Abstract
Rationale
Stress exposure has a lasting impact on motivated behavior and can exacerbate existing vulnerabilities for developing a substance use disorder. Several models have been developed to examine how stressful experiences shape drug reward. These range from locomotor sensitization and conditioned place preference to the propensity for drug self-administration or responding to drug-predictive cues. While self-administration studies are considered to have more translational relevance, most of the studies to date have been conducted in rats. Further, many self-administration studies are conducted in single-housed animals, adding the additional stressor of social isolation.
Objectives
We sought to establish how chronic social defeat stress (CSDS) and social housing conditions impact cocaine self-administration and cocaine-seeking behaviors in C57BL/6 mice.
Methods
We assessed self-administration behavior (cocaine or saline, 0.5 mg/kg/infusion) in C57BL/6 mice subjected to 10-day CSDS or in unstressed controls. Mice were housed either in pairs or in isolation during self-administration. We compared the effect of housing on acquisition of self-administration, seeking, extinction, drug-induced reinstatement, and after re-exposure to the social stressor.
Results
Pair-housing during self-administration revealed increased social avoidance after CSDS is associated with decreased cocaine intake. In contrast, single-housing revealed stress-sensitive cocaine intake, with increased social avoidance after CSDS associated with increased early cocaine intake. Pair-, but not single-housed mice are susceptible to drug-induced reinstatement independent of CSDS history. Stress re-exposure sensitized cocaine-seeking in stressed single-housed mice.
Conclusions
The social context surrounding cocaine intake can bidirectionally influence cocaine-related behaviors after psychosocial stress and should be considered when studying stress and drug cross-sensitization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute, moderate stress prepares animals to react to challenging stimuli by activating the hypothalamic pituitary adrenal (HPA) axis. However, repeated or severe stress has negative and debilitating effects often associated with the emergence of psychiatric disorders (Hollon et al. 2015; Sapolsky 2015). Psychosocial stress is a major risk factor for the development of drug addiction, where the cumulative number of stressful events is thought to predict future drug dependence (Lloyd and Turner 2008) (for review, see Sinha (2008)). In turn, drug intake increases HPA axis activation, supporting increased stress responses, amplifying drug craving, and promoting relapse (Newman et al. 2018; Wemm and Sinha 2019). The dynamic interplay between stress and drug exposure, whereby exposure to one increases the response to the other is usually termed “cross-sensitization.” Stress-sensitized responses to drugs of abuse play a major role in the development of addiction (Sinha 2008), particularly to psychostimulants (Kalivas and Stewart 1991; Newman et al. 2018).
Several rodent models have been developed to study the relationship between psychosocial stress exposure and substance abuse (Yap and Miczek 2008). Brief or intermittent social stress increases the acquisition of cocaine self-administration (Haney et al. 1995; Tidey and Miczek 1997), progressive ratio break points (Burke and Miczek 2015), or “binge” drug intake in rats (Covington 3rd and Miczek 2001). In contrast, when rats are exposed to prolonged social stress, the effects on cocaine intake are either subtle (Kabbaj et al. 2001), or cocaine self-administration is drastically reduced (Miczek et al. 2011) (for review, see Newman et al. (2018)). Cross-sensitization studies are more limited in mice. While social stress can promote cocaine self-administration in mice (Han et al. 2015; Han et al. 2017), some studies indicate that these effects are absent (Yap and Miczek 2007) or follow a bimodal distribution (Arena et al. 2019). Further, the effects of psychosocial stress appear to be dependent on mouse strain (CFW vs. C57BL/6) (Arena et al. 2019; Yap and Miczek 2007). To study the neurobiological substrates of drug and stress cross-sensitization, it is critical to fully characterize the influence of psychosocial stress on drug self-administration in C57BL/6 mice since many transgenic tools are preferentially developed in this strain (Gong et al. 2007).
Housing conditions provide an additional level of complexity when studying the influence of stress on drug intake. Both rats and mice are frequently single-housed during drug self-administration procedures (Arena et al. 2019; Griffin 3rd et al. 2007; Han et al. 2015; Holly et al. 2016; Kabbaj et al. 2001; McGrath et al. 2018; Ward and Walker 2009) for various reasons (catheter damage, specific tests, etc.). However, rats and mice are social animals and social isolation constitutes a stressor known to increase cocaine intake (Gipson et al. 2011; Green et al. 2010; Hofford et al. 2015; Neisewander et al. 2012; Westenbroek et al. 2013). Housing conditions thus become a factor that can impact drug-related behaviors and necessitate further investigation.
Here we used chronic social defeat stress (CSDS) in C57BL/6 mice to mimic the chronic adversity associated with increased vulnerability to drug use, craving, and relapse in humans (Sinha 2008). CSDS is a highly standardized procedure (Berton et al. 2006; Golden et al. 2011; Krishnan et al. 2007) widely used in mice (Chandra et al. 2017b; Fox et al. 2020; Highland et al. 2019; Nam et al. 2019; Nasca et al. 2019; Pagliusi Jr. et al. 2020) including for the study of social stress on drug self-administration (Arena et al. 2019). CSDS induces anhedonia and loss of motivated behaviors in the majority of rodents (> 60%, termed “stress-susceptible”) (Der-Avakian et al. 2014; Fox and Lobo 2019; Heshmati and Russo 2015; Krishnan et al. 2007), which are factors commonly associated with substance use disorders (Hatzigiakoumis et al. 2011). Following CSDS, we determined stress susceptibility with a social interaction test. We then compared the effect of pair- and single-housing on cocaine self-administration acquisition, seeking, extinction, drug-induced reinstatement, as well as after re-exposure to the social stressor in CSDS and undefeated mice. We found divergent responses to cocaine depending on housing conditions, where pair housing uncovered CSDS reduced motivation for cocaine self-administration but preserved sensitivity to drug-induced relapse. Conversely, single housing revealed stress-sensitive individual differences in early cocaine self-administration and promoted drug seeking following acute stress.
Methods
Animals
Studies were conducted in accordance with guidelines set up by the Institutional Animal Care and Use Committees at University of Maryland School of Medicine (UMSOM). All animals were housed in UMSOM animal facilities on a 12:12-h light:dark cycle and were given food and water ad libitum during the entire experiment. Wild-type C57BL/6 male mice (University of Maryland Veterinary Resources) were 7 to 8 weeks old at the beginning of the experiments. Male CD-1 retired breeders (Charles River, >4 months) were used as aggressors for CSDS.
Social defeat stress
Chronic social defeat stress was performed as previously described (Fox et al. 2020; Francis et al. 2017; Francis et al. 2019). For 10 consecutive days, mice were placed in hamster cages with perforated Plexiglas dividers containing a novel, aggressive CD1-resident. Mice were physically defeated by a new resident for 10 min and then housed opposite the resident for 24 h sensory interaction. After the last defeat, all mice were housed individually in cages with woodchip bedding. Twenty-four hours later, social avoidance was assessed in a social interaction test. Experimental mice were placed in an open field containing a perforated box. Time spent around the box (“interaction zone”) was acquired with a video tracking software (CleverSys, Reston, VA, USA) and compared between two 2.5-min trials during which the perforated box was empty or contained a novel CD-1. Social interaction ratios were calculated by dividing time spent in the interaction zone with and without the novel mouse present. After social interaction testing, mice were assigned to a self-administration group (saline or cocaine) based on individual social interaction ratios, such that each group contained a similar distribution of social interaction ratios. We did not divide CSDS mice into the traditional “susceptible” or “resilient” groups but instead show individual social interaction ratios in the correlation analysis due to the complexity of these experiments which necessitates a lower N.
Intravenous surgery
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (16 mg/kg) and implanted with long-term indwelling jugular catheters (Plastics One, Roanoke, VA) as described previously (Chandra et al. 2017a; Engeln et al. 2020). Mice were flushed daily with 50 IU/ml heparin (40%, in saline) and 2.27% Baytril (20%; Bayer, Shawnee, KS).
Housing
Following catheterization surgery, mice were either single- or pair-housed, using a perforated Plexiglas divider, in a cage with woodchip bedding. Pair-housed mice were housed with mice from the same stress and drug condition. Housing conditions were maintained throughout the remainder of the experiment.
Intravenous cocaine self-administration
After 5 days of recovery, mice underwent 10 days of cocaine (or saline) self-administration under a fixed ratio 1 (FR1) schedule of reinforcement (0.5 mg/kg/infusion in saline; 2 h/day) using a 10-s time out. Operant chambers (MED Associates, Fairfax, VT) had two nosepoke holes on one wall and a house-light on the middle of the opposite wall. Responses in the active nosepoke triggered a 10-μl infusion of cocaine and a 10-s illumination of the active nosepoke light. During this 10-s time out period, nosepokes in the active port were recorded but did not result in further infusions. Responses on the inactive nosepoke were also recorded but were without any programmed consequences. No prior training was used for FR1 acquisition. To avoid administering anesthetics for testing catheter patency, we instead assessed psychomotor activation at the end of each FR1 session. Mice that exhibited strong psychomotor activity after cocaine intake and differentiated between active and inactive nosepokes were considered to have acquired cocaine self-administration behavior. All responses made by cocaine self-administering mice were compared with those made by mice from an identical stress condition responding for saline. Twenty-four hours after the last FR1 session, a 1-h seeking test was performed under extinction conditions (i.e., a response resulted in cue presentation but no drug delivery).
Drug-induced reinstatement
Following the seeking test, one-half of mice were subjected to additional extinction sessions and drug-induced reinstatement as described previously (Engeln et al. 2020). Extinction sessions consisted of 4 to 5 distinct 1-h sessions separated by 5-min intervals during which mice were placed back in their home cage. Extinction sessions are identical to the seeking test in that each response on the active nosepoke resulted in cue presentation but no drug delivery. Here, we refer to “seeking” and “extinction” separately for the purposes of differentiating between the fully extinguished subgroup and the stress re-exposed subgroup (see below). On the following day, the extinguished mice were injected with cocaine (7.5 mg/kg in saline, intraperitoneal) or saline 5 min before a cocaine-induced reinstatement test where, again, each response resulted in cue presentation but no drug delivery.
Stress-modulated seeking
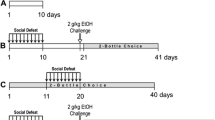
Following the seeking test, the other half of mice underwent a 1-day social defeat stress as described previously (Fox et al. 2020). Mice were defeated for 3 min by three different resident CD-1 mice on a single day, with each session separated by 15 min of sensory interaction. On the following day, mice were subjected to an additional seeking test. Experimental timelines are available in Figs. 1a and 3a.
Social avoidance after chronic social defeat stress predicts cocaine self-administration behavior in pair-housed mice. a Experimental timeline. After 10-day chronic social defeat stress (CSDS), mice undergo social interaction (SI) testing, followed by iv catheterization surgery. Mice are pair-housed for recovery and all subsequent procedures. Mice then self-administer cocaine or saline on a fixed ratio 1 (FR1) schedule for 10 days, are tested for drug seeking behavior, and then divided into two groups, undergoing either extinction and drug-induced reinstatement or a single day of social defeat stress (SSDS) followed by a second seeking test. b Social interaction ratio in CSDS or no-CSDS control mice after 10-day CSDS. Individual data points represent individual mice that go on to self-administer cocaine (filled symbols) or saline (empty symbols); ***p < 0.001. c Number of cocaine or saline infusions earned by no-CSDS (square) or CSDS (circle) mice during 10 days of self-administration procedure; *p < 0.05 for both cocaine groups compared with their respective saline controls starting at day 4. d Scatter plots and Pearson’s correlations of time spent interacting with social target during SI test and cumulative cocaine infusions earned after 3, 5, 7, and 10 days of cocaine self-administration procedure in no-CSDS and CSDS mice. P values for each correlation are reported next to the legend. Note the changing Y-axis in panel d
Statistics
Statistical analyses were performed using GraphPad Prism 7 and 8 software (GraphPad Software, San Diego, CA). Normality was assessed with Bartlett’s test. A two-tailed Student t test was used for social interaction. For cocaine-related experiments, 3-way analysis of variance (ANOVA), 3-way ANOVA with repeated measures (RM-ANOVA), and 2-way ANOVA were run when appropriate, followed by Tukey or Sidak post hoc tests. In the absence of main effect of chronic stress for data analyzed by 3-way RM ANOVA, we used drug and session as factors in a 2-way RM-ANOVA (extinction data in Figs. 2d and 4d). Pearson’s correlation was used to examine relationships between social interaction and cocaine-related behaviors. Mice were excluded from the entire study if they did not acquire cocaine self-administration or failed Grubbs’ outlier test. For single-housed mice, 1 no-CSDS-saline and 1 CSDS-cocaine mice were excluded. For pair-housed mice, 1 no-CSDS-saline, 1 CSDS-saline, and 1 CSDS-cocaine mice were excluded. Sample sizes were determined from previous studies (Engeln et al. 2020; Fox et al. 2020). The threshold for significance was set at p ≤ 0.05. All graphs represent mean ± SEM. In graphs, individual values are plotted to report that variation is similar between compared groups.
History of chronic social defeat stress does not alter cocaine seeking or drug-induced reinstatement in pair housed mice. a Number of active responses during the 1-h, non-reinforced seeking test in no-CSDS and CSDS mice. Individual data points represent individual mice who administered saline (empty symbols) or cocaine (filled symbols); ***p < 0.001 and ****p < 0.0001 vs. respective saline group. b Scatter plot and Pearson’s correlations comparing active responses during seeking and time spent interacting with social target during SI test in no-CSDS (square) and CSDS (circle) cocaine mice. c Scatter plot and Pearson’s correlation comparing active responses during seeking and cumulative cocaine infusions over 10 days of cocaine self-administration in no-CSDS- and CSDS-cocaine mice. P values for each correlation are reported below the legend. d Active responses over four, 1-h extinction sessions in the subgroup of cocaine and saline mice that underwent extinction; ****p < 0.0001 for both cocaine groups compared with their respective saline controls. e Comparison of active responses during the seeking test and the first extinction session in the extinguished subgroup of mice; ***p < 0.001. f Active responses after drug-induced reinstatement (7.5 mg/kg cocaine or saline, ip) in the extinguished subgroup of mice; *p < 0.05 vs. respective saline control. g Active responses during the second seeking test in mice exposed to single day social defeat stress. h Comparison of responses during the first seeking test and the second seeking test in the stress re-exposed subgroup; *p < 0.05
Results
Social stress is associated with lower cocaine intake during self-administration acquisition in pair-housed mice
After CSDS, mice showed reduced social interaction ratios (t test: t(37) = 4.10, p < 0.001; Fig. 1b) and were subsequently divided into subgroups later self-administering either cocaine or saline such that each group contained a similar distribution of social interaction ratios. Mice were paired with a mouse from the same drug and stress condition. The effect of stress was present in both subgroups of mice later taking cocaine or saline (2-way ANOVA, chronic stress F(1,35) = 16.59, p < 0.001; future drug group F(1,35) = 0.26, p = 0.61) demonstrating no differences in social interaction prior to self-administration (Fig. 1b). Pair-housed CSDS and no-CSDS mice both developed cocaine self-administration compared with their respective saline controls (3-way ANOVA: day × drug F(9,315) = 3.97, p < 0.0001; Tukey post hoc: p < 0.05 starting at day 4 (Fig. 1c). The number of cocaine doses taken by pair-housed CSDS and no-CSDS mice did not significantly differ over the 10 days (3-way ANOVA: chronic stress F(1,35) = 0.56, p = 0.46; day × chronic stress F(9,315) = 1.18, p = 0.31). Pair-housed CSDS and no-CSDS mice self-administering cocaine learned to differentiate between the active and inactive nosepoke to a similar extent (3-way RMANOVA: chronic stress, F(1,36) = 2.65, p = 0.11; day × nosepoke F(9,324) = 4.61, p < 0.0001; Tukey’s post hoc, active vs. inactive, p < 0.05 from day 5 for no-CSDS, p < 0.05 from day 7 for CSDS; Supplementary Fig. 1a). Pair-housed mice self-administering saline did not differentiate between the nosepokes (3-way RMANOVA: chronic stress F(1,34) = 0.95, p = 0.33; day × nosepoke F(9,306) = 0.48, p = 0.88; Supplementary Fig. 1a).
We next compared cumulative drug intake with individual social interaction behavior. Assessing pair-housed mice independent of their CSDS exposure, we found positive correlations between social interaction time and cumulative drug intake, indicating that mice with greater social interaction (i.e., the least stress-susceptible) administered more cocaine. The correlation was statistically significant after 3 and 5 days but was weakened at 7 and 10 days (r = 0.45; R2 = 0.20, p = 0.049 after 3 days, r = 0.44; R2 = 0.19, p = 0.05 after 5 days, r = 0.439; R2 = 0.19, p = 0.053 after 7 days and r = 0.38; R2 = 0.14, p = 0.095 after 10 days; Fig. 1d). Since stress exposure drives the differences in social interaction, we next assessed the social interaction-drug relationship in no-CSDS and CSDS mice separately. Again, we found a positive correlation between social interaction time and cumulative drug intake in pair-housed CSDS mice after 3 days of self-administration (r = 0.71; R2 = 0.50, p < 0.02; Fig. 1d). This correlation was not statistically significant in no-CSDS mice (r = 0.37; R2 = 0.14, p = 0.29; Fig. 1d). Over the course of the self-administration procedure, social interaction time and cocaine infusions remained positively correlated in pair-housed CSDS mice (r = 0.69; R2 = 0.48, p = 0.03 after 5 days, r: 0.68; R2 = 0.46, p = 0.03 after 7 days; r = 0.68; R2 = 0.46, p = 0.03 after 10 days; Fig. 1d), but in no-CSDS mice, this relationship remained non-significant (r = 0.42; R2 = 0.18, p = 0.23 after 5 days, r = 0.44; R2 = 0.20, p = 0.20 after 7 days and r = 0.50; R2 = 0.25, p = 0.14 after 10 days; Fig. 1d). Together, this suggests that the individual response to chronic stress impacts the acquisition of cocaine intake in pair-housed mice. Correlations were similar when comparing cocaine intake and social interaction ratio instead of social interaction time (Supplementary Table 1).
Chronic social defeat stress does not alter cocaine seeking but decouples drug intake from drug seeking in pair-housed mice
We next asked if chronic stress exposure would influence the motivation to seek drug under non-reinforced conditions. Twenty-four hours following the last cocaine self-administration session, pair-housed mice underwent a 1-h drug-seeking test. Both pair-housed CSDS and no-CSDS mice showed high responses in the active nosepoke compared with their respective saline controls (2-way ANOVA: drug, F(1,35) = 45.65, p < 0.0001; Sidak’s post hoc: p < 0.001 and p < 0.0001 respectively; Fig. 2a). CSDS had no significant effect on active responses in pair-housed mice (2-way ANOVA: chronic stress, F(1,35) = 0.38, p = 0.54). However, CSDS reduced inactive responses during seeking (2-way ANOVA: chronic stress, F(1,35) = 6.49, p = 0.02). Post hoc analysis revealed a difference in inactive responses only between pair-housed no-CSDS-saline and no-CSDS-cocaine mice (Sidak’s post hoc: p < 0.05; Supplementary Fig. 1b).
To determine if stress susceptibility influenced cocaine-seeking behavior, we next compared social interaction with drug seeking behavior. Unlike cumulative FR1 intake, we found no significant correlation in either pair-housed CSDS or no-CSDS mice (CSDS: r = − 0.41; R2 = 0.17, p = 0.24; no-CSDS: r = 0.40; R2 = 0.16, p = 0.25; Fig. 2b). To determine how drug-intake history influenced cocaine seeking, we compared cumulative cocaine intake with drug seeking behavior. We found a positive correlation in no-CSDS mice, such that mice with greater intake history exhibited greater active responses during drug seeking (r = 0.71; R2 = 0.51, p = 0.02; Fig. 2c). However, in CSDS mice, cocaine intake and seeking responses were not correlated, suggesting that CSDS decouples drug intake from drug seeking behavior (r = − 0.24; R2 = 0.06, p = 0.51; Fig. 2c).
Non-contingent cocaine reinstates drug seeking while re-exposure to social stress has limited impact in pair-housed mice
Following the seeking test, one-half of the pair-housed mice underwent 4 additional extinction sessions. Both CSDS and no-CSDS mice previously taking cocaine started with high responding compared with saline groups regardless of chronic stress history (3-way RM-ANOVA: drug, F(1,16) = 17.50, p < 0.001; chronic stress, F(1,16) = 0.037 p = 0.85; Fig. 2d). Cocaine-experienced no-CSDS mice decreased their responding between the seeking test and the subsequent extinction session (“extinction session 1,” 3-way RM-ANOVA: session × drug, F(1,16) = 7.48, p < 0.05; Sidak’s post hoc: no-CSDS-cocaine p < 0.001). This effect was not observed in the CSDS-cocaine mice (p = 0.40) suggesting slightly slower initial extinction in the stress-experienced group (Fig. 2e). However, all pair-housed cocaine-experienced mice stopped responding for non-reinforced actions by “extinction session 3” (2-way RM-ANOVA, session × drug, F(3,48) = 21.83, p < 0.0001, Sidak’s post hoc vs. saline controls: session 1, p < 0.0001; session 2, p = 0.03; all other sessions, p > 0.05; Fig. 2d). On the next day, the extinguished groups of mice were tested for drug-induced reinstatement. Both no-CSDS and CSDS mice showed increased responding compared with their saline controls (2-way ANOVA, drug, F(1,16) = 18.09, p < 0.001; Sidak’s post hoc: no-CSDS, p < 0.01; CSDS, p < 0.05). CSDS had no impact on responses, indicating that CSDS in pair-housed mice neither promotes nor impairs drug-induced reinstatement (2-way ANOVA: chronic stress, F(1,16) = 0.67, p = 0.42; Fig. 2f).
Instead of additional extinction sessions, the second half of pair-housed mice were subjected to a single day of social defeat stress and tested in a second seeking test the following day. Regardless of CSDS history, there were no significant differences in responding compared with saline mice (2-way ANOVA: drug, F(1,15) = 3.10, p = 0.1; chronic stress, F(1,15) = 0.41, p = 0.53; Fig. 2g). When we compared seeking behavior before and after the stress (re-)exposure, we found a significant effect of the drug condition on responding and a trending drug × session Interaction (3-way RM-ANOVA: drug, F(1,15) = 7.09, p < 0.05; drug × session, F(1,15) = 4.17, p = 0.06). Sidak’s post hoc revealed that only CSDS-cocaine mice significantly decreased responding following the acute stress re-exposure (p < 0.05 vs. CSDS-saline; Fig. 2h). Together, this suggests that exposure to stress only affected previously stressed mice. Importantly, this effect was not likely due to extinction of responding alone since CSDS mice from the extinguished subgroup did not show a significant decrease between seeking and extinction session 1 (see above; Fig. 2e). Of note, the subgroups tested for drug-induced reinstatement and stress-modulated seeking did not differ in their baseline seeking behavior (3-way ANOVA: future group, F(1,31) = 2.53, p = 0.12; future group × chronic stress, F(1,31) = 0.03, p = 0.86; Supplementary Fig. 1c).
Social stress is associated with inter-individual differences in cocaine intake during early stages of self-administration in single-housed mice
Previous work describing increased motivation for cocaine after CSDS mostly used single-housed animals. Since mice that displayed enhanced susceptibility to CSDS, as observed by lower social interaction ratios, had relatively decreased motivation for cocaine when pair-housed, we next repeated the same set of experiments in mice that would remain single-housed throughout all cocaine-related procedures. As before, mice subjected to CSDS had reduced social interaction ratios (t test: t(31) = 3.03, p < 0.01; Fig. 3b). Following social interaction, mice were divided into subgroups later self-administering either cocaine or saline such that both groups contained a similar distribution of social interaction ratios. The effect of stress was present in both subgroups but did not differ between single-housed mice later taking cocaine or saline (2-way ANOVA: chronic stress, F(1,29) = 8.56, p < 0.01; future drug group, F(1,29) = 0.24, p = 0.63; Fig. 3b) demonstrating no difference between the groups prior to self-administration. Following surgery and recovery, single-housed CSDS and no-CSDS mice acquired cocaine self-administration when compared with their respective saline controls (3-way RM-ANOVA: chronic stress, F(1,29) = 6.055, p < 0.05; drug, F(1,29) = 89.55, p < 0.0001. Day × drug, F(9,261) = 12.79, p < 0.0001; Tukey post hoc: CSDS-cocaine vs. CSDS-saline p < 0.05 starting at day 2; no-CSDS-cocaine vs. no-CSDS-saline p < 0.05 starting at day 4; Fig. 3c). There was no significant chronic stress × drug or day interaction (chronic stress × drug, F(1,29) = 0.9474, p = 0.3384; chronic stress × day, F(9,261) = 1.16, p = 0.32), and both CSDS and no-CSDS mice acquired similar cocaine self-administration behavior by the end of the FR1 procedure (Fig. 3c). Single-housed CSDS and no-CSDS mice self-administering cocaine learned to differentiate between the active and inactive nosepoke to a similar extent (3-way RMANOVA: chronic stress, F(1,32) = 0.49, p = 0.4882; day × nosepoke, F(9,288) = 8.00, p < 0.0001, Tukey’s post hoc, active vs. inactive, p < 0.05 from day 2 for no-CSDS, p < 0.05 from day 3 for CSDS; Supplementary Fig. 1d). Single-housed saline mice did not differentiate between the nosepokes, although single-housed CSDS mice made fewer responses overall (3-way RMANOVA: chronic stress, F(1,28) = 6.85, p = 0.014; day × nosepoke F(9,252) = 0.94, p = 0.493; Supplementary Fig. 1d). We also assessed if housing condition influenced FR1 cocaine intake. We compared FR1 cocaine intake between single- and pair-housed mice and found no overall differences, indicating similar acquisition between the groups regardless of housing (3-way ANOVA: housing × chronic stress, F(1,33) = 2.23, p = 0.14; day × housing × chronic stress, F(9,297) = 0.79, p = 0.62; Supplementary Fig. 2a).
Social avoidance after chronic social defeat predicts early cocaine self-administration behavior in single-housed mice. a Experimental timeline. After 10-day chronic social defeat stress (CSDS), mice undergo social interaction (SI) testing, followed by iv catheterization surgery. Mice are single-housed during recovery and all subsequent procedures. Mice then self-administer cocaine or saline on a fixed ratio 1 (FR1) schedule for 10 days, are tested for drug seeking behavior, and then divided into two groups, undergoing either extinction and drug-induced reinstatement or a single day of social defeat stress (SSDS) followed by a second seeking test. b Social interaction ratio in no-CSDS or CSDS mice during the SI test. Individual data points represent mice that go on to self-administer cocaine (filled symbols) or saline (empty symbols); **p < 0.01. c Number of cocaine or saline infusions earned by no-CSDS (square) or CSDS (circle) mice during 10 days of self-administration procedure; *p < 0.05 for no-CSDS-cocaine starting at day 4 and #p < 0.05 for CSDS-cocaine starting at day 2 compared with their respective saline controls. d Scatter plots and Pearson’s correlations of time spent interacting with social target during SI test and cumulative cocaine infusions earned after 3, 5, 7, and 10 days of cocaine self-administration procedure in no-CSDS and CSDS mice. P values for each correlation are reported next to the legend. Note the changing Y-axis in panel d
As before, we further assessed the relationship between stress and the development of cocaine self-administration, by comparing cumulative doses of cocaine taken by single-housed mice in the FR1 procedure with social interaction. Contrary to pair-housed mice, there was no relationship between social interaction and drug intake in single-housed mice independent of CSDS exposure (r = − 0.24; R2 = 0.06, p = 0.35 after 3 days, r = − 0.17; R2 = 0.03, p = 0.50 after 5 days, r = − 0.21; R2 = 0.04, p = 0.42 after 7 days and r = − 0.30; R2 = 0.09, p = 0.24 after 10 days; Fig. 3d). However, when we assessed single-housed CSDS and no-CSDS mice separately, we found a strong negative correlation between social interaction time and cumulative cocaine doses after 3 days of self-administration in CSDS mice only, with the most stress-susceptible mice taking the most cocaine (CSDS: r = − 0.89; R2 = 0.80, p < 0.01; no-CSDS: r = 0.38; R2 = 0.14, p = 0.32; Fig. 3d). As stable drug intake developed, the relationship between social interaction and cumulative cocaine infusions lost statistical significance in CSDS mice suggesting that chronic stress differentially impacts early, but not late cocaine intake in CSDS mice based on their response to stress (r = − 0.59; R2 = 0.35, p = 0.13 after 5 days, r = − 0.41; R2 = 0.17, p = 0.31 after 7 days; r = − 0.35; R2 = 0.12, p = 0.39 after 10 days; Fig. 3d). In no-CSDS mice, this relationship was never statistically significant (r = 0.20; R2 = 0.04, p = 0.61 after 5 days, r = 0.005; R2 = 0.005, p = 0.99 after 7 days; r = − 0.31; R2 = 0.09, p = 0.42 after 10 days; Fig. 3d). We found similar correlations when comparing cocaine intake and social interaction ratio as when comparing intake with social interaction time (Supplementary Table 1).
Chronic social defeat stress does not alter cocaine seeking in single-housed mice
Twenty-four hours after the last cocaine self-administration session, single-housed mice were tested for drug seeking. Both CSDS and no-CSDS mice showed high responding on the active nosepoke compared with their respective saline control group (2-way ANOVA: drug, F(1,29) = 24.81, p < 0.0001; Sidak’s post hoc: p < 0.005; Fig. 4a). The chronic stress condition had no significant effect on active responses (2-way ANOVA: chronic stress, F(1,29) = 2.25, p = 0.14). Similarly, CSDS had no impact on inactive responses during seeking (2-way ANOVA: chronic stress, F(1,29) = 0.60, p = 0.45; Supplementary Fig. 1e).
Single housing blocks drug-induced reinstatement independent of social defeat history. a Number of active responses during the 1-h, non-reinforced seeking test in no-CSDS and CSDS mice. Individual data points represent individual mice who administered saline (empty symbols) or cocaine (filled symbols); **p < 0.01 vs. respective saline control. b Scatter plot and Pearson’s correlations comparing active responses during seeking and time spent interacting with social target during SI test in no-CSDS (square) and CSDS (circle) cocaine mice. c Scatter plot and Pearson’s correlation comparing active responses during seeking and cumulative cocaine infusions over 10 days of cocaine self-administration in no-CSDS- and CSDS-cocaine mice. P values for each correlation are reported below the legend. d Active responses over five, 1-h extinction sessions in the subgroup of cocaine and saline mice that underwent extinction; **p < 0.01 vs. respective saline control. e Comparison of active responses during the seeking test and the first extinction session in the extinguished subgroup of mice. f Active responses after drug-induced reinstatement (7.5 mg/kg cocaine or saline) in the extinguished subgroup of mice. g Active responses during the second seeking test in mice exposed to single day social defeat stress; *p < 0.05, **p < 0.01 vs. respective saline control. h Comparison of responses during the first seeking test, and the second seeking test in the stress re-exposed subgroup; *p < 0.05
To determine if stress susceptibility influenced cocaine-seeking behavior in single-housed mice, we compared social interaction with drug seeking behavior. Unlike early FR1 intake, we found no significant correlation in either cocaine group (CSDS: r = 0.12; R2 = 0.02, p = 0.77; no-CSDS: r = − 0.47; R2 = 0.22, p = 0.21; Fig. 4b). To determine how drug-intake history influenced cocaine-seeking in single-housed mice, we compared cumulative cocaine intake with drug seeking behavior. Cumulative cocaine infusions over 10 days were not correlated with active responses during seeking in CSDS mice (r = 0.30; R2 = 0.09, p = 0.47; Fig. 4c). In single-housed no-CSDS mice, there was a trending positive correlation between cumulative cocaine intake and responses during seeking, similar to pair-housed no-CSDS mice; however, it failed to reach statistical significance (r = 0.55; R2 = 0.30, p = 0.12; Fig. 4c). We also assessed if single-housing impacted drug-seeking behavior and found comparable cocaine-seeking regardless of housing (3-way ANOVA: drug, F(1,64) = 67.80, p < 0.001; housing, F(1,64) = 0.09, p = 0.77; drug × housing, F(1,64) = 0.49, p = 0.49; Supplementary Fig. 2b).
Re-exposure to social stress maintains drug-seeking behavior, while non-contingent cocaine fails to reinstate drug seeking in single-housed mice
Following the seeking test, one-half of single-housed mice underwent 5 additional extinction sessions. Both single-housed CSDS and no-CSDS-cocaine mice started with high responding compared with saline control groups independent of stress history (3-way RM-ANOVA: drug, F(1,12) = 14.17, p < 0.005; chronic stress, F(1,12) = 1.095, p = 0.32; drug × chronic stress, F(1,12) = 0.1267, p = 0.73; Fig. 4d). Contrary to the pair-housed mice, there was no significant decrease in active responses during extinction session 1 when compared with seeking in either single-housed no-CSDS or CSDS mice (3-way RM-ANOVA: session × chronic stress, F(1,12) = 1.09, p = 0.32; Fig. 4e). However, all single-housed cocaine mice quickly stopped responding for non-reinforced actions when compared with their respective saline controls by “extinction session 2” (2-way RM-ANOVA: session × drug, F(4,56) = 27.88, p < 0.0001; Sidak’s post hoc vs. saline control, session 1 p < 0.005, other sessions p > 0.05; Fig. 4d). The following day, the extinguished mice were tested for drug-induced reinstatement. Contrary to pair-housed mice, neither single-housed CSDS nor no-CSDS-cocaine mice showed increased responding compared with saline controls independent of CSDS history (2-way ANOVA: drug, F(1,12) = 0.89, p = 0.36; chronic stress, F(1,12) = 0.43, p = 0.53; Fig. 4f). We also assessed if housing condition influenced responding during drug-induced reinstatement Although 52% lower, active responses in single-housed CSDS-cocaine mice did not significantly differ from pair-housed CSDS-cocaine mice (3-way ANOVA: drug, F(1,28) = 14.25, p < 0.001; drug × housing, F(1,28) = 6.97, p = 0.013; drug × chronic stress × housing, F(1,28) = 0.964, p = 0.334; Tukey post hoc: CSDS-cocaine single-housed vs. CSDS-cocaine pair-housed, p = 0.61; Supplementary Fig. 2c). There were also no differences in active responses between single-housed and pair-housed no-CSDS-cocaine mice during drug-induced reinstatement (Tukey post hoc: p = 0.926; Supplementary Fig. 2c).
As before, the other half of single-housed mice did not undergo extinction but were instead subject to 1 day of social defeat stress. As with the pair-housed mice, the subgroups of single-housed mice tested for drug-induced reinstatement and stress-modulated seeking did not differ in their baseline seeking behavior (3-way ANOVA: future group, F(1,25) = 1.62, p = 0.21; future group × chronic stress, F(1,25) = 0.11, p = 0.74; Supplementary Fig. 1f). When single-housed mice were retested for seeking after SSDS, both cocaine-experienced groups showed high responding compared with their respective saline control groups independent of the previous chronic stress condition (2-way ANOVA: chronic stress, F(1,13) = 1.78, p = 0.20; drug, F(1,13) = 24.06, p < 0.001; Sidak’s post hoc: p < 0.05 for no-CSDS and p < 0.005 for CSDS mice; Fig. 4g). When we compared seeking behavior of single-housed mice before and after the stress (re-)exposure, we found that acute stress decreased cocaine seeking behavior, but only in no-CSDS mice (3-way ANOVA: session, F(1,13) = 19.54, p < 0.001; Sidak’s post hoc p < 0.05 for no-CSDS mice, p > 0.05 for CSDS mice; Fig. 4h). Importantly, this effect was not likely due to extinction of responding, since no-CSDS mice subjected to extinction did not decrease responding between seeking and extinction session 1 (see above, Fig. 4e). Unlike drug-induced reinstatement, both no-CSDS and CSDS single-housed mice showed significant drug seeking after acute stress compared with saline controls. However, in no-CSDS mice, statistically significant seeking is partially driven by decreased responding in no-CSDS-saline mice, since they exhibited differences in seeking before and after acute stress (3-way RM-ANOVA: drug, F(1,13) = 29.07, p < 0.001, Sidak’s post hoc: p < 0.05 for saline no-CSDS, p > 0.05 for saline CSDS; Fig. 4h). We also assessed if housing condition influenced cocaine-seeking behavior after SSDS and found that single-housed mice did not differ from pair-housed mice (3-way ANOVA: drug, F(1,28) = 22.27, p < 0.001; housing, F(1,28) = 3.28, p = 0.08; drug × housing, F(1,28) = 5.96, p = 0.02; Tukey post hoc: pair-housed vs. single-housed no-CSDS-cocaine, p > 0.99 and pair-housed vs. single-housed CSDS-cocaine, p = 0.99; Supplementary Fig. 2d). Altogether, these data suggest that acute stress had limited impact on pair-housed mice or single-housed mice already exposed to chronic stress; however, it significantly decreased responding in single-housed and previously stress-naïve mice.
Discussion
Here we show that housing conditions during self-administration have a divergent effect on CSDS-sensitized cocaine intake and seeking. In pair-housed mice, cocaine intake throughout self-administration positively correlated with social interaction. In single-housed mice, early cocaine intake levels were instead negatively correlated with social interaction; however, this association was lost in late self-administration. When tested for relapse-like behaviors, pair-housed, but not single-housed mice showed drug-induced reinstatement of cocaine seeking. In contrast, only single-housed mice displayed sensitivity to stress re-exposure during cocaine seeking. Together, these observations further explain why studies on stress and drug cross-sensitization in mice have led to contradictory findings.
While many studies have established a link between psychosocial stress and subsequent cocaine self-administration, there are comparatively fewer studies using mice, especially C57BL/6 mice. To address this gap, we used well-defined social stress and drug intake procedures that reliably induce stress-related behaviors in C57BL/6 mice (Fox et al. 2020; Francis et al. 2017; Golden et al. 2011) and are effective for assessing cocaine intake or relapse-like behaviors (Chandra et al. 2017a; Engeln et al. 2020). Since social isolation can be a confounding factor in the development of cocaine self-administration (Gipson et al. 2011; Hofford et al. 2015; Westenbroek et al. 2013), we typically pair-house our animals using perforated dividers to allow for sensory interaction between self-administration sessions (Chandra et al. 2017a; Engeln et al. 2020). In our pair-housed mice, we found CSDS exposure had no major effect on cocaine intake using a FR1 schedule of reinforcement. In rats, CSDS promotes the acquisition of cocaine self-administration (Haney et al. 1995; Tidey and Miczek 1997); however, in C57BL/6 mice, CSDS can either promote or attenuate cocaine intake, generating two separate subgroups (Arena et al. 2019). Given that CSDS often divides mice into susceptible (low social interaction) and resilient (high social interaction) subgroups (Krishnan et al. 2007), we analyzed the relationship between social avoidance and the progressive development of cocaine intake. In line with decreased social interaction as a proxy for decreased motivated behavior (Fox and Lobo 2019; Krishnan et al. 2007), we found pair-housed mice with lower social interaction after CSDS made fewer responses for cocaine throughout the entire self-administration procedure. This finding agrees with reports of reduced cocaine “binge” intake following continuous social stress in rats (Miczek et al. 2011) or decreased cocaine self-administration in “low responders” to sucrose (Arena et al. 2019). Interestingly, we found lower cocaine intake in socially avoidant mice that were pair-housed between self-administration sessions, while the mice tested by Arena et al. were single-housed and underwent self-administration testing in the home cage (Arena et al. 2019). In the home cage, low cocaine intake is thought to be influenced by the mismatch between the positive emotional state associated with the “home” environment and the activating and potentially anxiogenic effect of cocaine (for review (Ahmed et al. 2020). CSDS may potentiate this anxiogenic effect, thereby reducing cocaine intake. While we did not measure anxiety-like behavior in our study, we may have created an anxiogenic environment by “forcing” social interaction via the pair housing of socially avoidant mice. This may have increased the overall anxiogenic effects, thereby leading to lower drug intake. Oppositely in mice with preserved social functions, pair-housing may have promoted development of cocaine self-administration due to peer influence (Smith 2012). Ultimately, our work agrees with the work of Arena et al., in that, in a subset of C57BL/6 mice, CSDS may decrease reward processing thereby reducing self-administration behavior. However, disentangling the value of the drug reward from the overall motivation to self-administer drug will require additional work varying the response requirement and/or the reward magnitude (Roberts et al. 2013).
After mice acquired self-administration, we tested them for cocaine seeking 24 h after the last session to determine if CSDS exposure influenced responding when cocaine was suddenly removed. At the group level, regardless of CSDS history or housing conditions, all cocaine-experienced mice showed similar levels of responding. In rats, cocaine seeking is enhanced by social stress after 2 weeks of forced abstinence and is associated with persistent elevation of corticotropin releasing factor (CRF) levels in the ventral tegmental area (VTA) (Holly et al. 2016). Aside from potential species differences, these changes may not be present at the 24-h time point used in our study but instead develop over more prolonged periods of abstinence. When we examined seeking behavior in individual pair-housed mice, we found cocaine seeking correlated with total drug intake in unstressed control (no-CSDS) mice, illustrating the reinforcing effects of cocaine. Along with high active responses, the pair-housed no-CSDS mice also exhibit high inactive responding during the seeking test, an indicator of strong drug seeking behavior. Pair-housed CSDS mice did not show high levels of inactive responding during seeking. Unlike pair-housed no-CSDS mice, previous cocaine intake was decoupled from cocaine seeking behavior in CSDS mice such that mice with lower drug intake showed seeking levels comparable to those with a history of high drug intake. It is possible the low intake CSDS mice were more sensitive to the anxiogenic effect of cocaine during FR1 (Paine et al. 2002), and removing cocaine during seeking could promote responding for the cue (i.e., a non-drug reward). In support of increased sensitivity to cocaine, socially defeated Swiss Webster mice show greater response rate for low cocaine doses (Han et al. 2015). Thus, a subset of mice in our study may be more sensitive to dysphoric effects induced by the higher dose used here (0.5 mg/kg/inf). Alternatively, the decoupling of intake history with seeking could reflect increased motivation for cocaine in some animals, since social stress can increase progressive ratio breakpoints in rats pair-housed prior to (but not during) cocaine self-administration (Burke and Miczek 2015). Whether the decoupling is due to reduced dysphoric state, increased motivation, or both will require further work.
After the seeking test, we subjected mice to either additional extinction sessions and drug-induced reinstatement or acute social stress followed by a second seeking test. Regardless of CSDS history in the extinguished group, pair-housed cocaine-experienced mice showed strong drug-induced reinstatement. This indicates that the moderate dose of cocaine (7.5 mg/kg) was sufficient to override any differences due to stress history. In contrast, we found no major effect on drug seeking in pair-housed no-CSDS mice after the acute stress; however, the stress re-exposure decreased responding in pair-housed CSDS mice. This indicates that the acute stress did not enhance relapse-like behaviors, and in CSDS-experienced mice, it suppressed responding, consistent with the relationship between lower intake and stress responsivity during FR1. These findings were surprising to us, given that acute stress exposure increases vulnerability to reinstatement of conditioned place preference for both cocaine (Ribeiro Do Couto et al. 2009) and morphine (Ribeiro Do Couto et al. 2006). However, Ribeiro Do Couto et al. used drug-primed reinstatement, while our acutely stressed mice remained cocaine abstinent. Our acute stress paradigm also differs from the stress-induced reinstatement methods commonly used in rats (yohimbine or footshock (Funk et al. 2006; Le et al. 2005; Shaham et al. 1997)) since mice were not first completely extinguished, and our stressor was not conditioned as in Manvich et al. (2016). Collectively, these data suggest that when mice are pair-housed, CSDS can reduce cocaine self-administered during FR1 acquisition depending on stress susceptibility. Similarly, stress re-exposure further decreases responding and blunts cocaine seeking. However, pair-housed CSDS-mice show preserved drug-induced reinstatement suggesting that drug re-exposure drives relapse under these conditions.
Many cocaine self-administration studies rely on single-housed animals. Since higher social avoidance was associated with lower drug intake in pair-housed mice, we asked if single housing would impact the same cocaine-related behaviors and explain the discrepancies between our study and others. When single housed during self-administration, CSDS-cocaine mice showed statistically significantly higher intake compared with their respective saline controls much earlier in FR1 than pair-housed CSDS mice (from day 2 vs. day 4 of FR1) and either pair- or single-housed no-CSDS-cocaine mice (both from day 4 of FR1). This difference illustrates two points. First, housing condition may explain why we failed to see differences in self-administration acquisition between pair-housed stressed and unstressed mice—i.e., the pair housing may have mitigated some effects of stress. Second, including a saline control group is important for self-administration experiments in mice. C57BL/6 mice are notorious for responding for visual cues even in the absence of food or drug reward (Baron and Kish 1962; Olsen and Winder 2009). Here, the single-housed CSDS-saline mice made fewer responses during early FR1, likely driven by stress and/or anxiety, causing reduced responding for the moderately rewarding light cue (see also seeking after acute stress below). Thus, early cocaine intake in single-housed CSDS mice appears much greater when considered against the background of depressed responding in the saline group. This difference in time it took to reach significant FR1 levels in single-housed CSDS mice is also supported by a strong negative correlation between social interaction and cocaine intake in the first 3 days of FR1. However, the correlation lost statistical significance at later FR1 time points. Together, this suggests that, when mice are single-housed, social stress may accelerate the development of cocaine intake during early FR1 but has limited effect on sustained drug consumption. This may also explain why previous studies reported no overall effect of social defeat on cocaine self-administration in mice (Yap and Miczek 2007).
As with pair-housed mice, we found that regardless of CSDS history, both cocaine groups showed comparable cocaine seeking at 24 h. Similar to the pair-housed mice, single-housed no-CSDS mice showed a trending positive correlation between cumulative drug intake and seeking. However, contrary to pair-housed mice, both single-housed CSDS and no-CSDS mice were insensitive to drug-induced reinstatement following extinction of responding. This observation in both defeated and undefeated mice suggests that the housing conditions have a greater influence than prior stress exposure. Drug intake can be influenced by social peers (Smith 2012) and reinstatement of nicotine seeking following extinction is enhanced by the social context (Wang et al. 2014). The absence of a cage mate serving as an additional drug cue could thus contribute to the lack of drug-induced reinstatement observed in single-housed mice.
Contrary to pair-housed mice, none of the single-housed mice showed significant drug-induced reinstatement after extinction of responding. However, both groups of single-housed mice showed significant cocaine seeking following acute stress exposure independent of CSDS history. Interestingly in no-CSDS mice, we found that active responding was decreased by acute stress between the two seeking tests. The global decrease in responding is consistent with preclinical and clinical work showing that acute stress can induce hedonic deficits for both non-drug and drug rewards (Bogdan and Pizzagalli 2006; Funk et al. 2005; van Erp and Miczek 2001) and is reminiscent of low responding levels in the early stages of FR1 in saline CSDS mice. Moreover, as this effect was absent in pair-housed mice, it suggests that acute stress potentially reduces motivation only when previously stress-naïve mice are single-housed. In CSDS mice, active responses were instead unaltered between the two seeking tests suggesting that previous stress exposure made stress-experienced animals insensitive to stress re-exposure. This could illustrate different parts of the inverted U-shaped curve described for stress (Sapolsky 2015): in stress-naïve mice, an acute stressor produces behavioral inhibition while in stress-experienced mice this effect is lost. Thus, a history of repeated stress may abolish the “protective,” anti-drug effects of stress to support a higher risk for stress-induced relapse. More detailed work on that aspect will however be needed as the number of animals in this task might have been too low to efficiently highlight differences between groups.
In summary, our work in C57BL/6 mice showed that while reduced social interaction/enhanced stress susceptibility was associated with decreased cocaine intake in pair-housed mice, reduced social interaction in single-housed animals was associated with greater early cocaine intake as compared with unstressed controls. Additionally, pair-housed mice were more susceptible to drug-induced reinstatement of cocaine seeking, while stress re-exposure sensitized cocaine seeking in single-housed mice. Together, this suggests that following psychosocial stress, the social context surrounding cocaine intake bidirectionally influences cocaine-related behaviors. Moreover, this work further demonstrates that CSDS can be a powerful tool to understand the interaction between stress and relapse-like behaviors when appropriate (stress-modulated or drug-induced) tests are considered. Although some of these conditions have a limited number of mice due to the complexity of these experiments, our correlation analyses revealed important relationships between social stress and cocaine intake. Future work will need to evaluate the neurobiological substrates of these discrepancies at different stages of this cross-sensitization process. Indeed, both stress and cocaine increase dopamine concentrations in the nucleus accumbens (NAc) (Di Chiara and Imperato 1988; Kalivas and Duffy 1995), produce common synaptic adaptations in dopaminergic neurons through CRF signaling (Newman et al. 2018; Saal et al. 2003), and alter synaptic strength in the NAc (Khibnik et al. 2016). Understanding how the social environment impacts these changes will be crucial for the development of new therapeutics. Moreover, assessing if our paradigm similarly produces divergent outcomes for drugs of abuse such as opioids and alcohol will provide information on the shared mechanisms involved in stress/drug cross-sensitization. Finally, it should be noted that this study was conducted exclusively in male mice. There are known sex differences in the development of drug dependence, craving, and relapse (Greenfield et al. 2010). In mice, sex differences in cocaine intake, extinction, and reinstatement have been reproduced using our self-administration paradigm (Engeln et al. 2020). Future work using new social stress models in female mice (Harris et al. 2018; Iniguez et al. 2018; Newman et al. 2019; Takahashi et al. 2017) in conjunction with drug self-administration procedures will allow for the study of how psychosocial stress impacts drug abuse in females.
References
Ahmed SH, Badiani A, Miczek KA, Muller CP (2020) Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev 110:3–27. https://doi.org/10.1016/j.neubiorev.2018.08.015
Arena DT, Covington HE 3rd, DeBold JF, Miczek KA (2019) Persistent increase of I.V. cocaine self-administration in a subgroup of C57BL/6J male mice after social defeat stress. Psychopharmacology 236:2027–2037. https://doi.org/10.1007/s00213-019-05191-6
Baron A, Kish GB (1962) Low-intensity auditory and visual stimuli as reinforcers for the mouse. J Comp Physiol Psychol 55:1011–1013. https://doi.org/10.1037/h0044286
Berton O et al (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. https://doi.org/10.1126/science.1120972
Bogdan R, Pizzagalli DA (2006) Acute stress reduces reward responsiveness: implications for depression. Biol Psychiatry 60:1147–1154. https://doi.org/10.1016/j.biopsych.2006.03.037
Burke AR, Miczek KA (2015) Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology 232:3067–3079. https://doi.org/10.1007/s00213-015-3947-5
Chandra R et al (2017a) Drp1 mitochondrial fission in D1 neurons mediates behavioral and cellular plasticity during early cocaine abstinence. Neuron 96:1327–1341 e1326. https://doi.org/10.1016/j.neuron.2017.11.037
Chandra R et al (2017b) Reduced Slc6a15 in nucleus accumbens D2-neurons underlies stress susceptibility, J Neurosci. 37:6527–6538. https://doi.org/10.1523/JNEUROSCI.3250-16.2017
Covington HE 3rd, Miczek KA (2001) Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology 158:388–398. https://doi.org/10.1007/s002130100858
Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A (2014) Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry 76:542–549. https://doi.org/10.1016/j.biopsych.2014.01.013
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274–5278
Engeln M, Mitra S, Chandra R, Gyawali U, Fox ME, Dietz DM, Lobo MK (2020) Sex-specific role for Egr3 in nucleus accumbens D2-medium spiny neurons following long-term abstinence from cocaine self-administration. Biol Psychiatry 87:992–1000. https://doi.org/10.1016/j.biopsych.2019.10.019
Fox ME, Lobo MK (2019) The molecular and cellular mechanisms of depression: a focus on reward circuitry. Mol Psychiatry 24:1798–1815. https://doi.org/10.1038/s41380-019-0415-3
Fox ME et al (2020) Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol Psychiatry 25:1022–1034. https://doi.org/10.1038/s41380-018-0211-5
Francis TC et al (2017) Molecular basis of dendritic atrophy and activity in stress susceptibility. Mol Psychiatry 22:1512–1519. https://doi.org/10.1038/mp.2017.178
Francis TC, Gaynor A, Chandra R, Fox ME, Lobo MK (2019) The selective RhoA inhibitor Rhosin promotes stress resiliency through enhancing D1-medium spiny neuron plasticity and reducing hyperexcitability. Biol Psychiatry 85:1001–1010. https://doi.org/10.1016/j.biopsych.2019.02.007
Funk D, Harding S, Juzytsch W, Le AD (2005) Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology 183:341–349. https://doi.org/10.1007/s00213-005-0194-1
Funk D, Li Z, Le AD (2006) Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: relationship to the reinstatement of alcohol seeking. Neuroscience 138:235–243. https://doi.org/10.1016/j.neuroscience.2005.10.062
Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT (2011) Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology 214:557–566. https://doi.org/10.1007/s00213-010-2060-z
Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6:1183–1191. https://doi.org/10.1038/nprot.2011.361
Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR (2007) Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27:9817–9823. https://doi.org/10.1523/JNEUROSCI.2707-07.2007
Green TA et al (2010) Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry 67:28–35. https://doi.org/10.1016/j.biopsych.2009.06.022
Greenfield SF, Back SE, Lawson K, Brady KT (2010) Substance abuse in women. Psychiatr Clin North Am 33:339–355. https://doi.org/10.1016/j.psc.2010.01.004
Griffin WC 3rd, Randall PK, Middaugh LD (2007) Intravenous cocaine self-administration: individual differences in male and female C57BL/6J mice. Pharmacol Biochem Behav 87:267–279. https://doi.org/10.1016/j.pbb.2007.04.023
Han X, Albrechet-Souza L, Doyle MR, Shimamoto A, DeBold JF, Miczek KA (2015) Social stress and escalated drug self-administration in mice II. Cocaine and dopamine in the nucleus accumbens. Psychopharmacology 232:1003–1010. https://doi.org/10.1007/s00213-014-3734-8
Han X, DeBold JF, Miczek KA (2017) Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology 234:2813–2821. https://doi.org/10.1007/s00213-017-4676-8
Haney M, Maccari S, Le Moal M, Simon H, Piazza PV (1995) Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res 698:46–52. https://doi.org/10.1016/0006-8993(95)00788-r
Harris AZ et al (2018) A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43:1276–1283. https://doi.org/10.1038/npp.2017.259
Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L (2011) Anhedonia and substance dependence: clinical correlates and treatment options. Front Psych 2:10. https://doi.org/10.3389/fpsyt.2011.00010
Heshmati M, Russo SJ (2015) Anhedonia and the brain reward circuitry in depression. Curr Behav Neurosci Rep 2:146–153. https://doi.org/10.1007/s40473-015-0044-3
Highland JN, Zanos P, Georgiou P, Gould TD (2019) Group II metabotropic glutamate receptor blockade promotes stress resilience in mice. Neuropsychopharmacology 44:1788–1796. https://doi.org/10.1038/s41386-019-0380-1
Hofford RS, Prendergast MA, Bardo MT (2015) Pharmacological manipulation of glucocorticoid receptors differentially affects cocaine self-administration in environmentally enriched and isolated rats. Behav Brain Res 283:196–202. https://doi.org/10.1016/j.bbr.2015.01.049
Hollon NG, Burgeno LM, Phillips PE (2015) Stress effects on the neural substrates of motivated behavior. Nat Neurosci 18:1405–1412. https://doi.org/10.1038/nn.4114
Holly EN, Boyson CO, Montagud-Romero S, Stein DJ, Gobrogge KL, DeBold JF, Miczek KA (2016) Episodic social stress-escalated cocaine self-administration: role of phasic and tonic corticotropin releasing factor in the anterior and posterior ventral tegmental area. J Neurosci 36:4093–4105. https://doi.org/10.1523/JNEUROSCI.2232-15.2016
Iniguez SD et al (2018) Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry 83:9–17. https://doi.org/10.1016/j.biopsych.2017.07.014
Kabbaj M, Norton CS, Kollack-Walker S, Watson SJ, Robinson TE, Akil H (2001) Social defeat alters the acquisition of cocaine self-administration in rats: role of individual differences in cocaine-taking behavior. Psychopharmacology 158:382–387. https://doi.org/10.1007/s002130100918
Kalivas PW, Duffy P (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675:325–328. https://doi.org/10.1016/0006-8993(95)00013-g
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244. https://doi.org/10.1016/0165-0173(91)90007-u
Khibnik LA, Beaumont M, Doyle M, Heshmati M, Slesinger PA, Nestler EJ, Russo SJ (2016) Stress and cocaine trigger divergent and cell type-specific regulation of synaptic transmission at single spines in nucleus accumbens. Biol Psychiatry 79:898–905. https://doi.org/10.1016/j.biopsych.2015.05.022
Krishnan V et al (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404. https://doi.org/10.1016/j.cell.2007.09.018
Le AD, Harding S, Juzytsch W, Funk D, Shaham Y (2005) Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology 179:366–373. https://doi.org/10.1007/s00213-004-2036-y
Lloyd DA, Turner RJ (2008) Cumulative lifetime adversities and alcohol dependence in adolescence and young adulthood. Drug Alcohol Depend 93:217–226. https://doi.org/10.1016/j.drugalcdep.2007.09.012
Manvich DF, Stowe TA, Godfrey JR, Weinshenker D (2016) A method for psychosocial stress-induced reinstatement of cocaine seeking in rats. Biol Psychiatry 79:940–946. https://doi.org/10.1016/j.biopsych.2015.07.002
McGrath AG, Lenz JD, Briand LA (2018) PKMzeta in the nucleus accumbens acts to dampen cocaine seeking. Neuropsychopharmacology 43:2390–2398. https://doi.org/10.1038/s41386-018-0170-1
Miczek KA, Nikulina EM, Shimamoto A, Covington HE 3rd (2011) Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci 31:9848–9857. https://doi.org/10.1523/JNEUROSCI.0637-11.2011
Nam H, Chandra R, Francis TC, Dias C, Cheer JF, Lobo MK (2019) Reduced nucleus accumbens enkephalins underlie vulnerability to social defeat stress. Neuropsychopharmacology 44:1876–1885. https://doi.org/10.1038/s41386-019-0422-8
Nasca C et al (2019) Multidimensional predictors of susceptibility and resilience to social defeat stress. Biol Psychiatry 86:483–491. https://doi.org/10.1016/j.biopsych.2019.06.030
Neisewander JL, Peartree NA, Pentkowski NS (2012) Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology 224:33–56. https://doi.org/10.1007/s00213-012-2853-3
Newman EL, Leonard MZ, Arena DT, de Almeida RMM, Miczek KA (2018) Social defeat stress and escalation of cocaine and alcohol consumption: Focus on CRF. Neurobiol Stress 9:151–165. https://doi.org/10.1016/j.ynstr.2018.09.007
Newman EL, Covington HE 3rd, Suh J, Bicakci MB, Ressler KJ, DeBold JF, Miczek KA (2019) Fighting females: neural and behavioral consequences of social defeat stress in female mice. Biol Psychiatry 86:657–668. https://doi.org/10.1016/j.biopsych.2019.05.005
Olsen CM, Winder DG (2009) Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology 34:1685–1694. https://doi.org/10.1038/npp.2008.226
Pagliusi M Jr, Bonet IJM, Brandao AF, Magalhaes SF, Tambeli CH, Parada CA, Sartori CR (2020) Therapeutic and preventive effect of voluntary running wheel exercise on social defeat stress (SDS)-induced depressive-like behavior and chronic pain in mice. Neuroscience 428:165–177. https://doi.org/10.1016/j.neuroscience.2019.12.037
Paine TA, Jackman SL, Olmstead MC (2002) Cocaine-induced anxiety: alleviation by diazepam, but not buspirone, dimenhydrinate or diphenhydramine. Behav Pharmacol 13:511–523. https://doi.org/10.1097/00008877-200211000-00001
Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J (2006) Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology 185:459–470. https://doi.org/10.1007/s00213-006-0345-z
Ribeiro Do Couto B, Aguilar MA, Lluch J, Rodriguez-Arias M, Minarro J (2009) Social experiences affect reinstatement of cocaine-induced place preference in mice. Psychopharmacology 207:485–498. https://doi.org/10.1007/s00213-009-1678-1
Roberts DC, Gabriele A, Zimmer BA (2013) Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neurosci Biobehav Rev 37:2026–2036. https://doi.org/10.1016/j.neubiorev.2013.04.017
Saal D, Dong Y, Bonci A, Malenka RC (2003) Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37:577–582. https://doi.org/10.1016/s0896-6273(03)00021-7
Sapolsky RM (2015) Stress and the brain: individual variability and the inverted-U. Nat Neurosci 18:1344–1346. https://doi.org/10.1038/nn.4109
Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J (1997) Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci 17:2605–2614
Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci 1141:105–130. https://doi.org/10.1196/annals.1441.030
Smith MA (2012) Peer influences on drug self-administration: social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology 224:81–90. https://doi.org/10.1007/s00213-012-2737-6
Takahashi A, Chung JR, Zhang S, Zhang H, Grossman Y, Aleyasin H, Flanigan ME, Pfau ML, Menard C, Dumitriu D, Hodes GE, McEwen BS, Nestler EJ, Han MH, Russo SJ (2017) Establishment of a repeated social defeat stress model in female mice. Sci Rep 7:12838. https://doi.org/10.1038/s41598-017-12811-8
Tidey JW, Miczek KA (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology 130:203–212. https://doi.org/10.1007/s002130050230
van Erp AM, Miczek KA (2001) Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav 73:301–311. https://doi.org/10.1016/s0031-9384(01)00458-9
Wang T, Han W, Wang B, Jiang Q, Solberg-Woods LC, Palmer AA, Chen H (2014) Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genes Brain Behav 13:202–212. https://doi.org/10.1111/gbb.12112
Ward SJ, Walker EA (2009) Sex and cannabinoid CB1 genotype differentiate palatable food and cocaine self-administration behaviors in mice. Behav Pharmacol 20:605–613. https://doi.org/10.1097/FBP.0b013e328331ba30
Wemm SE, Sinha R (2019) Drug-induced stress responses and addiction risk and relapse. Neurobiology of stress 10:100148. https://doi.org/10.1016/j.ynstr.2019.100148
Westenbroek C, Perry AN, Becker JB (2013) Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res 252:68–71. https://doi.org/10.1016/j.bbr.2013.05.040
Yap JJ, Miczek KA (2007) Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology 192:261–273. https://doi.org/10.1007/s00213-007-0712-4
Yap JJ, Miczek KA (2008) Stress and rodent models of drug addiction: role of VTA-accumbens-PFC-amygdala circuit. Drug Discov Today Dis Model 5:259–270. https://doi.org/10.1016/j.ddmod.2009.03.010
Funding
This work was funded by NIH grants R01MH106500, R01DA038613, R01DA047843 (to MKL), and K99DA050575 (to MEF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Studies were conducted in accordance with guidelines set up by the Institutional Animal Care and Use Committees at University of Maryland School of Medicine (UMSOM).
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 960 kb)
Rights and permissions
About this article
Cite this article
Engeln, M., Fox, M.E. & Lobo, M.K. Housing conditions during self-administration determine motivation for cocaine in mice following chronic social defeat stress. Psychopharmacology 238, 41–54 (2021). https://doi.org/10.1007/s00213-020-05657-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05657-y