of (R)-ketamine (Fig. 3l, m).
The buried food test has been used to check for the ability to smell volatile odors (Peng et al. 2016). Combined prophylactic and therapeutic use of (R)-ketamine significantly attenuated LPS (5 mg/kg, 24 h after injection)-induced increase in the latency of mice to eat the food as compared to the saline control (Fig. 3n). In contrast, a single administration of (R)-ketamine (i.e., 24 h before or 10 min after LPS injection) did not affect the latency to eat the food as compared to the saline control (Supplemental Fig. 1h, 2e).
Discussion
The major findings of this study are as follows: first, systemic administration with high dose of LPS (5 mg/kg) caused an increase in the systemic inflammation, neuroinflammation, and delirium-related cognitive impairment, consistent with the previous reports (Hoogland et al. 2018; Oliveira-Lima et al. 2019; Yang et al. 2020). Furthermore, we found a notable protective effects of (R)-ketamine on LPS-induced delirium-related cognitive impairment, which could be associated with substantially significant decrease in the pro-inflammatory cytokines in the plasma, and a decrease in the activated microglia and pro-inflammatory cytokines in both the PFC and hippocampus. Importantly, the treatment with (R)-ketamine (twice, 24 h before and 10 min after LPS injection) could reverse LPS-induced splenomegaly, systemic inflammation, neuroinflammation, and delirium-related cognitive impairment. In contrast, a single injection of (R)-ketamine (24 h before LPS injection or 10 min after LPS injection) did not protect against LPS-induced inflammatory events in mice. Collectively, it is likely that (R)-ketamine might have prophylactic and therapeutic effects for LPS-induced cognitive impairment.
The systemic administration of bacterial LPS to mice could trigger systemic inflammatory response, resulting in delirium and related cognitive impairment (Biesmans et al. 2013; Oliveira-Lima et al. 2019; Sultan et al. 2021; Yamanashi et al. 2021). Accumulating evidences suggested a close relationship between systemic inflammation-induced neuroinflammation and delirium-related cognitive impairment (Annane and Sharshar 2015; Jackson et al. 2009; Schmidt et al. 2002; Sultan et al. 2021; Yamanashi et al. 2021). The pathophysiology of delirium and its related cognitive impairment remains unclear. It has been reported that systemic administration of LPS could induce the release of pro-inflammatory cytokines and mediators in the brain, indicating that systemic inflammation could induce neuroinflammation in the brain (Hoogland et al. 2018; Sun et al. 2015; Yang et al. 2020). Activation of microglia cells and elevation of inflammatory cytokines [i.e., IL-1β, IL-6, IL-17A, TNF-α, IFN-γ, MCP-1, iNOS, and cyclooxygenase-2 (COX-2)] in the cortex and hippocampus after LPS administration have been suggested to play key roles in the pathophysiology of endotoxemia-derived cognitive impairment (Cunningham and Sanderson 2008; Curtin et al. 2009; Griffin et al. 2013; Hennessy et al. 2017; Hoogland et al. 2018; Jaehne et al. 2015; Lestage et al. 2002; Oliveira-Lima et al. 2019; Seemann and Lupp 2016; Skelly et al. 2019; Sun et al. 2015; Yang et al. 2020). It is reported that LPS-induced acute cognitive impairment can be reduced by systemic administration of IL-1 receptor antagonist (Cunningham and Sanderson 2008; Curtin et al. 2009; Griffin et al. 2013; Hennessy et al. 2017; Hoogland et al. 2018; Jaehne et al. 2015; Lestage et al. 2002; Oliveira-Lima et al. 2019; Seemann and Lupp 2016; Skelly et al. 2019; Sun et al. 2015; Yang et al. 2020). Systemic neutralization of IL-17A could attenuate LPS-induced activation of microglia cells, an increase in the hippocampal inflammatory mediators (i.e., IL-6, TNF-α, iNOS, and COX-2), and cognitive impairment (Sun et al. 2015). Microglia cells can produce various pro-inflammatory cytokines in the brain after LPS exposure (Fu et al. 2018; Peng et al. 2017). IL-17A plays important roles in the activation of microglia cells and it stimulates the synthesis and secretion of inflammatory cytokines (i.e., IL-6 and TNF-α) from activated microglia cells, in a dose-dependent manner (Fu et al. 2018; Peng et al. 2017). Collectively, the current data suggest that (R)-ketamine could produce potent anti-inflammatory effects in LPS (5 mg/kg)-treated mice although its precise mechanisms are currently unclear.
The PFC has been shown to play important roles in cognitive and behavioral functions (i.e., working memory, decision-making, autism, and depression) in humans and rodents (D’Esposito and Postle 2015; Pu et al. 2020; Yang et al. 2017; Zhang et al. 2018). Impairment in cognitive domains such as cognitive deficits and behavioral flexibility has been shown to be associated with a dysregulation of PFC activity (Karlsgodt et al. 2009). Hippocampus is also a key brain region involved in the pathogenesis of cognitive deficits (Lisman et al. 2017). Systemic LPS injection-induced neuroinflammation in the PFC and hippocampus contributes essentially to cognitive impairment (Griffin et al. 2013; Hennessy et al. 2017; Makinson et al. 2019; Sun et al. 2015). In this study, we found that treatment with (R)-ketamine (twice, 24 h before and 10 min after LPS injection) significantly attenuated LPS (5 mg/kg)-induced increase in the plasma levels of pro-inflammatory cytokines (IL-6, IL-17A, and IFN-γ), which was accompanied by a significant reduction in the protein expression of Iba-1, pro-inflammatory cytokines (IL-6, IL-17A, and IFN-γ), and iNOS in the PFC and hippocampus. The data suggest that combined prophylactic and therapeutic use of (R)-ketamine could improve LPS-induced cognitive impairment through potent anti-inflammatory actions in the periphery and CNS.
In contrast, a single prophylactic (24 h before LPS injection) or therapeutic (10 min after LPS injection) use of (R)-ketamine had no significant protective effects on splenomegaly, systemic inflammation, and cognitive impairment in LPS (5 mg/kg)-treated mice. Interestingly, the previous studies demonstrated that pre- or post-treatment with (R,S)-ketamine could exert protective effects against inflammatory responses induced by administration of LPS (0.5–1.0 mg/kg) (Huang et al. 2019a, b; Mastrodonato et al. 2020). Post-treatment with (R)-ketamine could also attenuate LPS (0.5 mg/kg)-induced depression-like behaviors in mice (Yamaguchi et al. 2018; Yang et al. 2017). In this study, we found that a single pre- or post-treatment with (R)-ketamine had no significant effects on systemic inflammation and cognitive impairment induced by LPS (5 mg/kg, i.p.), suggesting that single pre- or post-treatment with (R)-ketamine could not exert anti-inflammatory effects on LPS (5 mg/kg)-induced systemic inflammation and neuroinflammation. At present, the precise mechanisms underlying potent anti-inflammatory action by treatment with (R)-ketamine (twice, 24 h before and 10 min after LPS injection) are unclear. Further detailed study is needed.
From the current data, it is unclear whether (R)-ketamine could ameliorate inflammation in the periphery and brain independently. We previously reported that blockade of IL-6 receptor in the periphery promoted rapid and sustained antidepressant-like effects in a chronic social defeat stress model although intracerebroventricular injection of IL-6 receptor antibody did not exert antidepressant-like effects in the same model (Zhang et al. 2017). Thus, it is possible that anti-inflammatory effects in the periphery might contribute to anti-neuroinflammatory effects in the brain, resulting in improvement of depression-like behaviors. Collectively, it is likely that the close relationship between systemic inflammation-induced neuroinflammation and delirium-related cognitive impairment may play a role in the beneficial effects of (R)-ketamine although further study is needed.
Postoperative delirium is a form of delirium in patients who have undergone surgery and anesthesia, usually peaking 1–3 days after their operation. Furthermore, postoperative delirium and cognitive impairment have been associated with increased mortality (Deiner and Silverstein 2009; Duning et al. 2021; Whitlock et al. 2011). It is, therefore, very important to prevent the development of postoperative delirium and cognitive impairment. Since there are several intraoperative risk factors for delirium, it is of great interest to prevent postoperative delirium in patients who have undergone surgery. From the current data, it is possible that (R)-ketamine might have prophylactic effects for postoperative delirium and cognitive impairment in patients with surgery. A phase 1 study showed safety profile of (R)-ketamine in healthy subjects (Wei et al. 2021). Therefore, a future randomized double-blind placebo-controlled trial of (R)-ketamine is of great interest to prevent the development of postoperative delirium and cognitive impairment.
Conclusions
Combined prophylactic and therapeutic use of (R)-ketamine could attenuate systemic inflammation, neuroinflammation, and delirium-related cognitive impairment in mice after administration of high dose of LPS. Our understanding of beneficial actions of (R)-ketamine in the LPS-induced neuroinflammation will undoubtedly shed light on novel therapeutic strategies for the treatment of bacterial LPS-induced delirium and related cognitive impairment.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ELISA :
-
Enzyme-linked immunosorbent assay
- Iba-1 :
-
Ionized calcium-binding adapter molecule 1
- IFN :
-
Interferon
- IL :
-
Interleukin
- iNOS :
-
Inducible nitric oxide synthase
- LPS :
-
Lipopolysaccharide
- NMDAR :
-
N-Methyl-d-aspartate receptor
- PFC :
-
Prefrontal cortex
- TNF-α :
-
Tumor necrosis factor-α
References
Alqahtani F, Assiri MA, Mohany M, Imran I, Javaid S, Rasool MF, Shakeel W, Sivandzade F, Alanazi AZ, Al-Rejaie SS, Alshammari MA, Alasmari F, Alanazi MM, Alamri FF (2020) Coadministration of ketamine and perampanel improves behavioral function and reduces inflammation in acute traumatic brain injury mouse model. Biomed Res Int 2020:3193725. https://doi.org/10.1155/2020/3193725
Annane D, Sharshar T (2015) Cognitive decline after sepsis. Lancet Respir Med 3:61–69. https://doi.org/10.1016/S2213-2600(14)70246-2
Biesmans S, Meert TF, Bouwknecht JA, Acton PD, Davoodi N, De Haes P, Kuijlaars J, Langlois X, Matthews LJ, Ver DL, Hellings N, Nuydens R (2013) Systemic immune activation leads to neuroinflammation and sickness behavior in mice. Mediators Inflamm 2013:271359. https://doi.org/10.1155/2013/271359
Chang EI, Zarate MA, Arndt TJ, Richards EM, Rabaglino MB, Keller-Wood M, Wood CE (2018a) Ketamine reduces inflammation pathways in the hypothalamus and hippocampus following transient hypoxia in the late-gestation fetal sheep. Front Physiol 9:1858. https://doi.org/10.3389/fphys.2018.01858
Chang L, Toki H, Qu Y, Fujita Y, Mizuno-Yasuhira A, Yamaguchi JI, Chaki S, Hashimoto K (2018b) No sex-specific differences in the acute antidepressant actions of (R)-ketamine in an inflammation model. Int J Neuropsychopharmacol 21:932–937. https://doi.org/10.1093/ijnp/pyy053
Cunningham C, Maclullich AM (2013) At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun 28:1–13. https://doi.org/10.1016/j.bbi.2012.07.012
Cunningham C, Sanderson DJ (2008) Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain Behav Immun 22:1117–1127. https://doi.org/10.1016/j.bbi.2008.05.007
Curtin NM, Boyle NT, Mills KH, Connor TJ (2009) Psychological stress suppresses innate IFN-gamma production via glucocorticoid receptor activation: reversal by the anxiolytic chlordiazepoxide. Brain Behav Immun 23:535–547. https://doi.org/10.1016/j.bbi.2009.02.003
Deiner S, Silverstein JH (2009) Postoperative delirium and cognitive dysfunction. Bri J Anesth 103:i41-146. https://doi.org/10.1093/bja/aep291
De Kock M, Loix S, Lavand’Homme P (2013) Ketamine and peripheral inflammation. CNS Neurosci Ther 19:403–410. https://doi.org/10.1111/cns.12104
D’Esposito M, Postle BR (2015) The cognitive neuroscience of working memory. Annu Rev Psychol 66:115–142. https://doi.org/10.1146/annurev-psych-010814-015031
Draper A, Koch RM, van der Meer JW, Aj AM, Pickkers P, Husain M, van der Schaaf ME (2018) Effort but not reward sensitivity is altered by acute sickness induced by experimental endotoxemia in humans. Neuropsychopharmacology 43:1107–1118. https://doi.org/10.1038/npp.2017.231
Duning T, Ilting-Reuke K, Beckhuis M, Oswald D (2021) Postoperative delirium – treatment and prevention. Curr Opin Anaesthesiol 34:27–32. https://doi.org/10.1097/ACO.0000000000000939
Fu L, Zhu P, Qi S, Li C, Zhao K (2018) MicroRNA-92a antagonism attenuates lipopolysaccharide (LPS)-induced pulmonary inflammation and injury in mice through suppressing the PTEN/AKT/NF-kappaB signaling pathway. Biomed Pharmacother 107:703–711. https://doi.org/10.1016/j.biopha.2018.08.040
Fujita Y, Hashimoto K (2020) Decreased bone mineral density in ovariectomized mice is ameliorated after subsequent repeated intermittent administration of (R)-ketamine, but not (S)-ketamine. Neuropsychopharmacol Rep 40:401–406. https://doi.org/10.1002/npr2.12132
Fujita A, Fujita Y, Pu Y, Chang L, Hashimoto K (2020) MPTP-induced dopaminergic neurotoxicity in mouse brain is attenuated after subsequent intranasal administration of (R)-ketamine: a role of TrkB signaling. Psychopharmacology 237:83–92. https://doi.org/10.1007/s00213-019-05346-5
Fujita Y, Hashimoto Y, Hashimoto H, Chang L, Hashimoto K (2021) Dextran sulfate sodium-induced inflammation and colitis in mice are ameliorated by (R)-ketamine, but not (S)-ketamine: a role of TrkB signaling. Eur J Pharmacol 897:173954. https://doi.org/10.1016/j.ejphar.2021.173954
Griffin EW, Skelly DT, Murray CL, Cunningham C (2013) Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci 33:15248–15258. https://doi.org/10.1523/JNEUROSCI.6361-11.2013
Hashimoto K (2015) Inflammatory biomarkers as differential predictors of antidepressant response. Int J Mol Sci 16:7796–7801. https://doi.org/10.3390/ijms16047796
Hashimoto K (2019) Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci 73:613–627. https://doi.org/10.1111/pcn.12902
Hashimoto K (2020) Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem Pharmacol 177:113935. https://doi.org/10.1016/j.bcp.2020.113935
Hennessy E, Gormley S, Lopez-Rodriguez AB, Murray C, Murray C, Cunningham C (2017) Systemic TNF-alpha produces acute cognitive dysfunction and exaggerated sickness behavior when superimposed upon progressive neurodegeneration. Brain Behav Immun 59:233–244. https://doi.org/10.1016/j.bbi.2016.09.011
Hoogland I, Westhoff D, Engelen-Lee JY, Melief J, Valls SM, Houben-Weerts J, Huitinga I, van Westerloo DJ, van der Poll T, van Gool WA, van de Beek D (2018) Microglial activation after systemic stimulation with lipopolysaccharide and Escherichia coli. Front Cell Neurosci 12:110. https://doi.org/10.3389/fncel.2018.00110
Huang N, Hua D, Zhan G, Li S, Zhu B, Jiang R, Yang L, Bi J, Xu H, Hashimoto K, Luo A, Yang C (2019a) Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav 176:93–100. https://doi.org/10.1016/j.pbb.2018.12.001
Huang N, Wang Y, Zhan G, Yu F, Li S, Hua D, Jiang R, Li S, Wu Y, Yang L, Zhu B, Hua F, Luo A, Yang C (2019b) Contribution of skeletal muscular glycine to rapid antidepressant effects of ketamine in an inflammation-induced mouse model of depression. Psychopharmacology 236:3513–3523. https://doi.org/10.1007/s00213-019-05319-8
Inouye SK, Westendorp RG, Saczynski JS (2014) Delirium in elderly people. Lancet 383:911–922. https://doi.org/10.1016/S0140-6736(13)60688-1
Jackson JC, Hopkins RO, Miller RR, Gordon SM, Wheeler AP, Ely EW (2009) Acute respiratory distress syndrome, sepsis, and cognitive decline: a review and case study. South Med J 102:1150–1157
Jaehne EJ, Corrigan F, Toben C, Jawahar MC, Baune BT (2015) The effect of the antipsychotic drug quetiapine and its metabolite norquetiapine on acute inflammation, memory and anhedonia. Pharmacol Biochem Behav 135:136–144. https://doi.org/10.1016/j.pbb.2015.05.021
Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD (2009) Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res 108:143–150. https://doi.org/10.1016/j.schres.2008.12.025
Kealy J, Murray C, Griffin EW, Lopez-Rodriguez AB, Healy D, Tortorelli LS, Lowry JP, Watne LO, Cunningham C (2020) Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J Neurosci 40:5681–5696. https://doi.org/10.1523/JNEUROSCI.2876-19.2020
Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK (2008) One-year health care costs associated with delirium in the elderly population. Arch Intern Med 168:27–32. https://doi.org/10.1001/archinternmed.2007.4
Lestage J, Verrier D, Palin K, Dantzer R (2002) The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun 16:596–601. https://doi.org/10.1016/s0889-1591(02)00014-4
Lisman J, Buzsaki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD (2017) Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci 20:1434–1447. https://doi.org/10.1038/nn.4661
Lu Y, Ding X, Wu X, Huang S (2020) Ketamine inhibits LPS-mediated BV2 microglial inflammation via NMDA receptor blockage. Fundam Clin Pharmacol 34:229–237. https://doi.org/10.1111/fcp.12508
Makinson R, Lloyd K, Grissom N, Reyes TM (2019) Exposure to in utero inflammation increases locomotor activity, alters cognitive performance and drives vulnerability to cognitive performance deficits after acute immune activation. Brain Behav Immun 80:56–65. https://doi.org/10.1016/j.bbi.2019.02.022
Mastrodonato A, Cohensedgh O, LaGamma CT, McGowan JC, Hunsberger HC, Denny CA (2020) Prophylactic (R, S)-ketamine selectively protects against inflammatory stressors. Behav Brain Res 378:112238. https://doi.org/10.1016/j.bbr.2019.112238
McCusker J, Cole MG, Dendukuri N, Belzile E (2003) Does delirium increase hospital stay? J Am Geriatr Soc 51:1539–1546. https://doi.org/10.1046/j.1532-5415.2003.51509.x
Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C (2012) Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging 33:603-616.e3. https://doi.org/10.1016/j.neurobiolaging.2010.04.002
Oliveira-Lima OC, Carvalho-Tavares J, Rodrigues MF, Gomez MV, Oliveira A, Resende RR, Gomez RS, Vaz BG, Pinto M (2019) Lipid dynamics in LPS-induced neuroinflammation by DESI-MS imaging. Brain Behav Immun 79:186–194. https://doi.org/10.1016/j.bbi.2019.01.029
Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369:1306–1316. https://doi.org/10.1056/NEJMoa1301372
Peng M, Zhang C, Dong Y, Zhang Y, Nakazawa H, Kaneki M, Zheng H, Shen Y, Marcantonio ER, Xie Z (2016) Battery of behavioral tests in mice to study postoperative delirium. Sci Rep 6:29874. https://doi.org/10.1038/srep29874
Peng Z, Gong X, Yang Y, Huang L, Zhang Q, Zhang P, Wan R, Zhang B (2017) Hepatoprotective effect of quercetin against LPS/d-GalN induced acute liver injury in mice by inhibiting the IKK/NF-kappaB and MAPK signal pathways. Int Immunopharmacol 52:281–289. https://doi.org/10.1016/j.intimp.2017.09.022
Pu Y, Yang J, Chang L, Qu Y, Wang S, Zhang K, Xiong Z, Zhang J, Tan Y, Wang X, Fujita Y, Ishima T, Wang D, Hwang SH, Hammock BD, Hashimoto K (2020) Maternal glyphosate exposure causes autism-like behaviors in offspring through increased expression of soluble epoxide hydrolase. Proc Natl Acad Sci U S A 117:11753–11759. https://doi.org/10.1073/pnas.1922287117
Savage JC, St-Pierre MK, Hui CW, Tremblay ME (2019) Microglial ultrastructure in the hippocampus of a lipopolysaccharide-induced sickness mouse model. Front Neurosci 13:1340. https://doi.org/10.3389/fnins.2019.01340
Schedlowski M, Engler H, Grigoleit JS (2014) Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav Immun 35:1–8. https://doi.org/10.1016/j.bbi.2013.09.015
Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ (2002) Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol 52:168–174. https://doi.org/10.1002/ana.10265
Seemann S, Lupp A (2016) Administration of AMD3100 in endotoxemia is associated with pro-inflammatory, pro-oxidative, and pro-apoptotic effects in vivo. J Biomed Sci 23:68. https://doi.org/10.1186/s12929-016-0286-8
Skelly DT, Griffin EW, Murray CL, Harney S, O’Boyle C, Hennessy E, Dansereau MA, Nazmi A, Tortorelli L, Rawlins JN, Bannerman DM, Cunningham C (2019) Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry 24:1533–1548. https://doi.org/10.1038/s41380-018-0075-8
Sultan ZW, Jaeckel ER, Krause BM, Grady SM, Murphy CA, Sanders RD, Banks MI (2021) Electrophysiological signatures of acute systemic lipopolysaccharide-induced inflammation: potential implications for delirium science. Br J Anaesth 126:996–1008. https://doi.org/10.1016/j.bja.2020.12.040
Sun J, Zhang S, Zhang X, Zhang X, Dong H, Qian Y (2015) IL-17A is implicated in lipopolysaccharide-induced neuroinflammation and cognitive impairment in aged rats via microglial activation. J Neuroinflammation 12:165. https://doi.org/10.1186/s12974-015-0394-5
Walker AK, Wing EE, Banks WA, Dantzer R (2019) Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol Psychiatry 24:1523–1532. https://doi.org/10.1038/s41380-018-0076-7
Ward JL, Harting MT, Cox CJ, Mercer DW (2011) Effects of ketamine on endotoxin and traumatic brain injury induced cytokine production in the rat. J Trauma 70:1471–1479. https://doi.org/10.1097/TA.0b013e31821c38bd
Wei Y, Chang L, Hashimoto K (2020) A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol Biochem Behav 190:172870. https://doi.org/10.1016/j.pbb.2020.172870
Wei Y, Chang L, Hashimoto K (2021) Molecular mechanisms underlying the antidepressant actions of arketamine: beyond the NMDA receptor. Mol Psychiatry in Press. https://doi.org/10.1038/s41380-021-01121-1
Whitlock EL, Vannucci A, Avidan MS (2011) Postoperative delirium. Mineva Anesthesiol 77:448–456
Xiong Z, Chang L, Qu Y, Pu Y, Wang S, Fujita Y, Ishima T, Chen J, Hashimoto K (2020) Neuronal brain injury after cerebral ischemic stroke is ameliorated after subsequent administration of (R)-ketamine, but not (S)-ketamine. Pharmacol Biochem Behav 191:172904. https://doi.org/10.1016/j.pbb.2020.172904
Yamaguchi JI, Toki H, Qu Y, Yang C, Koike H, Hashimoto K, Mizuno-Yasuhira A, Chaki S (2018) (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology 43:1900–1907. https://doi.org/10.1038/s41386-018-0084-y
Yamanashi T, Malicoat JR, Steffen KT, Zarei K, Li R, Purnell BS, Najafi A, Saito K, Singh U, Toth BA, Lee S, Dailey ME, Cui H, Kaneko K, Cho HR, Iwata M, Buchanan GF, Shinozaki G (2021) Bispectral EEG (BSEEG) quantifying neuro-inflammation in mice induced by systemic inflammation: a potential mouse model of delirium. J Psychiatr Res 133:205–211. https://doi.org/10.1016/j.jpsychires.2020.12.036
Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K (2015) Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry 77:e19–e20. https://doi.org/10.1016/j.biopsych.2014.06.021
Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K (2017) (R)-Ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol Psychiatry 82:e43–e44. https://doi.org/10.1016/j.biopsych.2016.12.020
Yang C, Yang JJ, Luo A, Hashimoto K (2019) Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry 9:280. https://doi.org/10.1038/s41398-019-0624-1
Yang L, Zhou R, Tong Y, Chen P, Shen Y, Miao S, Liu X (2020) Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol Dis 140:104814. https://doi.org/10.1016/j.nbd.2020.104814
Zhang K, Hashimoto K (2019) Lack of opioid system in the antidepressant actions of ketamine. Biol Psychiatry 85:e25–e27. https://doi.org/10.1016/j.biopsych.2018.11.006
Zhang JC, Li SX, Hashimoto K (2014a) R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141. https://doi.org/10.1016/j.pbb.2013.11.033
Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, Li SX, Shirayama Y, Hashimoto K (2014b) Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol 18: pyu077. https://doi.org/10.1093/ijnp/pyu077.
Zhang JC, Yao W, Hashimoto K (2016) Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol 14:721–731. https://doi.org/10.2174/1570159x14666160119094646
Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, Hashimoto K (2017) Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry 7:e1138. https://doi.org/10.1038/tp.2017.112
Zhang K, Dong C, Fujita Y, Fujita A, Hashimoto K (2018) 5-Hydroxytryptamine-independent antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int J Neuropsychopharmacol 21:157–163. https://doi.org/10.1093/ijnp/pyx100
Zhang J, Qu Y, Chang L, Pu Y, Hashimoto K (2019) (R)-Ketamine rapidly ameliorates the decreased spine density in the medial prefrontal cortex and hippocampus of susceptible mice after chronic social defeat stress. Int J Neuropsychopharmacol 22:675–679. https://doi.org/10.1093/ijnp/pyz048
Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K (2020) A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry 10:186. https://doi.org/10.1038/s41398-020-00878-3
Funding
This study was supported by grant from Japan Agency for Medical Research and Development (AMED) (to K.H., JP20dm0107119).
Author information
Authors and Affiliations
Contributions
KH worked on experimental design. JCZ, LM, XYW, JJS, and YGQ performed the experiments. JCZ analyzed the data. KH and JCZ contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, USA, and approved by the Chiba University Institutional Animal Care and Use Committee (permission number: 2–308).
Consent for publication
Not applicable.
Competing interests
Dr. Hashimoto is the inventor of filed patent applications on “The use of R-ketamine in the treatment of psychiatric diseases,” “(S)-norketamine and salt thereof as pharmaceutical,” “R-ketamine and derivative thereof as prophylactic or therapeutic agent for neurodegeneration disease or recognition function disorder,” “Preventive or therapeutic agent and pharmaceutical composition for inflammatory diseases or bone diseases,” and “R-ketamine and its derivatives as a preventive or therapeutic agent for a neurodevelopmental disorder” by the Chiba University. Dr. Hashimoto also declares that he has received research support and consultant from Dainippon Sumitomo, Otsuka, and Taisho. The other authors have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiancheng Zhang and Li Ma contributed equally to this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Ma, L., Wan, X. et al. (R)-Ketamine attenuates LPS-induced endotoxin-derived delirium through inhibition of neuroinflammation. Psychopharmacology 238, 2743–2753 (2021). https://doi.org/10.1007/s00213-021-05889-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05889-6