Abstract
Rationale
Stressful life experiences can persistently increase the motivation for, and consumption of, intensely rewarding stimuli, like cocaine, over time. In rodents, intermittent versus continuous exposure to social stress engenders opposing changes to reward-related behavior, as measured by consumption of sucrose and cocaine.
Objective
The present study examines if the effects of intermittent versus continuous social stress on cocaine self-administration in mice parallel those seen in rats.
Methods
Both forms of social stress involve a brief daily physical confrontation with an aggressive resident for 10 consecutive days. Continuous social stress involves constant visual and olfactory exposure to an aggressive resident via habitation in a protected portion of the resident’s home cage, while exposure to an aggressive resident during intermittent social stress is limited to a single, physical encounter per day. Implementing a femoral vein catheterization method for the first time in mice, we determined divergent changes to intravenous cocaine self-administration.
Results
Modestly increased cocaine self-administration after intermittent social stress was confirmed. In a subset of animals, continuous social stress in mice substantially increased cocaine self-administration and sucrose intake. By stark contrast, another subpopulation had substantial attenuation of cocaine self-administration and sucrose intake after continuous social stress.
Conclusions
Bimodal divergence in responding for rewarding stimuli including cocaine after social stress experience likely reflects two opposing forms of coping to continuous social stress that promote either a sensitization or attenuation of reward-seeking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coping with stress is necessary for survival, but uncontrollable stressful events can greatly exacerbate the onset of psychopathologies like drug abuse (Kleber and Gawin 1984; Der-Avakian et al. 2006; Sapolsky 2015). For example, stressful life experiences can facilitate the acquisition of cocaine use and prompt cocaine craving or relapse in patients (Karlsgodt et al. 2003; Sinha 2008). Empirical attempts to model substance use disorders sensitive to stress experiences in animal models have examined the initiation and persistent increase of intense drug-taking, as well as reinstatement of drug-seeking after abstinence (Marinelli et al. 1996; Goeders 2002; Miczek et al. 2008; Han et al. 2017). Most behavioral and physiological findings from translational models of social stress–increased cocaine self-administration are established in non-human primates and rats, and less in mice (Morgan et al. 2002; Czoty et al. 2004; Miczek et al. 2011; Holly et al. 2012; Shimamoto et al. 2015). While long-term intravenous drug self-administration in mice is methodologically challenging, novel catheterization techniques enable the study of more prolonged and intense drug consumption, resulting in more clinically relevant models of substance use disorders (Thomsen and Caine 2005; Han et al. 2015). This study explores how intermittent social defeat stress (ISDS) and continuous social defeat stress (CSDS) alter cocaine-taking in mice, in order to determine how the intensity of social stress episodes alters the ensuing patterns of behavioral and neural plasticity.
Although the intensity, frequency, and probability of defensive-submissive reactions to aggression differ among strains, mice are a reliably pugnacious species (Parmigiani and Brain1983; Guillot and Chapouthier 1996; Miczek et al. 2001). Social stress in rodents, like humans, is characterized by increased circulating glucocorticoids, immune suppression, persistent dysregulation of cardiovascular function, and hyperthermia (Henry and Cassel 1969; Björkqvist2001; de Groot et al. 1999; Miczek et al. 2008). Like other types of stress, intermittent social stress in rats causes sensitization to psychomotor stimulation, hastens acquisition of cocaine self-administration, and augments cocaine-taking during a 24-h unlimited access binge (Miczek and Mutschler 1996; Tidey and Miczek 1997; Covington and Miczek 2001; Covington et al. 2008). Likewise, pulses of non-contingent footshock not only increase rates of cocaine self-administration but also facilitate maintenance of high response rates at lower doses of cocaine in rats (Goeders and Guerin 1994).
The effects of social defeat on cocaine reward in mice vary based on the age at which the stress is experienced (Montagud-Romero et al. 2015; Rodriguez-Arias et al. 2017), as well as the sex of the animal (Kikusui et al. 2005), and the nature of the social defeat stress. Overall, episodic stress intensifies the respond for cocaine, particularly during the acquisition and maintenance of drug-taking, which also includes a leftward shift in responding for intravenous unit doses (Han et al. 2015). Rodent models of continuous social stress often cause a robust depressive-like phenotype, which is characterized by social avoidance and blunted reward processing (Kudryavtseva et al. 1991; Berton et al. 2006). Rats show attenuated cocaine-seeking and cocaine-taking after continuous social stress, but these effects have yet to be confirmed in mice (Miczek et al. 2011; Shimamoto et al. 2015).
Experience with intermittent and continuous social defeat stress either exacerbates or attenuates cocaine self-administration and alcohol intake, respectively (Miczek et al. 2011; Holly et al. 2012; Norman et al. 2015; Han et al. 2015). Here, we implemented femoral vein catheterization in order to delineate persistent changes in intravenous cocaine self-administration in C57BL/6J mice. Our main goal was to extend our rat studies showing divergent effects of intermittent and continuous social defeat stress on subsequent cocaine self-administration to mice (Miczek et al. 2011; Shimamoto et al. 2015). The current results provide a basis for characterizing adaptive processes in neurobiological systems after intermittent and continuous social stress and for identifying individuals who are especially vulnerable to the potentiating effects of social stress on drug-taking.
Materials and methods
Subjects
Experimental animals
C57BL/6J male mice (Jackson Labs, Bar Harbor, ME) weighing 25–27 g upon arrival were individually housed in clear polycarbonate cages (28 × 17 × 14 cm) with stainless steel wire lids and lined with pine shavings within a temperature-controlled mouse vivarium (21 ± 1 °C, 30 to 40% humidity) that was kept on a reversed 12-h photocycle (lights off, 0600 h). Mice were adapted to the facilities for at least 7 days before experiments. Animals were given standard rodent chow and water ad libitum (Lab Diet 5001 Rodent Diet, PMI Nutrition International, Brentwood, MO, USA). All housing conditions were maintained throughout the experiment (social defeat, sucrose preference, and cocaine intravenous self-administration) with the exception of the chronic social defeat protocol (adapted from Kudryavtseva et al. 1991). All experimental procedures were reviewed and approved by the Tufts Institutional Animal Care and Use Committee, following the NIH Guide for the Care and Use of Laboratory Animals, 8th Edition (National Research Council 2011).
Residents
A separate group of male CFW mice (Charles River, Wilmington, MA) was pair-housed with females in clear polycarbonate cages (28 × 17 × 14 cm) for 1 month upon arrival at 8 weeks of age and served as aggressive stimulus mice (residents). Each resident was chosen for consistent aggressive behavior during confrontations with an intruder mouse (Miczek and O’Donnell 1978).
Drugs
Cocaine hydrochloride was obtained from the Research Technology Branch of the National Institute on Drug Abuse and dissolved in sterile 0.9% saline.
Experimental design
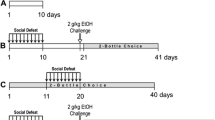
Upon arrival, mice were randomly assigned to three groups: intermittent social defeat stress, continuous social defeat stress, or non-stressed controls. The mice in both stress groups were socially defeated for 10 consecutive days (days 1–10). Seven to 10 days after the last defeat, all animals were examined for social interaction and sucrose preference and then began cocaine self-administration (Fig. 1).
Social defeat stress
Intermittent episodic social defeat
Intermittently stressed mice were defeated using the resident-intruder protocol as illustrated and described previously (Miczek and O’Donnell 1978; Miczek et al. 1982; Yap and Miczek 2007) on days 1 through 10. Each social defeat episode took place in the room adjacent to where they were housed. Experimentally stressed mice were placed in a perforated cage inside the home cage of an aggressive male resident to allow unrestricted visual, auditory, and olfactory contact with protection from injury. After 5 min of social threat in the perforated cage, stressed mice were removed from the perforated cage and returned to the resident home cage where attack by the resident occurred. Immediately following defeat (defined as having received 30 bites within a maximum of 5 min), intruder mice were then placed back in the perforated cage for another 5 min in the aggressor’s home cage. Intruder mice were then returned to the adjacent room where they were singly housed until their next defeat on the subsequent day. Intruder mice were exposed to a novel resident during each daily defeat. During each defeat episode, the latency to attack, number of bites received, and total duration of each encounter were recorded.
Continuous social defeat
Continuously stressed mice were defeated as described previously (adapted from Miczek and O’Donnell 1978; Kudryavtseva et al. 1991). Male resident mice were allowed 24 h to establish territory on one-half of a clear polycarbonate cage (46 × 24 × 16 cm), divided lengthwise by a perforated divider with a stainless steel wire lid and pine shavings as bedding (Golden et al. 2011). Similar to the intermittent stress protocol, the intruder mouse was placed directly within the resident aggressor’s home cage compartment. After 5 min of defeat (approximately 30 bites), the intruder was transferred across the perforated divider to the opposite compartment and housed there for the remainder of the day. Resident aggressors remained in their home cage throughout the 10-day defeat period, and intruder mice were exposed to a different resident aggressor daily to prevent habituation.
Sucrose preference
To examine sucrose preference, 50-mL bottles were filled with either 1% sucrose (w/v) in tap water or tap water alone. The bottles were introduced 4 h into the dark photoperiod and fluid levels were noted at 4, 6, and 8 h over 2 consecutive days into the dark photoperiod. The position of the tubes was changed, and bottles were reweighed, after the initial 24 h. To control for unintentional fluid loss and evaporation, bottle measurements were also recorded from an empty cage, and these values were subtracted from each mouse’s intake. Sucrose consumption was calculated as grams of sucrose consumed (g) and as a percent of total fluid intake (sucrose + H2O) averaged over the 2 days of testing.
Social interaction testing
Seven days after the last day of social defeat, social behavior was evaluated using a 3-chamber apparatus (85 × 30 × 35 cm) (Moy et al. 2004; Zanettini et al. 2010; Newman et al. 2016). The socially defeated or non-stressed male habituated to the central chamber of the 3-chamber apparatus for a 2.5-min period. An unfamiliar resident aggressor stimulus mouse was placed in a wire mesh cage in either the right or left chamber. Following this habituation period, the doors on either side of the central chamber were lifted, allowing the experimental male to move freely between the central chamber, the chamber with the resident stimulus male, and a third chamber with an empty stimulus cage during a 2.5-min social approach test. EthoVision XT software (Noldus, Wageningen, the Netherlands) tracked the male and recorded the duration of time it spent within each of the three chambers.
Surgical procedures
On the day following sucrose preference testing (day 23), intermittently defeated and control mice were permanently implanted with an indwelling catheter (Silastic silicon tubing, ID 0.012 in, OD 0.025 in) into the right jugular or femoral vein under a combination of ketamine (100 mg/kg i.p.) and xylazine (10 mg/kg i.p.) anesthesia (Han et al. 2015). The distal end of the catheter was passed subcutaneously from the insertion point to the area between the scapulae where it exited through a small incision and was affixed to a small plastic pedestal (Plastics One, Roanoke, VA). After surgery, mice were allowed 4 days to recover. Catheters were flushed daily with 0.05 mL of heparinized saline (20 IU/mL) to prevent clotting.
Cocaine self-administration
For cocaine self-administration, a panel with cue lights and two nose-poke operanda was inserted vertically into the home cage of the mouse (Miczek and de Almeida 2001). The top of the panel held a house light for cage illumination and a counterbalanced arm holding a liquid swivel (Instech Laboratories, Plymouth Meeting, PA). A 3-mL syringe in a computer-controlled pump was connected to the swivel and pedestal by PE20 tubing. A green cue light distinguished the operanda (the “active” hole had a light present, while the “inactive” hole remained unlit). The active side remained constant throughout the experiment and was counterbalanced across subjects. A response on the active side triggered an I.V. infusion of cocaine, and the stimulus light was then deactivated for 20 s (indicating the post-infusion time-out). All responses and infusions were recorded automatically using a computer interface and software from Med Associates (St. Albans, VT).
Daily 4-h sessions
Following recovery from surgery, mice were trained to self-administer cocaine (0.3 mg/kg/infusion) according to a fixed ratio 1 (FR1) schedule of reinforcement (Han et al. 2015). Two 2-h time blocks of access were separated by a 10-min interval in which all cue and house lights were extinguished to signal unavailability of drug. Each access block was terminated after the delivery of 50 infusions or after 2 h of access. Mice acquired and were maintained on 0.3 mg/kg/infusion for 7 days. Thereafter, mice were tested according to an FR1 schedule with descending unit doses (0.1, 0.03, 0.01, 0.003, 0.001 mg/kg/infusion), receiving each dose for 2 consecutive days. Depending on cocaine self-administration during acquisition, mice with continuous stress experience were separated into one of two groups for all subsequent analyses. Responding for cocaine during training sessions after CSDS produced two subgroups that are represented by non-overlapping modes. During days 2–7 of acquisition, animals either took less than 10 infusions per session most often or self-administered more than 90 infusions per session most often (Fig. 7). The group of “high responders” took an average of 23 mg/kg/session on days 2–7 of acquisition, while the “low responders” took an average of 3 mg/kg/session.
All intermittently defeated and contemporaneous control animals were catheterized via the jugular vein, while all continuously defeated and contemporaneous controls were catheterized via the femoral vein. A separate subset of animals void of SDS experience were catheterized via the jugular or femoral vein to determine differences in cocaine acquisition based on catheterization method.
Statistical analysis
Data were analyzed using Prism version 7.0 (Graphpad Software Inc.). Two-way repeated measures ANOVA were used to analyze repeated observations of social interaction and sucrose preference after either ISDS, CSDS, or daily handling, cocaine self-administration after ISDS and in non-stressed controls to test the effects of the catheterization method. Two-way repeated measures ANOVA were also used to test the effects of ISDS, CSDS, and route of catheterization on cocaine self-administration. All significant results were followed by post hoc analyses with Bonferroni’s corrections for multiple comparisons. In the cocaine self-administration after CSDS, Kruskal–Wallis tests were used to analyze the relationship between the control animals, those in the “high responders,” and those in the “low responders” group across days of acquisition and descending doses of drug. Pearson’s correlation coefficient (r) was generated to determine the relationship between sucrose and cocaine consumption after CSDS. The criterion for statistical significance was p < 0.05.
Results
Intravenous drug self-administration in mice: femoral versus jugular catheterization
By catheterizing a subset of stress-naive mice in either the jugular or femoral veins, we confirmed that acquisition and maintenance of cocaine self-administration on an FR1 schedule of responding at 0.3 mg/kg/infusion were not significantly affected by the route of catheterization. The benefit of the femoral vein catheterization in mice, as compared to jugular, was evident by considerably extended longevity of catheters (Fig. 2). A log-rank (Mantel–Cox) test revealed a significant effect of catheterization route on catheter longevity (X2(1, 28) = 18.37; p < 0.001).
CSDS produces social deficits
Using a 3-chamber apparatus, we examined the time spent in each of the three chambers when the stimulus resident male was present. When tested 10 days after the last social defeat, animals with a history of CSDS showed social deficits when compared to those with a history of ISDS or daily handling. A repeated measures two-way ANOVA revealed a significant effect of chamber (F2, 267 = 77.11; p < 0.001) and a significant interaction between stress condition and chamber (F4, 267 = 4.81; p < 0.001). There was no significant main effect of stress. Post hoc analyses revealed significant attenuation of time spent in the chamber with the stimulus male by mice with a history of CSDS when compared to controls (p < 0.05) (Fig. 3).
CSDS produces two distinct sucrose-preferring phenotypes
Although ISDS had minimal effects on subsequent consumption of and preference for 1% sucrose during a 48-h preference test, CSDS aggregated animals into two distinct subpopulations. There was a significant, direct relationship between the amount of cocaine self-administered during acquisition and the amount of sucrose consumed during the preference test in mice that underwent CSDS (r = 0.602, p < 0.05; Fig. 8). Based on cocaine intake during days 2–7 of acquisition, animals with a history of CSDS were either placed in the “high responders” or “low responders” group for analysis of sucrose consumption and preference.
A repeated measures two-way ANOVA revealed both a significant effect of fluid type (F3, 166 = 169.4; p < 0.001) and a significant interaction between stress condition and fluid type (F3, 166 = 4.22; p < 0.01) for 24-h sucrose and water consumption during a 2-bottle test. There was no significant main effect of stress. Post hoc analyses revealed significantly increased consumption of 1% sucrose in mice with a history of CSDS assigned to the high responders group when compared to controls (p < 0.05). To examine the effects of sucrose preference over water, a one-way ANOVA revealed a significant effect of fluid type (F3, 83 = 66.79; p < 0.001). Post hoc analyses found significant attenuation of sucrose preference in mice with a history of CSDS assigned to the low responders group (p < 0.001), as well as ISDS (p < 0.001), compared to controls (Fig. 4).
Twenty-four-hour consumption and preference for 1.0% sucrose solution versus water in a 2-bottle preference test by intermittently stressed mice ((I); n = 36), continuously stressed mice placed into the high responders group ((HC); n = 7), continuously stressed mice placed into the low responders group ((LC); n = 6), and controls ((C); n = 38). All values are mean ± SEM. * = p < 0.05, *** = p < 0.001 compared to the relevant control group
Intermittent SDS modestly increases cocaine self-administration
For analysis of cocaine acquisition after previous experience with ISDS, a repeated measures two-way ANOVA revealed a significant effect of day (F6, 138 = 6.91; p < 0.001), as well as a significant effect of stress (F2, 23 = 6.38; p < 0.05). There was no significant interaction of stress and day of acquisition. Post hoc analyses found significantly increased cocaine self-administration on day 1of acquisition after ISDS as compared to controls (p < 0.001; Fig. 5a).
a Acquisition of cocaine self-administration (0.3 mg/kg/inf) after intermittent SDS (n = 14, filled squares) and contemporaneous controls (n = 12, open circles). b Responding for various doses of intravenous cocaine in mice with a history of intermittent social defeat (n = 6) and contemporaneous controls (n = 9). Individual data were averaged over two sessions. Data are expressed as mean; *p < 0.05 vs. controls
Responding for varying doses of cocaine after ISDS was analyzed using a repeated measures two-way ANOVA to reveal a significant effect of stress (F1, 72 = 4.93; p < 0.05). There was no significant main effect of cocaine dosage or interaction of stress and cocaine dosage (Fig. 5b).
Continuous SDS persistently increases or attenuates cocaine self-administration
Acquisition of cocaine self-administration after previous experience with CSDS was analyzed as a whole group using a repeated measures two-way ANOVA to reveal a significant effect of day (F1, 140 = 10.37; p < 0.01). There was no significant effect of stress and no significant interaction of stress and day of acquisition (Fig. 6a).
a Acquisition of cocaine self-administration (0.3 mg/kg/inf) after continuous social defeat (n = 13, black squares) and contemporaneous controls (n = 9, open circles). b Acquisition of cocaine self-administration (0.3 mg/kg/inf) after continuous social defeat (high responders: n = 7, dark gray squares; low responders: n = 6, light gray triangles) and contemporaneous controls (n = 9, open circles).c Responding for various doses of intravenous cocaine in mice with a history of continuous social defeat (n = 9, black squares) and contemporaneous controls (n = 6, open circles). d Responding for various doses of intravenous cocaine in mice with a history continuous social defeat (high responders: n = 5, dark gray squares; low responders: n = 4, light gray triangles) and contemporaneous controls (n = 6, open circles). Data from each dose on the dose-effect curves were averaged over two sessions. Data are expressed as mean ± SEM
Responding for varying doses of cocaine after CSDS was also analyzed as a whole group using a repeated measures two-way ANOVA to reveal a significant effect of cocaine dosage (F5, 114 = 6.82; p < 0.001). There was no significant main effect of stress or interaction of stress and cocaine dosage (Fig. 6c).
Previous experience with CSDS aggregated mice into two distinct cocaine-taking phenotypes during days 2–7 of acquisition. Approximately half of the animals (n = 6) clustered around a group mean of 3 mg/kg/session, while the other half of the animals (n = 7) had a group mean of 23 mg/kg/session. Although responding for cocaine on day 1 was not indicative of subsequent cocaine-taking, no member of the “low responders” took more infusions/session than the minimum number of infusions/session taken by any member of the “high responders” within days 2–7 of acquisition (Fig. 7). Since cocaine self-administration data were clustered based upon the behavior itself, Kruskal–Wallis tests were run to compare cocaine self-administration between stress conditions. Responding for cocaine during acquisition after CSDS was analyzed by a Kruskal–Wallis test to reveal significant differences in the mean number of self-administered cocaine infusions across acquisition among the three conditions (H = 15.65, p = 0.001; Fig. 6b). Results of Kruskal–Wallis testing were also significant for the effect of cocaine dose (H = 12.32, p = 0.001; Fig. 6d).
a In animals with a history of continuous SDS (high responders: n = 7, dark gray bars; low responders: n = 6, light gray bars), the maximum number of cocaine infusions/session taken by any member of the low responders between days 2–7 of acquisition never exceeded the minimum number of cocaine infusions taken by high responders on corresponding days. b The number of cocaine infusions across days 2–7 of acquisition formed a continuous distribution in animals with no history of SDS (controls: n = 9), unlike the bimodal distribution produced after CSDS
Discussion
The present results confirm increased intravenous cocaine-taking in mice exposed to intermittent social defeat stress. We extend previous findings by comparing continuous to intermittent social stress followed by subsequent cocaine self-administration. We found that these two histories of social defeat had divergent effects on patterns of cocaine self-administration. Experience with continuous social defeat stress aggregated C57Bl/6J mice into two distinct subpopulations upon examination of subsequent cocaine intake during daily sessions. In fact, one subpopulation self-administered a mean of 23 mg/kg/session, whereas a second subpopulation averaged 3 mg/kg/session. The maximal number of infusions taken by any member of the low responders never exceeded the minimum number of infusions taken by any member of the high responders (Fig. 7). These patterns of cocaine-taking were closely correlated with those for sucrose preference, a putative measure of anhedonia (Willner et al. 1987; Berton et al. 2006; Krishnan et al. 2007). Preference for sucrose over water revealed overlap with patterns of cocaine-taking in continuously defeated mice. Specifically, the amount of sucrose consumed during the preference test predicted the amount of cocaine self-administered during acquisition (Fig. 8). After experiencing continuous SDS, mice showed either increased or attenuated intake of both sucrose and cocaine when compared to animals with a history of ISDS, or those with no social defeat experience. Taken together, the correlation in patterns of consumption for cocaine and sucrose suggests two hypotheses. First, chronic social stress may result in divergent neuroplastic processes to engender either increased consummatory behavior or an anhedonic-like phenotype. Second, anhedonia may present itself across behavior under the control of several highly reinforcing stimuli.
Twenty-four-hour intake of 1% sucrose during a preference test is predictive of cocaine consumption during acquisition in mice with a history of continuous SDS ((n = 17) (r = 0.34; p = 0.014)). Open circles denote non-stressed controls (n = 4), light gray squares denote animals placed in the low responders group (n = 6), and dark gray squares denote high responders (n = 7)
The results from the current set of experiments extend previous findings regarding the effects of exposure to continuous SDS. Continuous SDS in mice has produced phenotypes that translate to symptoms indicative of anhedonia in humans (Björkqvist 2001). Continuous SDS effectively blunts reward processing as measured by reduced intake of sucrose in both rats and mice (Willner et al. 1987; Rygula et al. 2005; Harris et al. 2018; Macedo et al. 2018), and attenuated cocaine self-administration in both male and female rats (Miczek et al. 2011; Shimamoto et al. 2015). Remarkably, continuous SDS engendered differentiated intake of both sucrose and cocaine, with approximately half of the mice increasing intake (Fig. 8). It remains to be determined how the intermittency, intensity, and controllability of stress alter the motivation for, and consumption of, rewarding stimuli, such as sucrose and cocaine. While continuous SDS consistently produced animals with social deficits, it resulted in two starkly contrasting phenotypes of sucrose and cocaine self-administration. These data may indicate a vulnerability to cocaine use based upon the individual’s response to stress experience (Charney 2004; Krishnan et al. 2007).
The translational value of stress-increased cocaine use in animal models depends heavily on drug access conditions (Ahmed and Koob 1998; Kawa et al. 2016; Ahmed et al. 2018). Until now, jugular catheters were limited in patency preventing the study of persistent and intense drug intake in mouse models of intravenous drug self-administration. The approach to the femoral vein extends the patency of catheters by several weeks and increases the time to accumulate drug intake. Allowing for long-term drug self-administration in mice enables the study of higher and longer rates of consumption, motivation, and more translationally relevant intermittent access conditions (Mantsch et al. 2007; Zimmer, Oleson and Roberts 2012). Earlier work demonstrated that the pharmacokinetics of cocaine depend not on the route of intravenous catheterization for the study of self-administered drugs, as long as the distance from the insertion site to the heart is properly accounted for (Collins and Kantak 2002; Czoty et al. 2004; Nader and Reboussin 1994). Minor changes to the pharmacodynamics of intravenous cocaine self-administration will alter susceptibility and sensitization of the drug (Samaha et al. 2002), and the current set of experiments in mice confirms there is an insignificant difference in responding for cocaine based upon the method of catheterization. Limited patency of jugular catheters has hindered our ability to study drug self-administration in the same mouse both before and after SDS, but femoral catheterization techniques render this feasible approach (Fig. 2).
Individuals are known to vary considerably in their behavioral, neural, and endocrine responses to social stressors (Wingfield and Sapolsky 2003; de Boer et al. 2017; Wood and Valentino 2017). In line with bimodal population distributions, two points emerge from the overall results of our study. First, 10 days of continuous social defeat stress significantly reduced social interactions in all of our B6 mice. Beyond this general deficit in social behavior, which is an important measure of stressor vulnerability, our chronically stressed mice did however diverge, with regard to reward sensitivity. As described above, either an increase or decrease in cocaine- and sucrose-taking was phenotypically expressed after chronic social stress. Thus, our data indicate that subpopulations exist beyond a distinct depressive-like phenotype, including completely opposite responses to reward sensitivity. This evidence may further explain the biological complexities of affective syndromes, which often occur with different outcomes to antidepressant treatment, even in patients with the same diagnosis (Labonté et al. 2017).
Second, intermittently stressed mice, when compared to non-stressed controls, generated significantly more response for cocaine under the current limited access self-administration conditions. However, elevated cocaine-taking by intermittently stressed mice was far less than the amount self-administered by high-responding (HC) chronically stressed mice. Taken together, these data indicate that social stress can gradually lead to the development of a distinct reward-seeking phenotype, whether it be intensely augmented or robustly diminished. Specifically, brief intermittent stress modestly increases cocaine-taking, while more intense stress exposures lead to a divergence in cocaine reward, either further increasing, or conversely, completely compromising drug-taking. In line with our behavioral data, molecular results have indicated a recruitment of many molecular neural mechanisms during the process of developing a resiliency to chronic social stress (Krishnan et al. 2007; Hultman et al. 2018; Lorsch et al. 2018). Future experiments are warranted to determine whether or not neural adaptations are associated with differential patterns of cocaine-taking across a range of individuals after chronic, intense social stress experiences.
References
Ahmed SH, Badiani A, Miczek KA, Müller CP (2018) Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282(5387):298–300
Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762):864–868
Björkqvist K (2001) Social defeat as a stressor in humans. Physiol Behav 73(3):435–442
Charney DS (2004) Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 161(2):195–216
Collins SL, Kantak KM (2002) Neuronal nitric oxide synthase inhibition decreases cocaine self-administration behavior in rats. Psychopharmacology 159(4):361–369
Covington HE, Miczek KA (2001) Repeated social-defeat stress, cocaine or morphine. Psychopharmacology 158(4):388–398
Covington HE, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA (2008) NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology 197(2):203–216
Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA (2004) Characterization of dopamine D 1 and D 2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology 174(3):381–388
de Boer SF, Buwalda B, Koolhaas JM (2017) Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci Biobehav Rev 74:401–422
de Groot J, van Milligen FJ, Moonen-Leusen BW, Thomas G, Koolhaas JM (1999) A single social defeat transiently suppresses the anti-viral immune response in mice. J Neuroimmunol 95(1–2):143–151
Der-Avakian A, Bland ST, Schmid MJ, Watkins LR, Spencer RL, Maier SF (2006) The role of glucocorticoids in the uncontrollable stress-induced potentiation of nucleus accumbens shell dopamine and conditioned place preference responses to morphine. Psychoneuroendocrinology 31(5):653–663
Goeders NE (2002) The HPA axis and cocaine reinforcement. Psychoneuroendocrinology 27(1–2):13–33
Goeders NE, Guerin GF (1994) Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology 114(1):63–70
Golden SA, Covington HE, Berton O, Russo SJ (2011) A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6(8):1183–1191
Guillot PV, Chapouthier G (1996) Intermale aggression and dark/light preference in ten inbred mouse strains. Behav Brain Res 77(1–2):211–213
Han X, Albrechet-Souza L, Doyle MR, Shimamoto A, DeBold JF, Miczek KA (2015) Social stress and escalated drug self-administration in mice II. Cocaine and dopamine in the nucleus accumbens. Psychopharmacology 232(6):1003–1010
Han X, DeBold JF, Miczek KA (2017) Prevention and reversal of social stress-escalated cocaine self-administration in mice by intra-VTA CRFR1 antagonism. Psychopharmacology 234(18):2813–2821
Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, Abbas AI, Leonardo ED, Bolkan SS, Hen R, Gordon JA (2018) A novel method for chronic social defeat stress in female mice. Neuropsychopharmacology 43(6):1276–1283
Henry JP, Cassel JC (1969) Psychosocial factors in essential hypertension recent epidemiologic and animal experimental evidence. Am J Epidemiol 90(3):171–200
Holly EN, Shimamoto A, DeBold JF, Miczek KA (2012) Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology 224(1):179–188
Hultman R, Ulrich K, Sachs BD, Blount C, Carlson DE, Ndubuizu N, Bagot RC, Parise EM, Vu MAT, Gallagher NM, Wang J (2018) Brain-wide electrical spatiotemporal dynamics encode depression vulnerability. Cell 173(1):166–180
Karlsgodt KH, Lukas SE, Elman I (2003) Psychosocial stress and the duration of cocaine use in non-treatment seeking individuals with cocaine dependence. The American journal of drug and alcohol abuse 29(3):539–551
Kawa AB, Bentzley BS, Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233(19–20):3587–3602
Kikusui T, Faccidomo S, Miczek KA (2005) Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology 178(2–3):202–210
Kleber HD, Gawin FH (1984) The spectrum of cocaine abuse and its treatment. J Clin Psychiatry 45:18–23
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Ghose S (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131(2):391–404
Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA (1991) Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav 38(2):315–320
Parmigiani S, Brain PF (1983) Effects of residence, aggressive experience and intruder familiarity on attack shown by male mice. Behav Process 8(1):45–57
Macedo GC, Morita GM, Domingues LP, Favoretto CA, Suchecki D, Quadros IMH (2018) Consequences of continuous social defeat stress on anxiety-and depressive-like behaviors and ethanol reward in mice. Horm Behav 97:154–161
Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR (2007) Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res 1167:101–111
Marinelli M, Le Moal M, Piazza PV (1996) Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res 724(2):251–255
Miczek KA, Mutschler NH (1996) Activational effects of social stress on IV cocaine self-administration in rats. Psychopharmacology 128(3):256–264
Miczek KA, de Almeida RM (2001) Oral drug self-administration in the home cage of mice: alcohol-heightened aggression and inhibition by the 5-HT 1B agonist anpirtoline. Psychopharmacology 157(4):421–429
Miczek KA, Thompson ML, Shuster L (1982) Opioid-like analgesia in defeated mice. Science 215(4539):1520–1522
Miczek KA, Maxson SC, Fish EW, Faccidomo S (2001) Aggressive behavioral phenotypes in mice. Behav Brain Res 125(1–2):167–181
Miczek KA, Yap JJ, Covington HE (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120(2):102–128
Miczek KA, Nikulina EM, Shimamoto A, Covington HE (2011) Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci 31(27):9848–9857
Miczek KA, O’Donnell JM (1978) Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology 57(1):47–55
Montagud-Romero S, Aguilar MA, Maldonado C, Manzanedo C, Miñarro J, Rodríguez-Arias M (2015) Acute social defeat stress increases the conditioned rewarding effects of cocaine in adult but not in adolescent mice. Pharmacol Biochem Behav 135:1–12
Morgan D, Brebner K, Lynch WJ, Roberts DCS (2002) Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol 13(5):389–396
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN (2004) Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3(5):287–302
Nader MA, Reboussin DM (1994) The effects of behavioral history on cocaine self-administration by rhesus monkeys. Psychopharmacology 115(1–2):53–58
Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA (2015) Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology 232(6):991–1001
Newman EL, Gunner G, Huynh P, Gachette D, Moss SJ, Smart TG, Rudolph U, DeBold JF, Miczek KA (2016) Effects of Gabra2 point mutations on alcohol intake: increased binge-like and blunted chronic drinking by mice. Alcohol Clin Exp Res 40(11):2445–2455
Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, Scarpa JR, Moy G, Loh YH, Cahill M, Lorsch ZS (2017) Sex-specific transcriptional signatures in human depression. Nat Med 23(9):1102–1111
Lorsch ZS, Loh YHE, Purushothaman I, Walker DM, Parise EM, Salery M, Cahill ME, Hodes GE, Pfau ML, Kronman H, Hamilton PJ (2018) Estrogen receptor α drives pro-resilient transcription in mouse models of depression. Nat Commun 9(1):1116
Rodríguez-Arias M, Montagud-Romero S, Rubio-Araiz A, Aguilar MA, Martín-García E, Cabrera R, Maldonado R, Porcu F, Colado MI, Miñarro J (2017) Effects of repeated social defeat on adolescent mice on cocaine-induced CPP and self-administration in adulthood: integrity of the blood–brain barrier. Addict Biol 22(1):129–141
Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U (2005) Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162(1):127–134
Samaha AN, Li Y, Robinson TE (2002) The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci 22(8):3244–3250
Sapolsky RM (2015) Stress and the brain: individual variability and the inverted-U. Nat Neurosci 18(10):1344–1346
Shimamoto A, Holly EN, Boyson CO, DeBold JF, Miczek KA (2015) Individual differences in anhedonic and accumbal dopamine responses to chronic social stress and their link to cocaine self-administration in female rats. Psychopharmacology 232(4):825–834
Sinha R (2008) Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences 1141(1):105–130
Thomsen M, Caine SB (2005) Chronic intravenous drug self-administration in rats and mice. Current Protocols in Neuroscience Chapter 9: Unit 9.20
Tidey JW, Miczek KA (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology 130(3):203–212
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93(3):358–364
Wingfield JC, Sapolsky RM (2003) Reproduction and resistance to stress: when and how. J Neuroendocrinol 15(8):711–724
Wood SK, Valentino RJ (2017) The brain norepinephrine system, stress and cardiovascular vulnerability. Neurosci Biobehav Rev 74:393–400
Yap JJ, Miczek KA (2007) Social defeat stress, sensitization, and intravenous cocaine self-administration in mice. Psychopharmacology 192(2):261–273
Zanettini C, Carola V, Iacono LL, Moles A, Gross C, d’Amato FR (2010) Postnatal handling reverses social anxiety in serotonin receptor 1A knockout mice. Genes Brain Behav 9(1):26–32
Zimmer BA, Oleson EB, Roberts DC (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37(8):1901–1910
Acknowledgments
The authors would like to thank J. Thomas Sopko for his assistance during manuscript preparation.
Funding
This work was funded by NIDA grant DA031734 to KAM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arena, D.T., Covington, H.E., DeBold, J.F. et al. Persistent increase of I.V. cocaine self-administration in a subgroup of C57BL/6J male mice after social defeat stress. Psychopharmacology 236, 2027–2037 (2019). https://doi.org/10.1007/s00213-019-05191-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05191-6