Abstract
Rationale
Basic and clinical studies have reported rapid and long-lasting antidepressant effects of ketamine. Although previous studies have proposed several mechanisms underlying the antidepressant effects of ketamine, these mechanisms have not been completely elucidated.
Objectives
The present study evaluated the effects of systemically administered ketamine treatment in a lipopolysaccharide (LPS)-induced mouse model of depression.
Methods
Non-targeted metabolomics, western blotting, and behavioral tests (locomotion, tail suspension, and forced swimming tests) were performed.
Result
Ketamine significantly attenuated the abnormally increased immobility time in a lipopolysaccharide (LPS)-induced mouse model of depression. Aminomalonic acid, glutaraldehyde, glycine, histidine, N-methyl-l-glutamic acid, and ribose levels in skeletal muscle were altered following ketamine administration. Furthermore, ketamine significantly decreased the LPS-induced increase in glycine receptor A1 (GlyA1) levels. However, the glycine receptor antagonist strychnine did not elicit any pharmacological effects on ketamine-induced alterations in behaviors or muscular GlyA1 levels. Exogenous glycine and l-serine significantly improved depression-like symptoms in LPS-induced mice.
Conclusions
Our findings suggest that skeletal muscular glycine contributes to the antidepressant effects of ketamine in inflammation. Effective strategies for improving skeletal muscular glycine levels may be a novel approach to depression treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is characterized by significant and persistent low mood (Smith 2014; Xia et al. 2018). Currently, depression treatment relies on conventional antidepressants that increase serotonin and norepinephrine levels in the synaptic cleft (Brunello et al. 2002). However, approximately one-third of the patients with depression do not gain the benefits or satisfactory effects of conventional antidepressants (Zhang et al. 2016; Zhu et al. 2016). Ketamine—an N-methyl-d-aspartic acid receptor (NMDAR) antagonist—produces rapid and long-lasting therapeutic effects against refractory major depressive disorder and bipolar depression (Dang et al. 2014; Zhu et al. 2016). Although an increasing number of studies are investigating the therapeutic mechanisms underlying the antidepressant effects of ketamine, precise mechanisms have not been completely elucidated.
Physical exercise is beneficial for recovery from depression (Archer et al. 2014). A meta-analysis including 23 randomized controlled trials and 977 participants showed that physical exercise is an effective therapeutic alternative for pharmaceutical approaches to depression treatment (Kvam et al. 2016). Improvements in the peroxisome proliferator-activated receptor γ coactivator α (PGC-1α)-dependent kynurenine metabolism contribute to the resilience of stress-induced depression (Agudelo et al. 2014). Furthermore, we have previously reported that the upregulation of the PGC-1α-fibronectin type III domain-containing protein 5–brain-derived neurotrophic factor (BDNF) signaling pathway in skeletal muscles is associated with stress resilience in mice subjected to chronic social defeat (Zhan et al. 2018). These findings suggest that depression onset is associated with the degradation of muscle function and that improvements of this function may alleviate depression.
Glycine confers important physiological effects, particularly in the muscle and brain tissues (Ito 2016; Koopman et al. 2017). The present study employed metabolomics and sequencing analysis in LPS-induced mouse models of depression to investigate the contribution of the skeletal muscular glycine signaling in antidepressant effects of ketamine. We hypothesized that skeletal muscular glycine levels can be a novel target for depression treatment and that ketamine can serve as a novel antidepressant.
Materials and methods
Animals
Male C57BL/6 mice (age, 2 months; body weight, 20–25 g) were used. All animals were obtained from the Animal Center of Tongji Hospital (Wuhan, China). Animals were housed in polypropylene cages under a 12/12 h light/dark cycle at 24 °C–26 °C. Food and water were provided ad libitum. Experimental protocols and animal handing conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised in 1996), and the study was approved by the Experimental Animal Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Drugs and treatment
Ketamine hydrochloride (Cat No. 1707032) was purchased from Fujian Gutian Pharmaceutical Co., Ltd. (Fujian, China). Glycine was purchased from Guangzhou Saiguo Biotech Co., Ltd. (Berlin, Germany). l-serine was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Strychnine was purchased from Shanghai Research & Development Co., Ltd. (Shanghai, China). Strychnine (1.0 μg/kg) or equal volume of vehicle was intravenously (i.v.) administrated after 55 min of intraperitoneal (i.p.) lipopolysaccharide (LPS) (0.5 mg/kg, Sigma-Aldrich Co., St Louis, MO, USA) administration. Ketamine (10 mg/kg, i.p.), glycine (120 mg/kg, i.p.), l-serine (168 mg/kg, i.p.), or equal volume of vehicle was singly administered after 1 h of LPS (0.5 mg/kg, i.p.) administration. Behavioral assessments, including the locomotion test (LMT) (24 h after LPS administration), tail suspension test (TST) (26 h after LPS administration), and forced swimming test (FST) (2 h after TST or 24 h after LPS administration), were performed. Body weight of each mouse was measured at the baseline and immediately after behavioral tests. Tissue samples were collected immediately after the mice were sacrificed.
Behavioral tests
LMT
The locomotor ability of each mouse was measured using YH-OF-M/R, which is an animal behavior analysis system (Yihong Co., Ltd., Wuhan, China), as previously described (Huang et al. 2019). The animals were placed in polypropylene cages (1000 × 1000 × 45 mm3) for 5 min. Behavioral traces and data were recorded.
TST
TST is an animal test for the efficacy of antidepressant treatment (Yang et al. 2017). The mice were suspended on a hook using a piece of adhesive tape. The duration of mouse immobility was measured during a 10-min period. The mouse was considered immobile while hanging passively and completely motionless (Cryan et al. 2005).
FST
FST was assessed using the automated forced swim apparatus YH-FST (Yihong Co., Ltd., Wuhan, China), as previously described (Huang et al. 2019). The mice were placed in an open cylinder (25 × 35 cm2) filled with water (depth, 20 cm) at 23 °C ± 1 °C, and the mouse immobility time was recorded during a 5-min period.
Nontargeted metabolomics tests
Samples preparation
After the mice were deeply anesthetized with 10% chloral hydrate, the right hindlimb muscle was collected from the control, LPS + vehicle, and LPS + ketamine groups (n = 8 for each group). Approximately 30 mg of muscle samples was harvested and placed into 1.5-mL Eppendorf tubes. A few small metal beads were added to the tube. Internal standard (2-chloro-l-phenylalanine in methanol, 20 μL; 0.3 mg/mL) and extraction solvent with methanol/water (v:v = 4:1) (600 μL) were added to the samples. Samples were homogenized at 60 Hz for 2 min, and 120 μL of chloroform was added. The samples were vortexed thoroughly, subjected to ultrasound-associated extraction for 10 min in ice water, stored at − 20 °C for 10 min, and centrifuged (12,000 rpm for 10 min) at 4 °C. The supernatant was transferred to Eppendorf tubes. Quality control (QC) samples were prepared by mixing 75-μL aliquots of each biological sample. Finally, the samples were analyzed via gas chromatography–mass spectrometry (GC–MS), as previously described (Li et al. 2017).
Metabolomics
Raw data in .D format were converted to .CDF format using ChemStation (version E.02.02.1431, Agilent, USA). The .CDF data were processed using ChromaTOF (version 4.34, LECO, St. Joseph, MI, USA) to obtain sample information, peak names (retention times and m/z), and peak intensities. Metabolites were annotated using the Fiehn (Kind et al. 2009) or National Institute of Standards and Technology (Halket et al. 1999) database. Internal standards, pseudo-positive peaks due to noise, column bleed, and derivatization procedure were removed, and multiple peaks from a single metabolite were combined to generate a final dataset for statistical analysis. All data were transformed by the LOG2 function in Excel 2007 (Microsoft, USA) prior to statistical analysis, and the data matrix was imported into the SIMCA software package (14.0, Umetrics, Umeå, Sweden). Data were analyzed using multivariate and univariate methods. First, the clustering of QC samples was examined by unsupervised principal component analysis (PCA). Thereafter, orthogonal partial least squares discriminant analysis (OPLS-DA) (Li et al. 2017) was performed to assess metabolic differences between the treatment groups. In the OPLS-DA model, variable importance in projection (VIP) scores were used to estimate the difference between individual metabolites. Two criteria were used to identify the significantly altered metabolites among the three groups: (1) A VIP score > 1.0 in the OPLS-DA model and (2) a P value < 0.05 and false discovery rate < 0.05 between two treatment groups.
Western blotting
After tissue collection, metal beads and RIPA buffer were added to the samples, and the samples were homogenized by sonication. The samples were centrifuged (12,000 rpm for 15 min) at 4 °C, and the supernatant was transferred to Eppendorf tubes. Protein concentration in the supernatant was detected using a BCA protein assay kit (Boster, Wuhan, China). Each sample was separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and bands were transferred to a PVDF membrane. All membranes were blocked with 5% milk in 0.1% Tween-20 in Tris-buffered saline (TBST) for 2 h at room temperature. After blocking, membranes were incubated with rabbit anti-GlyA1 (1:1000, 17951-1-AP, Proteintech Goup, Inc., Wuhan, China), rabbit anti-GlyB (1:1000, 15371-1-AP, Proteintech Goup, Inc., Wuhan, China), or rabbit anti-GAPDH (1:1000, #AF7021, Affinity, Cincinnati, USA) primary antibodies overnight at 4 °C. All membranes were washed with TBST and then incubated with goat anti-rabbit IgG horseradish peroxidase (1:5000, Promoter, Wuhan, China) secondary antibody for 2 h at room temperature. The membranes were then washed with TBST and imaged with enhanced chemiluminescence reagents (Qidongzi, Wuhan, China) using Chemi-Doc-XRS system (Bio-Rad, Hercules, USA).
Statistical analysis
Data are shown as mean and standard error. Analyses were performed using GraphPad Prism 7.0 (GraphPad Software, CA, USA). Results were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. Body weight was analyzed by two-way ANOVA. P < 0.05 was considered statistically significant.
Results
Behavioral effects of ketamine in the LPS-induced mouse model of depression
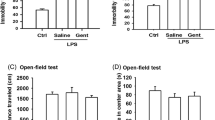
Ketamine (10 mg/kg) was administered to each mouse 1 h after LPS (0.5 mg/kg) administration (Fig. 1a). There were no significant differences in locomotion scores among the groups (Fig. 1b). However, ketamine significantly decreased the LPS-induced increase in FST immobility time (s. 1c). Furthermore, there were no significant changes in body weight before or after the behavioral tests across the three groups (Fig. 1d).
Behavioral effects of ketamine in the LPS-induced mouse model of depression. (a) Experimental schedule: Ketamine (10 mg/kg, i.p.) was injected 1 h after LPS (0.5 mg/kg, i.p.) administration. LMT and FST were conducted 23 and 25 h after LPS administration, respectively. Body weight was measured before and after the behavioral tests, and the tissues were collected. b Locomotion [treatment, F(2, 21) = 1.003, P > 0.05]. c Immobility time [treatment, F(2, 21) = 35.05, P < 0.001]. d Body weight [Group, F(1, 42) = 0.1123, P > 0.05; Column, F(2, 42) = 0.03743, P > 0.05; Interaction, F(2, 42) = 0.262, P > 0.05]. **P < 0.01 or ***P < 0.001. CONT, control group; FST, forced swimming test; i.p., intraperitoneal injection; i.v., intravenous injection; Ket, ketamine; LMT, locomotion test; LPS, lipopolysaccharide; N.S., not significant; Veh, vehicle
Global metabolite profiles among the groups
In PCA, the dissimilarity dots among the three groups were far away from each other, suggesting that the composition of metabolites in skeletal muscle were distinct among the groups (Fig. 2a–c, Table 1). PLS-DA and OPLS-DA demonstrated differential profiles of metabolites in the skeletal muscles of each group (Fig. 2d–i and Table 1).
Global metabolic profiles in the three groups. a PCA (All). b PCA (Control versus LPS + Saline). c PCA (LPS + Saline versus LPS + ketamine). d PLS-DA (Control versus LPS + Saline). e PLS-DA (LPS + Saline versus LPS + ketamine). f OPLS (Control versus LPS + Saline). g OPLS (LPS + Saline versus LPS + ketamine). h OPLS-DA with permutation 200 (Control versus LPS + Saline). i OPLS-DA with permutation 200 (LPS + Saline versus LPS + ketamine). OPLS, optimized potentials for liquid simulations; OPLS-DA, orthogonal partial least square-discriminate analysis; PCA, principal component analysis; PLS-DA, partial least squares discriminant analysis
Differential levels of metabolites in skeletal muscles
Heatmaps showed that metabolite levels in skeletal muscle were significantly different between the groups (Fig. 3a, b). LPS administration significantly decreased aminomalonic acid, glutaraldehyde, glycine, N-methyl-l-glutamic acid, and ribose levels and significantly increased histidine levels. These alterations were significantly attenuated following ketamine treatment (Table 2).
Heatmaps with hierarchical cluster analysis of metabolomics in skeletal muscle. a LPS + Veh group versus CONT group. b LPS + Ket group versus LPS + Veh group. The green rectangles represent a significant decrease in metabolite levels, and the red rectangles represent a significant increase in metabolite levels. Dendrograms present the relationships among different metabolites. a, control group; b, LPS + vehicle group; c, LPS + ketamine group; CONT, control; Ket, ketamine; LPS, lipopolysaccharide; Veh, vehicle
Effects of ketamine on GlyA1 and GlyB levels in the brain and skeletal muscles of the LPS-induced mouse model of depression
No significant difference was observed in GlyA1 and GlyB levels in the medial prefrontal cortex (mPFC) or hippocampus among the groups (Fig. 4a and b). However, in skeletal muscles, LPS significantly increased GlyA1 levels, and ketamine attenuated the increase in GlyA1 levels. However, there were no significant differences in skeletal muscle GlyB levels among the treatment groups (Fig. 4c).
GlyA1 and GlyB levels in mPFC, hippocampus, and muscle. a mPFC: GlyA1 [F(2, 21) = 0.09697, P > 0.05] and GlyB [F (2, 21) = 0.05426, P > 0.05]. b Hippocampus: GlyA1 [F (2, 21) = 0.7717, P > 0.05] and GlyB [F (2, 21) = 0.1928, P > 0.05]. c Muscle: GlyA1 [F (2, 21) = 6.516; P < 0.05] and GlyB [Group: F (2, 15) = 3.413, P > 0.05]. *P < 0.05. CONT, control; Ket, ketamine; LPS, lipopolysaccharide; mPFC, medial prefrontal cortex; N.S., not significant; Veh, vehicle
Strychnine did not affect the antidepressant effects of ketamine or GlyA1 and GlyB levels
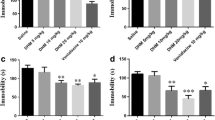
Strychnine was administered (i.v.) 5 min before ketamine administration to the LPS-induced mouse model of depression (Fig. 5a). Strychnine did not affect the ketamine-induced changes in TST and FST immobility times (Fig. 5b, c). Moreover, strychnine did not affect the protein levels of GlyA1 and GlyB induced by ketamine in mPFC, hippocampus, and skeletal muscles (Fig. 5d–f).
Effects of strychnine on behavior and GlyA1 and GlyB levels. a Experimental protocol: a single dose of strychnine (1.0 μg/kg, i.v.) was injected 5 min before ketamine treatment. Behavioral tests, including TST and FST, were conducted 24 and 26 h after LPS administration, respectively. The tissues were collected immediately after behavioral tests. b TST [F(3, 20) = 13.67, P < 0.001]. c FST [F(3, 20) = 7.906, P < 0.01]. d mPFC: GlyA1 [F(3, 20) = 0.2326, P > 0.05] and GlyB [F(3, 20) = 0.2057, P > 0.05]. e Hippocampus: GlyA1 [F(3, 20) = 0.5996, P > 0.05] and GlyB [F(3, 20) = 1.06, P > 0.05]. f Muscle: GlyA1 [F(3, 20) = 3.873, P < 0.05] and GlyB [F(3, 20) = 1.426, P > 0.05]. *P < 0.05, **P < 0.01 or ***P < 0.001. CONT, control group; FST, forced swimming test; i.p., intraperitoneal injection; i.v., intravenous injection; Ket, ketamine; LPS, lipopolysaccharide; mPFC, the medial prefrontal cortex; N.S., not significant; Str, strychnine; TST, tail suspension test; Veh, vehicle
Effects of glycine and l-serine on FST immobility time in the LPS-induced mouse model of depression
Glycine or l-serine was administered 1 h after LPS administration (Fig. 6a). LPS administration significantly increased FST immobility time, whereas glycine and l-serine administration significantly decreased FST immobility time; these results indicate the antidepressant effects of glycine and l-serine possess.
Effects of glycine or l-serine on FST immobility time in the LPS-induced mouse model of depression. a Experimental schedule: a single dose of glycine (120 mg/kg, i.p.) or l-serine (168 mg/kg, i.p.) was injected 1 h after LPS (0.5 mg/kg, i.p.) administration. FST was tested 23 h after LPS injection. b Glycine: FST [F(2, 15) = 14.08, P < 0.001]. cl-serine: FST [F(2, 15) = 9.237, P < 0.01]. *P < 0.05, **P < 0.01, or ***P < 0.001. CONT, control; FST, forced swimming test; i.p., intraperitoneal injection; Ket, ketamine; LPS, lipopolysaccharide; N.S., not significant; Veh, vehicle
Discussion
Ketamine produces rapid and long-lasting antidepressant effects (Huang et al. 2019; Li et al. 2010a; Yang et al. 2018a; Yang et al. 2017; Yang et al. 2015). Moreover, ketamine significantly improved depression-like symptoms in LPS-induced inflammation (Huang et al. 2019), chronic social defeat stress (Yang et al. 2018b), and learned helplessness (Shirayama and Hashimoto 2017). In the present study, we demonstrated beneficial effects of ketamine in the LPS-induced mouse model of depression and revealed that the mechanism underlying these therapeutic effects may be related to alterations in glycine metabolism in skeletal muscles. These findings suggest that skeletal muscles play pivotal roles in rapid antidepressant effects of ketamine in LPS-induced inflammation models. To the best of our knowledge, this may be the first study demonstrating roles of skeletal muscular glycine in the antidepressant effects of ketamine.
An imbalance in the inflammatory response plays a critical role in the mechanisms of depression (Dantzer et al. 2008). Patients with chronic inflammation are prone to develop depression. In addition, the activation of pro-inflammatory responses may increase the risk of depression (Dantzer et al. 2008). Abnormalities in the metabolism of peripheral blood (Li et al. 2010b) and brain tissues (Ni et al. 2008), such as cerebral cortex, hippocampus, and thalamus, have been observed in individuals with depression. Wu et al. (2017b) have indicated that metabolic dysfunctions of amino acids and purines in the hypothalamus might underlie the mechanisms of inflammation-induced depression. Collectively, these findings suggest that there is a causal link between depression, inflammation, and metabolism, although the precise mechanisms remain ambiguous.
Metabolomics is a method for quantitatively analyzing all metabolites in living organisms to investigate the association between metabolites and physiological and/or pathological alterations (Bujak et al. 2015). The dissimilarity dots among the three groups in PCA, PLS, and OPLS were far away from each other, indicating that the composition of skeletal muscle metabolites was rather distinct among the groups. Furthermore, these results suggest that skeletal muscle function plays a critical role in the pathogenesis of depression as well as in mechanisms underlying the antidepressant effects of ketamine. A cross-sectional study of 1046 elderly patients with depression demonstrated that muscle mass and strength are both negatively correlated with depressive symptoms in the Chinese population (Wu et al. 2017a). Previous studies have shown that BDNF downregulation in skeletal muscle promotes depression onset (Heyman et al. 2012; Zhan et al. 2018). On the contrary, physical exercise is an effective treatment strategy for depression and could be prescribed in a clinical setting as an adjunct treatment with antidepressants (Danielsson et al. 2013). These results regarding physical exercise further support our findings that the antidepressant effects of ketamine are associated with skeletal muscle improvements.
An increasing number of studies have suggested that glycine supplementation effectively improves muscle mass and function in models of cancer cachexia, sepsis, and reduced caloric intake (Koopman et al. 2017). In this study, we found that skeletal muscular glycine was significantly decreased in the depression-like phenotype, whereas ketamine attenuated these decreased glycine levels. This finding suggests that decreased skeletal muscular glycine levels can serve as a predisposing factor for depression onset and that the rapid antidepressant effects of ketamine are associated with increased skeletal muscular glycine levels. Although the exact mechanism of action of skeletal muscular glycine in the antidepressant effects of ketamine remains unclear, GLYX-13, which is an NMDAR glycine-site partial agonist, has been reported to produce antidepressant effects (Burgdorf et al. 2015). Collectively, these findings suggest that the skeletal muscular NMDAR glycine-binding site is a target in the antidepressant effects of ketamine.
The glycine receptor (GlyR) is an important inhibitory receptor in the central nervous system. GlyR is a Cl−-based selective channel protein, a member of the ligand-gated ion channel superfamily (Lynch et al. 2017), and comprises α and β subunits (Lynch 2004). We found that skeletal muscular GlyA1 levels were significantly increased after LPS exposure, whereas ketamine attenuated this change. These findings indicate that a nonisomorphic glycine–GlyR signaling pathway in skeletal muscles may be involved in the antidepressant effects of ketamine. What causes glycine levels to increase and the GlyR expression to decrease? This phenomenon may arise because the major binding site for glycine is not located on GlyRs but on NMDARs (Nong et al. 2003; Zhang et al. 2014), which are also the primary binding targets for the antidepressant effects of ketamine (Chaki 2017; Wohleb et al. 2017).
Strychnine is a specific antagonist of GlyR, which selectively excites the spinal cord, enhances the tension of skeletal muscle, and is clinically used for the treatment of palsy or amblyopia (Dutertre et al. 2012). In the present study, we evaluated the pharmacological impact of strychnine on the antidepressant effects of ketamine and observed no significant changes. Therefore, the antidepressant effect of ketamine is presumably related to glycine but not strychnine-sensitive GlyR in skeletal muscle. Glycine is localized to the strychnine-insensitive site associated with NMDARs (Nong et al. 2003; Zhang et al. 2014). However, the involvement of skeletal muscular glycine-NMDAR signaling in the antidepressant effects of ketamine requires further validation.
We found that glycine administration significantly improved depressive behaviors in LPS-induced mouse models. Interestingly, glycine and l-serine are reversibly metabolized by the enzyme serine hydroxymethyltransferase 1 (Shmt1) (Hashimoto 2014). Accordingly, we observed that l-serine administration alone exerted rapid antidepressant effects in the LPS-induced mouse model of depression. Although we did not conduct a thorough investigation of the antidepressant effects of d-serine, we infer that GlyR does not play a critical role in the antidepressant effects of ketamine via the activation of downstream effectors l-serine or d-serine and is physiologically bound to NMDAR.
In conclusion, rapid antidepressant effects of ketamine are likely related to increased glycine levels in skeletal muscles but not in brain tissues. Treatment strategies for increasing skeletal muscular glycine levels are a viable target for the research and development of rapid-onset antidepressants. Further studies of the mechanisms underlying the antidepressant effects of ketamine are warranted.
References
Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DMS, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL (2014) Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159:33–45
Archer T, Josefsson T, Lindwall M (2014) Effects of physical exercise on depressive symptoms and biomarkers in depression. CNS Neurol Disord Drug Targets 13:1640–1653
Brunello N, Mendlewicz J, Kasper S, Leonard B, Montgomery S, Nelson J, Paykel E, Versiani M, Racagni G (2002) The role of noradrenaline and selective noradrenaline reuptake inhibition in depression. Eur Neuropsychopharmacol 12:461–475
Bujak R, Struck-Lewicka W, Markuszewski MJ, Kaliszan R (2015) Metabolomics for laboratory diagnostics. J Pharm Biomed Anal 113:108–120
Burgdorf J, Zhang XL, Weiss C, Gross A, Boikess SR, Kroes RA, Khan MA, Burch RM, Rex CS, Disterhoft JF, Stanton PK, Moskal JR (2015) The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neuroscience 308:202–211
Chaki S (2017) Beyond ketamine: new approaches to the development of safer antidepressants. Curr Neuropharmacol 15:963–976
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
Dang YH, Ma XC, Zhang JC, Ren Q, Wu J, Gao CG, Hashimoto K (2014) Targeting of NMDA receptors in the treatment of major depression. Curr Pharm Des 20:5151–5159
Danielsson L, Noras AM, Waern M, Carlsson J (2013) Exercise in the treatment of major depression: a systematic review grading the quality of evidence. Physiother Theory Pract 29:573–585
Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56
Dutertre S, Becker CM, Betz H (2012) Inhibitory glycine receptors: an update. J Biol Chem 287:40216–40223
Halket JM, Przyborowska A, Stein SE, Mallard WG, Down S, Chalmers RA (1999) Deconvolution gas chromatography/mass spectrometry of urinary organic acids--potential for pattern recognition and automated identification of metabolic disorders. Rapid Commun Mass Spectrom 13:279–284
Hashimoto K (2014) Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin Ther Targets 18:1049–1063
Heyman E, Gamelin FX, Goekint M, Piscitelli F, Roelands B, Leclair E, Di Marzo V, Meeusen R (2012) Intense exercise increases circulating endocannabinoid and BDNF levels in humans--possible implications for reward and depression. Psychoneuroendocrinology 37:844–851
Huang N, Hua D, Zhan G, Li S, Zhu B, Jiang R, Yang L, Bi J, Xu H, Hashimoto K, Luo A, Yang C (2019) Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol Biochem Behav 176:93–100
Ito S (2016) GABA and glycine in the developing brain. J Physiol Sci 66:375–379
Kind T, Wohlgemuth G, Lee DY, Lu Y, Palazoglu M, Shahbaz S, Fiehn O (2009) FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem 81:10038–10048
Koopman R, Caldow MK, Ham DJ, Lynch GS (2017) Glycine metabolism in skeletal muscle: implications for metabolic homeostasis. Curr Opin Clin Nutr Metab Care 20:237–242
Kvam S, Kleppe CL, Nordhus IH, Hovland A (2016) Exercise as a treatment for depression: a meta-analysis. J Affect Disord 202:67–86
Li H, Cai J, Chen R, Zhao Z, Ying Z, Wang L, Chen J, Hao K, Kinney PL, Chen H, Kan H (2017) Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation 136:618–627
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010a) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
Li ZY, Zheng XY, Gao XX, Zhou YZ, Sun HF, Zhang LZ, Guo XQ, Du GH, Qin XM (2010b) Study of plasma metabolic profiling and biomarkers of chronic unpredictable mild stress rats based on gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 24:3539–3546
Lynch JW (2004) Molecular structure and function of the glycine receptor chloride channel. Physiol Rev 84:1051–1095
Lynch JW, Zhang Y, Talwar S, Estrada-Mondragon A (2017) Glycine receptor drug discovery. Adv Pharmacol 79:225–253
Ni Y, Su M, Lin J, Wang X, Qiu Y, Zhao A, Chen T, Jia W (2008) Metabolic profiling reveals disorder of amino acid metabolism in four brain regions from a rat model of chronic unpredictable mild stress. FEBS Lett 582:2627–2636
Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW (2003) Glycine binding primes NMDA receptor internalization. Nature 422:302–307
Shirayama Y, Hashimoto K (2017) Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur Arch Psychiatry Clin Neurosci 267:177–182
Smith K (2014) Mental health: a world of depression. Nature 515:181
Wohleb ES, Gerhard D, Thomas A, Duman RS (2017) Molecular and cellular mechanisms of rapid-acting antidepressants ketamine and scopolamine. Curr Neuropharmacol 15:11–20
Wu H, Yu B, Meng G, Liu F, Guo Q, Wang J, Du H, Zhang W, Shen S, Han P, Dong R, Wang X, Ma Y, Chen X, Niu K (2017a) Both muscle mass and muscle strength are inversely associated with depressive symptoms in an elderly Chinese population. Int J Geriatr Psychiatry 32:769–778
Wu Y, Li Y, Jia Y, Wei C, Xu H, Guo R, Li Y, Jia J, Qi X, Gao X (2017b) Imbalance in amino acid and purine metabolisms at the hypothalamus in inflammation-associated depression by GC-MS. Mol BioSyst 13:2715–2728
Xia CY, Wang ZZ, Yamakuni T, Chen NH (2018) A novel mechanism of depression: role for connexins. Eur Neuropsychopharmacol 28:483–498
Yang C, Kobayashi S, Nakao K, Dong C, Han M, Qu Y, Ren Q, Zhang JC, Ma M, Toki H, Yamaguchi JI, Chaki S, Shirayama Y, Nakazawa K, Manabe T, Hashimoto K (2018a) AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-Norketamine. Biol Psychiatry 84:591–600
Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017) Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry 7:1294
Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, Hashimoto K (2018b) Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry 83:18–28
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632
Zhan G, Huang N, Li S, Hua D, Zhang J, Fang X, Yang N, Luo A, Yang C (2018) PGC-1alpha-FNDC5-BDNF signaling pathway in skeletal muscle confers resilience to stress in mice subjected to chronic social defeat. Psychopharmacology 235:3351–3358
Zhang JC, Yao W, Hashimoto K (2016) Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol 14:721–731
Zhang XY, Ji F, Wang N, Chen LL, Tian T, Lu W (2014) Glycine induces bidirectional modifications in N-methyl-D-aspartate receptor-mediated synaptic responses in hippocampal CA1 neurons. J Biol Chem 289:31200–31211
Zhu W, Ding Z, Zhang Y, Shi J, Hashimoto K, Lu L (2016) Risks associated with misuse of ketamine as a rapid-acting antidepressant. Neurosci Bull 32:557–564
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (A.L. [81771159 and 81571047]; C.Y. [81703482]) and supported in part by the Program of the Bureau of Science and Technology Foundation of Changzhou (B.Z. [CJ20159022]; L.Y. [CJ20160030]) and Major Science and Technology Projects of Changzhou Municipal Committee of Health and Family Planning (B.Z. [ZD201505]; L.Y. [ZD201407]).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Dr. Chun Yang received research support from B. Braun Medical Inc. Other authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 257 kb)
Rights and permissions
About this article
Cite this article
Huang, N., Wang, Y., Zhan, G. et al. Contribution of skeletal muscular glycine to rapid antidepressant effects of ketamine in an inflammation-induced mouse model of depression. Psychopharmacology 236, 3513–3523 (2019). https://doi.org/10.1007/s00213-019-05319-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05319-8