Abstract
Background
Previous studies found that environmental enrichment protects against the initiation of stimulant self-administration in rats, but it is unclear if enrichment also protects against the escalation of stimulant use with long-term exposure.
Objective
The current study examined the effects of environmental enrichment on escalation of cocaine self-administration using an extended access procedure.
Methods
Rats were raised from 21 days in an enriched condition (EC) with social cohorts and novel objects, a social condition with only social cohorts (SC), a novelty condition (NC) with novel objects in isolated cages, or an isolated condition (IC) without social cohorts or novel objects. In young adulthood, EC, SC, NC, and IC rats were separated into short access (ShA) or long access (LgA) groups that received either 1 or 6 h, respectively, of daily cocaine self-administration (0.1 mg/kg/infusion) for 14 days. In a second experiment, EC and IC rats were used to assess differences in acquisition and escalation of cocaine self-administration at a 0.5 mg/kg/infusion unit dose.
Results
With ShA sessions, EC rats acquired cocaine self-administration at a slower rate than IC rats at both unit doses; however, with extended training, both groups eventually reached similar rates. At the 0.1 mg/kg/infusion dose, only NC and IC rats escalated in amount of intake when switched to the LgA sessions. At the 0.5 mg/kg/infusion dose, rates of cocaine self-administration escalated in LgA groups over 14 days regardless of EC or IC rearing condition; however, EC rats escalated at a faster rate, eventually reaching the same level of intake observed in IC rats.
Conclusions
Although environmental enrichment protects against escalation of a low unit dose of cocaine, it may not protect against escalation with a higher unit dose. In addition, at a lower unit dose, this protective mechanism appears to be due to the presence of social cohorts rather than novel objects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of individuals who experiment with drugs of abuse do not become addicted (Adams et al. 1999; Wagner and Anthony 2002). The term “addiction,” according to Olmstead (2006), is defined as maladaptive drug use and the obsession with obtaining and consuming the drug. Although only a percentage of the population develops drug abuse or dependence, it is unclear what precise factors contribute to susceptibility to substance abuse disorders. Thus, it is important to determine what genetic and environmental factors contribute to individual differences in drug abuse vulnerability.
The role of environmental factors in drug abuse vulnerability has been examined in both clinical and preclinical research. According to McGue et al. (1996), adolescent alcohol use is affected by sibling environmental effects. In an adoption study, McGue et al. (1996) found that being reared with a sibling who uses alcohol influences adolescent alcohol involvement. Further, it has also been found that genetic factors involved in substance abuse vulnerability are modifiable by environmental contexts (Hopfer et al. 2003). Environmental factors such as parental influence and enriching stimuli can decrease substance use among genetically predisposed individuals (Hopfer et al. 2003).
Environmental enrichment (EE) has been suggested as a possible treatment for cocaine abuse. Thiel et al. (2009) examined the effect of EE following established addiction-related behaviors on the impact of cocaine-associated environmental stimuli in rats. Thiel et al. (2009) found that EE attenuated the impact of cocaine-associated environmental stimuli involved in relapse. In addition, Solinas et al. (2008) found that EE can eliminate previously established addiction-related behaviors in mice, such as cocaine behavioral sensitization and conditioned place preference (CPP). Stressors, such as isolation from peers, also exacerbate behavioral sensitization (also termed locomotor sensitization) in rats (Ahmed et al. 1995). Taken together, these results indicate that the environmental context (either positive or negative) in which an individual resides may contribute to the success of abstaining from addiction-related behaviors.
The behavioral effects induced by EE during development have been examined in nonhuman animals. Solinas et al. (2009) found that mice reared in EE with novel objects have reduced sensitivity to the reinforcing effects of cocaine and reduced cocaine CPP compared to standard housed mice. In addition, enriched mice are less sensitive than standard housed mice to the locomotor stimulant effects of repeated cocaine injections (Solinas et al., 2009). Bowling et al. (1993) found that rats raised in an enriched condition (EC) show a decreased basal level of locomotor activity compared to rats raised in an isolated condition (IC). When amphetamine (AMPH) is administered, however, EC rats show a greater increase in locomotor activity compared to IC rats. Bardo et al. (1995) also found that EC rats are more sensitive to acute locomotor and rewarding effects of AMPH, but that IC rats are more sensitive to locomotor sensitization following repeated AMPH injections. Differential rates of stimulant self-administration have also been found, with EC rats self-administering less AMPH than IC rats at low unit doses (Bardo et al. 2001; Green et al. 2002). Thus, EE appears to have beneficial effects on behavior in multiple preclinical drug abuse paradigms.
Although previous studies found that EE protects against the initiation of stimulant self-administration in rats (Bardo et al. 2001), it is unclear if it also protects against the escalation of stimulant use with long-term exposure. Koob and Kreek (2007) defined compulsive drug abuse as the switch from low levels of regulated intake to increasing levels of dysregulated intake. Preclinical models of the transition from moderate to excessive drug intake have been developed in which rats first are exposed to short access (ShA) sessions (1 h/day) and then are switched to long access (LgA) sessions (e.g., 6 h/day). Stimulant self-administration during the LgA sessions leads to an increase in the rate of intake of cocaine (Ahmed and Koob 1998, 1999, 2004; Ahmed et al. 2003), methamphetamine (Kitamura et al. 2006; Schwendt et al. 2009), AMPH (Gipson and Bardo 2009), and methylphenidate (Marusich et al. 2010). Escalation of drug self-administration can be found in both the total intake amount and the first hour of drug intake across LgA sessions (Ahmed and Koob 1998).
The purpose of the current study was to determine if EE during development protects against escalation of cocaine self-administration in rats. At 21 days of age, rats were raised in either EC or IC environments until young adulthood and then were trained to self-administer cocaine (0.1 mg/kg/infusion unit dose) during ShA 1-h sessions. For comparison, a group of rats raised in a social condition (SC) without novel objects, as well as a group of rats raised in isolated cages with only novel objects (a novel control, or NC, group), were also assessed. A second experiment tested the effects of a higher dose of cocaine (0.5 mg/kg/infusion) in EC and IC rats. The cocaine doses used in these experiments were chosen in order to minimize EC/IC differences in initial acquisition of cocaine self-administration in ShA sessions; thus, differences in rates of escalation between groups would not be attributable to differences in baseline intake (see Green et al., 2010). In both experiments, rats then were continued on the ShA 1-h sessions or were switched to LgA 6-h sessions to determine the effect of EE on escalation of cocaine self-administration.
Method
Subjects
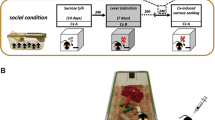
Seventy two male Sprague–Dawley rats were obtained from Harlan (Indianapolis, IN) at 21 days of age and were housed in an EC, SC, NC, or IC environment. EC and SC rats were housed 6–12 rats per cage and were handled daily. EC and SC cages were large steel wire cages (122 x 61 x 45.5 cm), with solid steel floors and pine bedding changed weekly. NC and IC rats were single-housed in stainless steel hanging cages (17 × 24 × 20 cm) and were not handled. EC rats were given an assortment of hard plastic objects (14 per cage) that were replaced and rearranged daily to maximize novelty. These objects included plastic tubes, various children's toys, metal running wheels, and large plastic balls. The objects varied in color, size, shape, and some toys had holes for rats to explore inside the toys. At least three toys were hung by a metal chain to maximize exploration and exercise. SC rats were not exposed to plastic objects, and NC rats were given two plastic toys rotated daily; these toys were smaller in size to account for the small isolated cages, although they also varied in size and color. IC rats were not exposed to plastic objects. Rats were raised in these conditions from 21 to 51 days of age and were maintained in these conditions for the duration of the experiment. All rats were housed in a colony room held at a constant temperature. Light and dark phases were on a 12:12-h cycle, and all experimentation occurred in the light phase. Rats were on food restriction (20 g food per day) for the duration of the experiment, as it has been well established that food restriction increases stimulant self-administration in rats (for a review, see Carroll and Meisch 1984). Rats had unlimited access to water in their home cage. Rats in social housing conditions (EC and SC rats) were food-restricted by providing 20 g of food per rat, and all of the food was placed in the same dish within the home cage at the end of the LgA sessions (both ShA and LgA rats were fed at the same time at the end of the day). Rats maintained stable, food-restricted body weights with this method. Rats were cared for in accordance with the 1996 edition of the Guide for the Care and Use of Laboratory Animals (NIH), and procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
Twelve operant conditioning chambers (ENV-001; MED Associates, St. Albans, VT) located inside sound-attenuating chambers were used. The front and back walls of the operant chambers were made of aluminum, while the side walls were made of Plexiglas. There was a recessed food tray (5 × 4.2 cm) located in the bottom-center of the front wall. A retractable response lever was located on each side of the recessed food tray on the front wall. A 28-V white cue light was located 6 cm above each response lever. A white house light was mounted in the center of the back wall of the chamber. All responses and scheduled consequences were recorded and controlled by a computer interface. A computer controlled the experimental sessions using Med-IV software.
Surgery
At 52 days of age, EC, SC, NC, and IC rats underwent surgery in which indwelling jugular catheters were implanted. One day prior to surgery and 3 days afterwards, rats were treated with the non-opioid analgesic carprofen (5 mg/kg, s.c.). While under anesthesia (100 mg/kg ketamine, 5 mg/kg diazepam, i.p.), an indwelling catheter was inserted into the jugular vein and exited via a metal cannula stabilized in a head mount made of dental acrylic. A silastic leash attached an infusion pump located outside of the chamber to the head mount during the self-administration sessions. Rats were given 1 week to recover, and a mixture of gentamicin (0.2 ml), heparin (0.6 ml), and saline was used to flush catheters daily.
Self-administration procedure
Acquisition phase
Following recovery from surgery, all rats received seven sessions of autoshaping (Carroll and Lac 1993). Each 1-h autoshaping session consisted of inactive lever presentation for the entire duration of the session; responses on this lever resulted in no programmed consequence. The first 15 min of this session also consisted of the presentation of the active lever (counterbalanced for position among subjects) on a random-time 60-s schedule. Following a response on the lever or following 15 s of the lever presentation, both cue lights were presented along with a 0.1 ml infusion of cocaine delivered across 5.9 s. Following the delivery of an infusion, the active lever retracted, and this pattern cycled until 15 min elapsed. The last 45 min of the session consisted of presentation of the inactive lever only.
Cocaine self-administration sessions occurred 2 h after the autoshaping session each day. These sessions consisted of 1 h access to contingent cocaine, either 0.1 or 0.5 mg/kg/infusion, on a fixed ratio-1 schedule of reinforcement. Sessions began with the presentation of both the active and inactive levers (same position as those presented in the autoshaping sessions). Responses on the active lever resulted in illumination of both cue lights and a 0.1-ml infusion of cocaine delivered across 5.9 s, followed by a 20-s time-out period in which both cue lights were illuminated. Responses to the inactive lever had no programmed consequence, and responses on either lever during the time-out period also had no programmed consequence. Following the end of the time-out period, the cue lights turned off, and responses on the active lever again resulted in a cocaine infusion.
Extended access phase
Following the 7-day acquisition phase, rats were divided randomly into ShA and LgA groups (1- and 6-h sessions, respectively). With the 0.1 mg/kg/infusion dose, there were six rats in both the EC ShA and LgA groups, nine rats in the SC ShA and six in the SC LgA groups, seven in the NC ShA and nine in the NC LgA groups, and five rats in both the IC ShA and LgA groups. With the 0.5 mg/kg/infusion dose, there were five rats in the EC ShA and six in the EC LgA groups, and six rats in the IC ShA and six in the IC LgA groups. Rats in the ShA group were given 14 daily cocaine self-administration sessions (no autoshaping) identical to those in training. Rats in the LgA group were treated similarly, except that the cocaine self-administration session length was increased to 6 h.
Specific experiments
Experiment 1
The purpose of experiment 1 was to determine the effect of EE on acquisition and escalation of cocaine self-administration at the 0.1 mg/kg/infusion unit dose. Rats were raised in either the EC, SC, NC, or IC environment until young adulthood and remained in their respective environments for the duration of the study. EC and IC rats were run simultaneously for comparison, whereas NC and SC rats were run separately as two follow-up groups.
Experiment 2
The purpose of experiment 2 was to determine the effect of EE on acquisition and escalation of cocaine self-administration at a higher unit dose of cocaine (0.5 mg/kg/infusion). Based on findings from experiment 1 (the protective effect of EE against escalation of cocaine self-administration), the purpose of using a higher dose of cocaine was to examine if this protective effect could be surmounted. SC and NC groups were not used at this higher dose because differences in rates of escalation between the extreme groups (EC and IC) were found at the lower dose, thus these groups were used with the 0.5 mg/kg/infusion dose to determine if the difference in rate of escalation due to EE is surmountable with a higher dose. The experiment was similar to experiment 1, except that the cocaine dose was 0.5 mg/kg/infusion. EC and IC rats were run simultaneously.
Statistical analyses
Separate repeated-measures ANOVAs were performed on the self-administration data from the acquisition and extended access phases for each experiment, with environmental condition and access duration (ShA vs. LgA) as between-subjects factors, and session and 5-min intervals as within-subjects factors. Pairwise comparisons were performed on the extended access phase data to compare intake on subsequent sessions to the first session of extended access. Linear trend analyses (also known as linear regression, see Montgomery et al. 2006) were also performed on the self-administration data from the extended access phase. Pairwise comparisons were performed on the time course data, comparing the number of infusions in ShA and LgA groups during 5-min intervals across the session (first hour only for LgA group). All tests were considered significant at p < 0.05, except for post hoc Bonferroni corrected pairwise comparisons on self-administration data from the acquisition phase that were considered significant at p < 0.007 (with seven comparisons), from the extended access phase at p < 0.004 (with 13 comparisons), and from the first hour of self-administration from the first and last session of the extended access phase at p < 0.004 (with 12 comparisons).
Results
Experiment 1
Acquisition
Acquisition of cocaine self-administration at the 0.1 mg/kg/infusion dose for EC, SC, NC, and IC rats is shown in Fig. 1a–c. An omnibus three-way repeated-measures ANOVA (4 × 2 × 7; environmental group × lever × session) revealed a significant main effect of lever [F(1,66) = 19.15, p < 0.0001], as well as a significant main effect of session [F(6,396) = 13.76, p < 0.0001] and lever × session interaction [F(6,396) = 19.23, p < 0.0001].
Since EC and IC groups were run simultaneously, data from these two groups are presented together (Fig. 1a) and are analyzed further together. To determine differences in acquisition of cocaine self-administration between the two environmental groups, a 2 × 2 × 7 (environmental group × lever × session) repeated-measures ANOVA revealed significant main effects of lever [F(1,34) = 5.21, p < 0.05] and session [F(6,204) = 3.67, p < 0.01], as well as a significant lever × session interaction [F(6,204) = 6.55, p < 0.001]. For both groups, pairwise comparisons revealed that active lever presses on the last session of acquisition were significantly higher than inactive lever presses. Active lever presses were not significantly different at any session between EC and IC groups.
To determine acquisition of cocaine self-administration in the SC group (Fig. 1b), a 2 × 7 (lever × session) repeated-measures ANOVA revealed significant main effects of lever [F(1,17) = 14.78, p < 0.001] and session [F(6,102) = 4.74, p < 0.001], as well as a significant lever × session interaction [F(6,102) = 4.25, p = 0.001]. Subsequent pairwise comparisons revealed significantly higher active lever presses than inactive lever presses on the last two sessions of acquisition. In the NC group, there was a significant main effect of lever [F(1,17) = 9.44, p < 0.01] and session [F(6,102) = 5.41, p < 0.001], as well as a significant lever × session interaction [F(6,102) = 9.38, p < 0.001]. Subsequent pairwise comparisons between active and inactive lever presses on each session revealed that active lever presses were significantly higher than inactive lever presses on sessions 5–7.
Escalation
Self-administration rates from the extended access phase at the 0.1 mg/kg/infusion dose for EC, SC, NC, and IC groups are illustrated in Fig. 2a–c. An omnibus three-way repeated-measures ANOVA (4 × 2 × 14; environmental group × access duration × session) revealed a significant main effect of session [F(13,585) = 6.77, p < 0.001], as well as an access duration × session interaction [F(13,585) = 3.52, p < 0.001].
A three-way repeated-measures ANOVA (2 × 2 × 14; environmental group × access duration × session) on the data from EC and IC groups alone (Fig. 2a) revealed a significant main effect of session [F(13,416) = 16.15, p < 0.001], a significant environmental group × session interaction (F(13,416) = 1.75, p < 0.05), a significant access duration × session interaction [F(13,416) = 11.91, p < 0.001], and a significant environmental group × access duration × session interaction [F(26,624) = 2.27, p < 0.001]. Post hoc Bonferroni corrected pairwise comparisons revealed that compared to session 8 (the first session of the extended access phase), cocaine intake was increased from LgA sessions 14 through 21 in IC rats, but not EC rats. A 2 × 2 × 14 ANOVA (environmental group × access duration × session) conducted on inactive lever presses from the extended access phase for ShA and LgA groups revealed no significant main effects or interactions (results not shown).
In the SC group (Fig. 2b), a 2 × 14 repeated-measures ANOVA revealed a significant main effect of session [F(13,442) = 15.16, p < 0.001] and a significant access duration × session interaction [F(13,442) = 11.18, p < 0.001]. Post hoc Bonferroni corrected pairwise comparisons revealed no significant increase in intake on any LgA session compared to session 8. However, there were significant increases in intake on ShA sessions 11–21 compared to session 8. In the NC group (Fig. 2c), a 2 × 14 repeated-measures ANOVA revealed a significant main effect of session [F(13,182) = 2.36, p < 0.01]. Post hoc Bonferroni corrected pairwise comparisons revealed no significant increases in intake on LgA sessions compared to session 8. However, there were significant increases in intake on ShA sessions 15–21 compared to session 8. Repeated-measures ANOVAs conducted on inactive lever presses during the extended access phase for the SC and NC groups revealed no significant main effects or interactions (results not shown).
Linear trend analyses were also conducted on the results shown in Fig. 2a–c to determine if escalation was obtained in each group. There was significant escalation in the IC LgA group [t(68) = 3.56, p < 0.001], but not in the EC LgA group. In the ShA groups, a linear trend was also obtained in the EC group [t(82) = 2.46, p < 0.05], but not in the IC group. In the SC groups, there was no significant escalation in the LgA group; however, there was significant escalation in the SC ShA group [t(82) = 3.85, p < 0.001]. There was also significant escalation in both ShA and LgA NC groups [(t(124) = 3.62, p < 0.001) and (t(95) = 3.98, p < 0.0001), respectively].
Time course
An omnibus three-way ANOVA (4 × 2 × 12; environmental group × session × time interval) was conducted on LgA data in 5-min intervals during the first hour of session 8 and 21 (i.e., the first and last LgA session; Fig. 3a–c). There was a significant main effect of session [F(1,22) = 6.83, p < 0.05], as well as a significant session × time interval [F(11,242) = 2.97, p < 0.001] and environmental group × session × time [F(33,242) = 1.65, p < 0.05] interaction.
A 2 × 2 × 12 (environmental group × session × time interval) ANOVA for EC and IC groups alone (Fig. 3a) revealed a significant main effect of session [F(1,9) = 7.19, p < 0.05], as well as a significant session × time interval [F(11,99) = 3.03, p < 0.01] interaction. Separate ANOVAs for the EC and IC groups revealed no significant effects during ShA sessions (results not shown). There were also no significant effects in the EC LgA group; however, the IC LgA ANOVA revealed a significant main effect of session [F(1,4) = 5.94, p < 0.05], as well as a significant session × time interval interaction [F(11,44) = 2.18, p < 0.05]. Pairwise comparisons revealed an elevation of cocaine intake on session 21 compared to session 8 after the first 10 min of the session, although this elevation was not statistically significant at all 5-min intervals.
In the SC group (Fig. 3b), a separate 2 × 12 (session × time interval) ANOVA revealed a significant main effect of session [F(1,5) = 6.63, p < 0.05], as well as a significant session × time interval interaction [F(11,55) = 3.85, p < 0.001] in the ShA group (results not shown), but no significant effects in the LgA group. Pairwise comparisons revealed an elevation of cocaine intake on ShA session 21 compared to ShA session 8 at various 5-min intervals (at the 5, 10, 15, 20, 25, and 30-min time intervals) across the hour of self-administration (results not shown). In the NC group (Fig. 3c), separate 2 × 12 (session × time interval) ANOVAs revealed a significant main effect of session [F(1,8) = 5.70, p < 0.05] and time interval [F(11,88) = 2.29, p < 0.05] in the ShA group (results not shown). In the NC LgA group, however, there was only a significant main effect of session [F(1,6) = 6.52, p < 0.05]. Pairwise comparisons revealed significantly elevated cocaine intake on session 21 compared to session 8 only during the last two 5-min intervals.
Experiment 2
Acquisition
Acquisition of cocaine self-administration at the 0.5 mg/kg/infusion dose in EC and IC groups is shown in Fig. 4. A three-way ANOVA (2 × 2 × 7; environmental group × lever × session) revealed a significant main effect of lever (F(1,22) = 31.6, p < 0.001), a significant environmental group × lever interaction [F(1,22) = 24.21, p < 0.001], a significant lever × session interaction [F(6,132) = 7.51, p < 0.001], and a significant environmental group × lever × session interaction [F(6,132) = 6.82, p < 0.05]. Pairwise comparisons revealed that active lever presses were significantly higher than inactive lever presses in the IC group from sessions 4 through 7. EC active and inactive lever presses did not differ significantly on any session.
Escalation
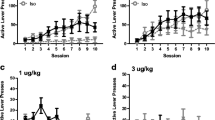
Figure 5 illustrates the number of cocaine infusions during the extended access phase for EC and IC ShA and LgA groups at the 0.5 mg/kg/infusion dose. A three-way repeated-measures ANOVA (2 × 2 × 14) revealed a significant main effect of session [F(13,260) = 5.91, p < 0.001], as well as a significant access duration × session interaction [F(13,260) = 4.16, p < 0.001]. Bonferroni corrected t tests revealed that intake on sessions 13–21 in the EC LgA group was significantly higher than intake on session 8. Separate 2 × 2 × 14 ANOVAs for ShA and LgA groups revealed no significant effects on inactive lever presses (results not shown).
Linear trend analyses were also conducted on the results in Fig. 5 to determine if escalation was obtained in each group. There was a significant effect of session in both the EC [t(81) = 5.87, p < 0.001] and IC [t(77) = 3.16, p < 0.01] LgA groups; however, the EC LgA group escalated at a greater rate than the IC LgA group [t(308) = 3.47, p < 0.001]. There was also significant escalation in the EC ShA group [t(68) = 2.26, p < 0.05].
Time course
As shown in Fig. 6, a 2 × 2 × 12 ANOVA (environmental group × session × time interval) across 5-min intervals during the first hour of the session revealed significant main effects of session [F(1,10) = 8.97, p < 0.05] and time interval [F(11,110) = 2.21, p < 0.05], as well as an environmental group × time interval [F(11,110) = 1.88, p < 0.05] interaction. Separate ANOVAs for EC and IC groups revealed a significant main effect of time interval in the EC ShA group [F(11,88) = 4.53, p < 0.001] and the IC ShA group [F(11,110) = 9.21, p < 0.001], but no significant session × time interaction; these main effects were due to a decrease in infusions across the session (results not shown). In the EC LgA group, there was a significant main effect of session [F(1,110) = 18.59, p < 0.05], as well as a significant session × time interval interaction [F(11,110) = 1.96, p < 0.05]. In the IC LgA group, there was a significant main effect of session [F(1,110) = 4.61, p < 0.05] and time interval [F(11,110) = 3.04, p < 0.001]. Pairwise comparisons revealed an elevation in intake across intervals in the first hour of intake on session 21 compared to session 8 in the EC LgA groups, although this elevation was not statistically significant at all 5-min intervals.
Discussion
The main finding from these experiments is that EE protected against escalation of cocaine self-administration, but only at a low unit dose (0.1 mg/kg/infusion), and that social cohorts, rather than novelty, appears to be the primary factor contributing to this protection. At the 0.1 mg/kg/infusion unit dose, EC and IC rats acquired cocaine self-administration to a similar rate after seven sessions. When switched to the LgA sessions, however, only IC rats escalated their intake across sessions at this dose. Cocaine self-administration in the first hour from the first to last LgA session indicated an altered pattern of intake in IC rats, with level of intake increasing primarily during the latter portion of the last session compared to the first session. This altered time-dependent pattern of intake was not found in EC rats. Similar to EC LgA rats, SC LgA rats also did not escalate at the 0.1 mg/kg/infusion unit dose, indicating that social interaction, rather than exposure to novel objects per se, plays an important role in the protective effect of environmental enrichment on escalation. In further support of this interpretation, an NC group, which was exposed to only novel toys in an isolated cage, significantly escalated cocaine intake at the 0.1 mg/kg/infusion unit dose. Thus, it appears that social cohorts contribute to the protective effect against cocaine escalation at this low unit dose. The NC group, however, was raised in isolated cages rather than larger cages (with restricted space to explore); thus, the possibility that exercise plays a role in this protective effect cannot be excluded.
At the higher unit dose (0.5 mg/kg/infusion), environmental enrichment did not protect against escalation. To the contrary, when switched to LgA sessions, EC rats showed a steeper increase in cocaine self-administration across sessions than IC rats. However, one complication in interpreting these latter results relates to the differences observed between EC and IC groups during the initial acquisition phase (sessions 1–7). EC rats failed to acquire cocaine self-administration at the 0.5 mg/kg/infusion unit dose compared to IC rats. It is possible that because EC rats did not reliably acquire cocaine self-administration at this dose, self-administration rates on the first day of extended access were so low that there was more room for EC LgA rats to escalate compared to IC LgA rats. The decreased acquisition rate of cocaine self-administration in EC rats at the 0.5 mg/kg/infusion unit dose is consistent with previous findings in which EC rats self-administered less AMPH than IC rats during 1-h sessions (Bardo et al. 2001; Green et al. 2002). In the current report, cocaine self-administration in the first hour from the first to last LgA session also indicated an elevation of intake across most of the session in the EC LgA group. Thus, the present results indicate that extended access to a high unit dose of cocaine may overcome the protective effects of EE against escalation of cocaine self-administration in rats.
While EC LgA rats likely escalated at a faster rate than IC LgA rats at the 0.5 mg/kg/infusion unit dose because EC rats failed to show reliable acquisition, cocaine intake did not differ between groups at the end of the extended access phase. Thus, EC LgA rats never escalated to a level of cocaine intake greater than that observed in IC LgA rats. The initial low rates of responding during the acquisition phase in the EC group at the higher dose may be due to increased sensitivity to the rewarding, aversive, or response suppressant effects of cocaine early in training. With amphetamine, for example, EC rats are more sensitive than IC rats to the amphetamine-induced increase in dopamine release in nucleus accumbens (Bardo et al. 1999). Although little is known about environmentally induced differential sensitivity to cocaine in the EE rodent model, this may contribute to differences in acquisition of cocaine self-administration.
While it is possible that differences in escalation during the extended access phase may be due to incomplete acquisition of stable rates of responding with the high unit dose (0.5 mg/kg/infusion), results from the lower unit dose (0.1 mg/kg/infusion) are not consistent with this explanation. In particular, although similar acquisition was evident in EC and IC rats on session 7 of the acquisition phase using the low unit dose (0.1 mg/kg/infusion), IC LgA rats escalated, whereas EC LgA rats did not.
A surprising finding of this study was the escalation observed in EC, SC, and NC groups given 0.1 mg/kg/infusion cocaine in ShA sessions. This effect was also observed in the EC group at the higher unit dose (0.5 mg/kg/infusion). Previous studies have found escalation with extended access only (Ahmed and Koob 1998, 1999, 2004; Gipson and Bardo, 2009). The escalation found in ShA groups in the current study may be due to differential acquisition across groups. Relative to the seven acquisition sessions used here, previous studies have employed a more extensive training schedule prior to the extended access phase (Ahmed 2009; Gipson and Bardo 2009; Kitamura et al. 2006); however, at least one other report has used as few as ten sessions of 1-h access prior to the extended access phase (Schwendt et al. 2009). In addition, previous studies of escalation have typically used a food training paradigm prior to self-administration, in which rats respond initially for sucrose pellets (Ahmed and Koob 1999; Kitamura et al. 2006). In contrast, to avoid the possibility of differential sensitivity to food reward, the current study employed a cocaine autoshaping procedure, rather than a food reinforcement procedure, to directly examine acquisition of cocaine self-administration among the environmental groups. To our knowledge, this is the first study of escalation to use an autoshaping procedure prior to drug self-administration. Thus, the lack of acquisition of stable rates of responding prior to extended access may be due to the short acquisition phase with autoshaping instead of food-reinforced pretraining.
The first hour of cocaine self-administration from LgA groups revealed time-dependent changes in pattern of intake due to escalation of cocaine self-administration across the extended access phase. The elevation of intake across 5-min intervals in the NC and IC LgA groups at the 0.1 mg/kg/infusion unit dose, as well as the EC LgA group at the 0.5 mg/kg/infusion unit dose, indicate a change in pattern of intake, as rats in these groups escalated from the first to the last session of the extended access phase. At the low unit dose, escalated cocaine intake occurred primarily during the latter portion of the first hour; at the higher unit dose, escalation tended to occur across the entire portion of the first hour. Profound changes in pattern of intake have been found in previous studies examining escalation of stimulant self-administration using the extended access procedure (Ahmed and Koob 1998; 1999; 2004), such that rats exhibit an increase in intake during the first 5 to 10 min of the session following escalated intake. The change in pattern of intake found in the current study differs from previous reports. Multiple procedural differences may explain the discrepant time-dependent changes observed in the current and previous reports, including environmental differences during development, as well as differences in initial training.
Although the protective mechanism of EE against escalation of cocaine self-administration appears to be due to social interaction, previous research on EE has found utility of novel objects in producing behavioral effects such as protection against behavioral sensitization and CPP (Solinas et al. 2008) as well as reducing AMPH self-administration (Green et al. 2002). In addition, social group housing alone does not account for brain anatomical and chemical changes caused by EE with both social interaction and novel objects (Renner and Rosenzweig 1987; Rosenzweig et al. 1978). Thus, both novelty and social interaction are important in examining influences on behavior and neurobiology.
In conclusion, the current findings indicate that although EE protects against initial stimulant intake, it may not protect against the escalation that characterizes the process of addiction to high doses of drug. The protective effect of EE against escalation of cocaine self-administration at low unit doses appears to reflect the social interaction component of EE, rather than novelty per se, and this effect may be overcome by higher unit doses. Previous clinical and preclinical research found that drug abuse can be decreased with nondrug alternative reinforcers (Carroll 1996), such as money or video game playing in humans (Vuchinich and Tucker 1983; Landau 1986, respectively) or sucrose in rats (Kanarek and Marks-Kaufman 1988). This study, however, illustrates the importance of social interaction as a nondrug reinforcer that may reduce substance use and it extends previous preclinical work showing that social interaction also may protect against relapse (Fritz et al. 2010). Although there are limitations involved with the utility of the rodent EE model in examining drug abuse vulnerability, the current study is the first attempt to use extended drug access, thus furthering the utility of this model in examining the environmental factors involved in drug abuse vulnerability.
References
Adams J, Bowman K, Burke B, Casson L, Caviness L, Coffey LE (1999) National household survey on drug abuse data collection. Final report
Ahmed SH (2009) Escalation of drug use. In: Olmstead MC (ed) Neuromethods: animal models of drug addiction. Humana, Totowa
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282(5387):298–300
Ahmed SH, Koob GF (1999) Long-lasting increase in the set point for cocaine self administration after escalation in rats. Psychopharmacology 146(3):303–312
Ahmed SH, Koob GF (2004) Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology 172(4):450–454
Ahmed SH, Lin D, Koob GF, Parsons LH (2003) Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem 86:102–113
Ahmed SH, Stinus L, Le Moal M, Cador M (1995) Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology 117(1):116–124
Bardo MT, Bowling SL, Rowlett JK, Manderscheid P (1995) Environmental enrichment attenuates locomotor sensitization, but not in vitro dopamine release, induced by amphetamine. Pharmacol Biochem Behav 51(2–3):397–405
Bardo MT, Valone JM, Robinet PM, Shaw WB, Dwoskin LP (1999) Environmental enrichment enhances the stimulant effect of intravenous amphetamine: search for a cellular mechanism in the nucleus accumbens. Psychobiology 27(2):292–299
Bardo MT, Valone KJE, JM DC (2001) Environmental enrichment decreases intravenous self administration of amphetamine in female and male rats. Psychopharmacology 155(3):278–284
Bowling SL, Rowlett JK, Bardo MT (1993) The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology 32(9):885–893
Carroll ME (1996) Reducing drug abuse by enriching the environment with alternative nondrug reinforcers. In: Green L, Kagel J (eds) Advances in behavioral economics. Ablex, Norwood, p 3
Carroll ME, Meisch RA (1984) Increased drug-reinforced behavior due to food deprivation. In: Thompson T, Barrett JE (eds) Advances in behavioral pharmacology, 4th edn. Academic, New York, pp 47–88
Carroll ME, Lac ST (1993) Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology 110(1–2):5–12
Fritz M, El Rawas R, Ahmad S, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G (2010) Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addict Biol (in press)
Gipson CD, Bardo MT (2009) Extended access to d-amphetamine self-administration increases impulsive choice in a delay discounting task in rats. Psychopharmacology 207:391–400
Green T, Gehrke B, Bardo MT (2002) Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology 162:373–378
Green TA, Alibhai IN, Roybal N, Winstanley CA, Theobald DEH, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ (2010) Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry 67:28–35
Hopfer CJ, Crowley TJ, Hewitt JK (2003) Review of twin and adoption studies of adolescent substance abuse. J Am Acad Child Adolesc Psych 42(6):710–719
Kanarek RB, Marks-Kaufman R (1988) Dietary modulation of oral amphetamine intake in rats. Physio & Beh 44(4–5):501–505
Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L (2006) Escalation of methamphetamine self administration in rats: a dose effect function. Psychopharmacology 186(1):48–53
Koob GF, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159
Landau D (1986) The effects of changes and constraints on access to video game playing on alcohol consumption. Diss Abstr Int 48:1174B
Marusich JA, Beckmann JS, Gipson CD, Bardo MT (2010) Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp Clin Psychopharmacol 18(3):257–266
McGue M, Sharma A, Benson P (1996) Parent and sibling influences on adolescent alcohol use and misuse: evidence from a U.S. adoption cohort. J Stud Alcohol 57(1):8–18
Montgomery DC, Peck A, Vining GG (2006) Introduction to linear regression analysis, 4th edn. Wiley, New York
Olmstead MC (2006) Animal models of drug addiction: where do we go from here? Q J Exp Psychol 59(4):625–653
Renner MJ, Rosenzweig MR (1987) Enriched and impoverished environments: effects on brain and behavior. Springer, New York
Rosenzweig MR, Bennet EL, Hebert M, Morimoto H (1978) Social grouping cannot account for cerebral effects of enriched environments. Brain Res 153(3):563–576
Solinas M, Chauvet C, Thiriet N, El Rawas R, Jaber M (2008) Reversal of cocaine addiction by environmental enrichment. Proc Natl Acad Sci 105(44):17145–17150
Solinas M, Thiriet N, El Rawas R, Lardeux V, Jaber M (2009) Environmental enrichment during early states of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacology 34:1102–1111
Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW (2009) Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther 331(2):555–562
Thiel KJ, Sanabria F, Pentkowski NS, Neisewander JL (2009) Anti-craving effects of environmental enrichment. Int J Neuropsychopharmacol 12(9):1151–1156
Vuchinich RE, Tucker JA (1983) Behavioral theories of choice as a framework for studying drinking behavior. J Abnormal Psy 92(4):408–416
Wagner FA, Anthony JC (2002) From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine and alcohol. Neuropsychopharmacol 26:479–488
Acknowledgements
We would like to thank William T. McCuddy, Luke Holderfield, Kate Fischer, Justin Yates, Emily Denehy, and Kristin Alvers for technical assistance. Supported by NIH grants R01 DA12964 and T32 DA007304.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gipson, C.D., Beckmann, J.S., El-Maraghi, S. et al. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology 214, 557–566 (2011). https://doi.org/10.1007/s00213-010-2060-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2060-z