Abstract
Rationale
Modafinil is a wake-promoting agent and has been reported to be effective in improving attention in patients with attentional disturbance. However, neural substrates underlying the modafinil effects on attention are not fully understood.
Objectives
We employed a functional magnetic resonance imaging (fMRI) study with the attention network test (ANT) task in healthy adults and examined which networks of attention are mainly affected by modafinil and which neural substrates are responsible for the drug effects.
Methods
We used a randomized placebo-controlled within-subjects cross-over design. Twenty-three healthy adults participated in two series of an fMRI study, taking either a placebo or modafinil. The participants performed the ANT task, which is designed to measure three distinct attentional networks, alerting, orienting, and executive control, during the fMRI scanning. The effects of modafinil on behavioral performance and regional brain activity were analyzed.
Results
We found that modafinil enhanced alerting performance and showed greater alerting network activity in the left middle and inferior occipital gyri as compared with the placebo. The brain activations in the occipital regions were positively correlated with alerting performance.
Conclusions
Modafinil enhanced alerting performance and increased activation in the occipital lobe in the alerting network possibly relevant to noradrenergic activity during the ANT task. The present study may provide a rationale for the treatment of patients with distinct symptoms of impaired attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention is an essential cognitive function for almost all aspects of behaviors. Through the “top-down” and “bottom-up” processes of attention, we detect targets with alerting features, select relevant inputs, ignore irrelevant inputs, and maintain appropriate task sets (Sarter et al. 2001). Posner and Petersen (1990) have proposed that the attention system can be divided into three networks: alerting, orienting, and executive control. These three networks have been theorized to be functionally and anatomically segregated (Fan et al. 2002; Posner and Rothbart 2007). In addition, pharmacological studies have revealed that each network is preferentially affected by a specific neurotransmitter system. Noradrenaline controls the efficiency of alerting, whereas acetylcholine and dopamine affect orienting and executive control, respectively (Green et al. 2008). To simultaneously study these three networks, the attention network test (ANT) has been developed (Fan et al. 2002). Through ANT, attentional functions have been studied during drug treatments (Conen et al. 2013; Ikeda et al. 2012; Thienel et al. 2009). Dysfunctions of attentional networks have also been reported in patients with attention deficit hyperactivity disorder (ADHD) (Johnson et al. 2008) and schizophrenia (Backes et al. 2011).
Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) is a wake-promoting agent that is structurally distinct from central nervous stimulants such as amphetamine and methylphenidate (Rugino 2007), and is prescribed worldwide for the treatment of excessive daytime sleepiness associated with narcolepsy (Minzenberg and Carter 2008). In addition, modafinil has also been prescribed for off-label uses in a variety of conditions including neuropsychiatric disorders (Peñaloza et al. 2013). Of note, modafinil has been reported to be effective in improving attention in patients with attentional disturbance, including ADHD (Turner 2006) and schizophrenia (Turner et al. 2004) for off-label uses. The mechanism of action of modafinil is unique and complex, and is distinct from that of traditional psychostimulants such as amphetamine (Mereu et al. 2013; Minzenberg and Carter 2008). Modafinil acts on dopaminergic and noradrenergic systems in the brain. Modafinil increases extracellular levels of dopamine in rodents (Murillo-Rodríguez et al. 2007) and humans (Volkow et al. 2009) possibly through binding to and inhibiting dopamine transporter (DAT) (Kim et al. 2014; Madras et al. 2006; Volkow et al. 2009). Modafinil also binds to norepinephrine transporter (NET) in monkey thalamus (Madras et al. 2006). Animal studies have suggested roles of D1 and D2 dopaminergic (Qu et al. 2008) and α and β adrenergic receptors (Lin et al. 1992) in the effects of modafinil. In addition to the dopaminergic and noradrenergic systems, modafinil affects other neurotransmitter systems including glutamate, γ-aminobutyric acid (GABA), serotonin, and histamine (Ferraro et al. 1999, 2000; Ishizuka et al. 2010). Therefore, modafinil is suggested to exert its effects via multiple neurotransmitter systems in the brain, although the exact action mechanism has not yet been clearly identified.
As expected from its wide range of action mechanisms, modafinil has been shown to enhance many cognitive functions (Minzenberg and Carter 2008). Interestingly, modafinil improves attention in healthy individuals (Caviola and Faber 2015) as well as in patients with cognitive dysfunctions. Modafinil has been reported to enhance sustained attention (Randall et al. 2005), attention switching (Marchant et al. 2009), and divided attention (Hart et al. 2006) in healthy individuals. On the other hand, other reports have shown no significant effects of modafinil on divided attention (Theunissen et al. 2009) and sustained attention (Randall et al. 2003). Thus, the reported effects of modafinil on attention remain inconsistent in spite of its clinical use for patients with attentional deficits.
To further characterize the effects of modafinil on attention, we therefore employed a functional magnetic resonance imaging (fMRI) study while healthy adults performed the ANT task. We attempted to reveal the networks of attention that are mainly affected by modafinil as well as the neural substrates that are responsible for the drug effects. Further, through the results from the present study, we also tried to provide a rationale for the choice of drug for the treatment of patients with attention deficits or dysfunction.
Materials and methods
Participants
Thirty-four healthy volunteers participated in the study, and the data of 23 subjects with normal weight were analyzed (Fig. 1). All subjects were right-handed according to the Edinburgh Handedness Inventory (Oldfield 1971). As for the excluded subjects, those with a medical history of psychiatric disorders, cardiac disorders, epilepsy, or allergy to modafinil; those with any diseases such as hypertension, hepatic dysfunction, or renal dysfunction; those who had taken any drugs for at least 2 weeks prior to the experiment; those with any contraindications to fMRI; and those with excessive consumption of alcohol or caffeine, smokers, and pregnant women were ruled out. In addition, the baseline mood and subjective states of the participants were assessed, and those with Beck Depression Inventory (BDI) score ≥ 11, Epworth Sleepiness Scale (ESS) score ≥ 10, State–Trait Anxiety Inventory (STAI)-state ≥42, and STAI-trait ≥45 were also excluded. BDI is widely used to quantify the severity of depressive moods (Beck et al. 1996), ESS is a standardized tool for assessing daytime sleepiness (Johns 1991), and STAI is valuable for screening of anxiety disorders, consisting of two separate rating scales, one for “state” anxiety and the other for “trait” anxiety (Spielberger 1983). BDI, ESS, and STAI consist of 21, 8, and 40 question items rated by 4-point scales, respectively. Participants were asked to abstain from caffeine and alcohol intake for 2 days and 24 h, respectively, before the experiment. They were instructed not to eat or drink anything except water for 2 h prior to and throughout the experiment. The characteristics of the participants are summarized in Table 1. The present study was approved by the ethics committee of Nippon Medical School (approval number 223019). All participants gave written informed consent prior to the experiment.
Study design and procedure
This study adopted a randomized placebo-controlled within-subjects cross-over design. To carefully monitor adverse drug effects in the participants, we conducted the study, including the fMRI data analyses, in a single-blind manner. Each participant was tested on two separate days at least 2 weeks apart to allow for complete clearance of drugs. In the first series, modafinil (200 mg as a Modiodal 100-mg tablet formula, Alfresa Pharma, Japan; Minzenberg et al. 2008) or a placebo tablet was administered orally with water. In the second series, participants were crossed over to receive the opposite medication. The modafinil and placebo tablets were identical in appearance and taste so that participants were not aware which tablet was being administered. The order of administration was randomly decided. Any demographic characteristics were not significantly different between participants who were allocated to placebo first or modafinil first (Table 1). We asked the participants to infer which drug arm they were in. Ten of the 23 participants correctly answered the arm to which they belonged, indicating that their inference was at chance level (p = 0.136: binomial distribution). The fMRI scan was started 2.5 h after administration of the placebo or modafinil, based on pharmacokinetic data that a 200-mg dose of modafinil reaches a peak plasma level at 2.5 h after oral administration (Robertson and Hellriegel 2003). Blood oxygenation level-dependent (BOLD) fMRI and behavioral data were acquired during performance of the ANT task. To assess subjective drug effects, subjective ratings were obtained before and at 2 h (just before fMRI scan) after the administration.
Drug effects on subjective ratings
Subjective assessments were collected using Profile of Mood States (POMS) (McNair et al. 1971), Hamilton Rating Scale for Depression (HAM-D) (Hamilton 1960) and Hamilton Rating Scale for Anxiety (HAM-A) (Hamilton 1959), and visual analogue scales (VAS) (Bond and Lader 1974). POMS was used to assess various mood states by a 5-point self-administered scale consisting of 65 items measuring 6 factors: Tension–Anxiety, Depression–Dejection, Anger–Hostility, Vigor, Fatigue, and Confusion. Assessments of depression and anxiety status included HAM-D and HAM-A, which consisted of 21 and 14 items, respectively. VAS was applied to assess subjective mood by using 16 lines with an opposing adjective at either end: (1) alert–drowsy, (2) calm–excited, (3) strong–feeble, (4) muzzy–clear-headed, (5) well-coordinated–clumsy, (6) lethargic–energetic, (7) contented–discontented, (8) troubled–tranquil, (9) mentally slow–quick-witted, (10) tense–relaxed, (11) attentive–dreamy, (12) incompetent–proficient, (13) happy–sad, (14) antagonistic–amicable, (15) interested–bored, and (16) withdrawn–gregarious. For each scale, participants were asked to mark each line between the antonyms to indicate how they felt at the present time. Each mood VAS was measured in millimeters from the end of positive mood. Scores ranged from 0 to 100, with lower scores indicating more positive mood. These sub-scales were grouped into three factors: Alertness (1, 3, 4, 5, 6, 9, 11, 12, 15), Contentedness (7, 8, 13, 14, 16), and Calmness (2, 10). Item scores were summed and averaged to create total scores for each respective factor.
Attention network test
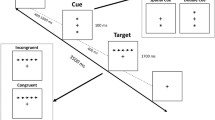
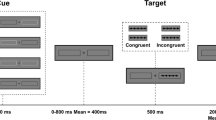
We used the attention network test (ANT) task designed as an event-related fMRI study (Fan et al. 2005; Thienel et al. 2009). The task paradigm is illustrated in Fig. 2. Each trial consisted of a cue period (150 ms), an interval (400 ms), a target period displaying target and flanker arrows (1550 ms), and an offset period (400–4900 ms). A fixation cross was shown in the center of the screen throughout the trial. The participants were instructed to maintain their gaze on the fixation cross. At the cue period, one of three cue types was presented: (1) no cue, only the central fixation cross; (2) the center cue, an asterisk displayed in the center with the fixation cross, alerting participants to the target onset; and (3) spatial cue, an asterisk displayed either above or below the fixation cross, predicting both the onset and spatial location of the target. At the target period, the target arrow was flanked on both sides by two distracter arrows, pointing in the same (congruent condition) or opposite (incongruent condition) direction to the target. This set of arrows appeared at the same location (above or below the fixation cross) as the spatial cue was presented at the cue period in each trial. Subjects were instructed to identify whether the central (target) arrow pointed to the left or the right and to respond as quickly and accurately as possible by pressing a button for the left direction with the left index finger or another button for the right direction with the right index finger. After a 24-trial practice session for about 2 min outside the MRI scanner room, participants performed three experimental sessions with 240 trials within a total of 20 min with breaks. Throughout the sessions, 24 possible stimulus combinations (3 cue conditions, 2 flanker conditions, 2 target presentation sides, and 2 target pointing directions) were presented equally in the trials (10 times for each combination) in a pseudorandom order. The task was created and presented on a personal computer using E-prime (version 2.0; Psychology Software Tools, USA), displayed on a monitor situated at the foot of the scanner bed, and viewed by participants via mirrors mounted to a head coil.
Schema of ANT task. Stimulus material and example for the course of one trial are presented. In each trial, a fixation cross was seen constantly in the center of the screen. A cue (no, center, or spatial cue) appeared randomly. After inter-stimulus interval, the target (the center arrow) and flankers of the left and right two arrows (congruent or incongruent flankers) were presented. During the target period, the participant was asked to indicate the direction of the arrow head of the target with a button press. After the target period, an inter-trial interval was given
Statistical analyses of demographic characteristics, subjective ratings, and behavioral data
The demographic characteristics were compared between the placebo first and the modafinil first groups. Unpaired t test and non-parametric Mann–Whitney’s U test were used for the data with normal and non-normal distributions, respectively. The gender data were analyzed by chi-square test. As behavioral measures, responses and reaction times (RTs) of the trials were recorded on E-prime, and the mean percent of correct responses (accuracy) and mean RT in correct responses for no-cue and center-cue conditions were calculated, respectively. According to previous studies (Fan et al. 2005), the alerting effect was calculated by subtracting the mean RT value in the center-cue condition from that in the no-cue condition. For the subjective ratings and behavioral data, paired t test and non-parametric Wilcoxon signed-rank test were used for data with normal and non-normal distributions, respectively, between the placebo and modafinil treatments. The threshold for significance was set at p < 0.05.
MRI data acquisition
Brain imaging data were obtained using an Intera Achieva 1.5 T Nova scanner (Philips Electronics, The Netherlands). Functional images were acquired with the following parameters: repetition time (TR) = 3000 ms, echo time (TE) = 60 ms, flip angle = 90°, field of view (FOV) = 200 mm, and matrix = 64 × 64 (Thienel et al. 2009). A total of 339 functional images were acquired from each participant with a T2*-weighted gradient-echo echo-planer imaging sequence sensitive to BOLD contrast. Whole brain coverage was obtained with 4-mm slice thickness and 35 axial slices. After the functional scans, a high-resolution T1-weighted structural image was also acquired for accurate spatial normalization with the following parameters: TR = 9.3 ms, TE = 4.6 ms, flip angle = 8°, FOV = 250 mm, matrix = 256 × 256, slice thickness = 1.2 mm, and number of slices = 160.
fMRI data analysis
Imaging data were preprocessed and analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, UK) running with MATLAB (Mathworks, USA). The structural T1 image and the functional images were manually reoriented to the anterior commissure–posterior commissure line. To correct for between-scan movements, the functional images were realigned to the first image of each session and again realigned to the mean image created after the first realignment. No participant had moved more than 2 mm in any direction during fMRI acquisition. The individual structural T1 image was then co-registered to the mean functional image. The transformed structural image was then segmented to create spatial normalization parameters that were applied to functional images in the next normalization step. The functional images were spatially normalized into the standard space defined by the Montreal Neurological Institute (MNI) template. After normalization, all scans were resampled at a resolution of 2 × 2 × 2 mm. The functional images were spatially smoothed with an isotropic Gaussian kernel (full width at half maximum of 8 mm) to increase the signal-to-noise ratio.
For subject-level statistical analyses, correct trials were modeled in the context of the general linear model using δ functions convolved with a canonical hemodynamic response function. Six regressors (related to the onset of the targets) were defined according to the permutation of all conditions comprising three cues (no, center, and spatial) and two flankers (congruent and incongruent). Incorrect and no-response trials were modeled separately as a regressor of non-interest. The six head movement parameters estimated during realignment were included as confounds. Low frequency noise was removed by applying a high-pass filter (cutoff period, 128 s) to the fMRI time-series data of each voxel. The statistical parametric map for each contrast, (a) alerting (central cue > no cue), (b) orienting (spatial cue > center cue), and (c) executive control (incongruent flanker > congruent flanker) of the t statistic, was calculated on a voxel × voxel basis.
For group-level analyses, the one-sample t test was performed to determine group-level activation for each contrast. Then, for group comparisons, paired t test was performed to assess the difference between the placebo and modafinil administrations. The contrast images obtained from subject-level statistical analyses were entered into paired t test analyses. All results on activation in the whole brain were reported at p < 0.001, uncorrected, with a minimum cluster size of 20 voxels. The cluster size threshold was determined using a cluster-extent correction procedure as implemented in SPM8. Only results surviving the cluster correction (p < 0.05) were reported.
According to the group comparison analysis, we focused on the changes in BOLD responses in the alerting contrast because significant difference in brain activity was shown between placebo and modafinil treatments in alerting contrast, but not in orienting and executive control contrasts. We set a priori ROIs with a sphere of 10 mm radius, centered at the maximum peak coordinates of the regions showing significant differences between the placebo and modafinil treatments using the Wake Forest University (WFU) PickAtlas. These ROIs were similar to a priori regions set in previous fMRI studies using the ANT task in healthy untreated controls (Backes et al. 2011; Muto et al. 2012; supplementary Table 1). The percent signal changes within the ROIs were calculated for the alerting contrast using MarsBar and were analyzed using the paired t test between the placebo and modafinil treatments and linear correlation (as below).
To investigate the correlation between BOLD activation (percent signal changes) and behavioral measures (RT and accuracy), we performed linear correlation analysis. Pearson’s correlation was used for RT analysis, and Spearman’s rank correlation method was used for accuracy analysis because these values did not follow normal distribution. The threshold for significance was set at p < 0.05.
Results
Effects of modafinil on subjective ratings
Changes in subjective ratings were calculated by subtracting the values before the drug administration from those at 2 h after the drug administration. There were no significant drug effects on the depression status as measured by HAM-D (p = 0.147) or the anxiety status as measured by HAM-A (p = 1) between the placebo and modafinil treatments. With regard to mood states as measured by POMS (Table 2), modafinil treatment demonstrated a significant increase in the score for vigor compared to the placebo treatment (p = 0.021). With regard to subjective mood as measured by VAS (Table 2), subjects with the modafinil treatment were significantly more alert (p = 0.016), energetic (p = 0.018), and quick-witted (p = 0.015) compared to those with the placebo treatment. Among the three mood factors, the modafinil treatment resulted in a significant increase in alertness factor compared to the placebo treatment (p = 0.005). The results were consistent with previous reports (Pringle et al. 2013; Rasetti et al. 2010). Thus, the results from subjective ratings confirmed that the modafinil treatment increased vigor and alertness.
Effects of modafinil on task performance
The effects of modafinil on reaction time, accuracy, and alerting effect were investigated during performance of the ANT task during MRI. Modafinil treatment showed significantly faster reaction times in no-cue condition (placebo 595.4 ± 18.6 ms; modafinil 531.5 ± 10.7 ms; p = 0.002) and in center-cue condition (placebo 565.2 ± 19.9 ms; modafinil 496.9 ± 10.9 ms; p = 0.001) (Fig. 3a). The modafinil treatment showed higher accuracy (placebo 94.1 ± 2.1%, median 98.1%; modafinil 98.3 ± 0.6%, median 98.8%; p < 0.001) than the placebo treatment (Fig. 3b). We also assessed the alerting effect by subtracting the mean RT values in the center-cue condition from those in the no-cue condition. The alerting effect was 30.2 ± 4.4 ms (median 34.0 ms) in the placebo treatment, consistent with a previous report (Abdullaev et al. 2010), and 34.6 ± 3.8 ms (median 34.8 ms) in the modafinil treatment. No treatment difference was found for the alerting effect (p = 0.230) (Fig. 3c). As for orientation, the orienting effect was 57.7 ± 5.5 ms (median 55.4 ms) and 36.2 ± 3.3 ms (median 32.3 ms) in the placebo and modafinil treatments (Supplementary Fig. 1), respectively, showing that modafinil decreased the orienting effect. In addition, there was no difference in executive control effect between the modafinil (14.3 ± 3.3 ms, median 17.8 ms) and the placebo treatments (17.6 ± 5.6 ms, median 17.2 ms).
Effects of modafinil on task performance. There were significant differences between the placebo and modafinil treatments for reaction time (a) and accuracy (b) in the no- and center-cue conditions, indicating that modafinil increased attention performance. c The score of the alerting effect did not show a significant difference between placebo and modafinil treatments. Error bars show standard error of mean. Each symbol indicates individual subject. Horizontal lines indicate median values. **p < 0.01; ***p < 0.001
Effects of modafinil on BOLD activation in the alerting network
For each treatment, whole-brain analysis was performed to determine brain regions showing BOLD activation in the alerting network. In the placebo treatment (Fig. 4a and Table 3), alerting network activity was found in the right superior parietal lobule, left inferior parietal lobule, bilateral middle occipital gyri, left fusiform gyrus, bilateral temporo-occipital cortices, and left cerebellum, consistent with previous fMRI studies using the ANT task in healthy untreated controls (Backes et al. 2011; Fan et al. 2005; Liu et al. 2013; Muto et al. 2012; Zheng et al. 2012; supplementary Table 1). The placebo treatment also showed activation in the left supplementary motor area, bilateral middle temporal gyri, right inferior temporal gyrus, right postcentral gyrus, right precuneus, left inferior occipital gyrus, and right caudate (p < 0.001, uncorrected). In the modafinil treatment (Fig. 4b and Table 3), a similar, but more pronounced activation pattern was shown as compared with that of the placebo treatment. Modafinil treatment showed alerting network activity in the left inferior temporal gyrus, bilateral middle occipital gyri, right inferior occipital gyrus, bilateral lateral temporo-occipital cortices, and bilateral cerebellum, activations that were also observed in previous fMRI studies using the ANT task in healthy untreated controls (supplementary Table 1). Modafinil treatment also activated the left middle temporal gyrus, right inferior temporal gyrus and left inferior occipital gyrus (p < 0.001, uncorrected).
Neural responses in alerting network. Group-level activations are shown in the alerting network in placebo (a) and modafinil (b) treatments. Axial slices are shown at z = −26 to z = 62 in the MNI template. The left side of these images corresponds to the left hemisphere. p < 0.001, uncorrected, with a minimum cluster size of 20 voxels. Color bar indicates t statistics. CBL cerebellum, FG fusiform gyrus, IOG inferior occipital gyrus, IPL inferior parietal lobule, ITG inferior temporal gyrus, LTOC lateral temporo-occipital cortex, MOG middle occipital gyrus, MTG middle temporal gyrus, PCUN precuneus, PoCG postcentral gyrus, SMA supplementary motor area, SPL superior parietal lobule
Next, whole-brain paired t test analysis was performed to compare alerting network activity between the placebo and modafinil treatments. The modafinil treatment showed significantly stronger activation in the left middle and inferior occipital gyri than the placebo treatment (p < 0.001, uncorrected; Fig. 5a and Table 4). No regions showed greater alerting network activity in the placebo than in the modafinil treatments. In the orienting and executive control contrasts in fMRI, there were no differences in regional brain activation between the placebo and modafinil treatments (threshold for significance, p < 0.001, uncorrected).
Effects of modafinil on brain activations in alerting network. The modafinil treatment showed significantly stronger activation in the alerting network than the placebo treatment. a Colored regions show the areas identified by group comparison analysis. Color bar indicates t statistics. b Percent BOLD signal changes for the alerting network calculated from a priori ROIs. Error bars indicate the standard error of mean. **p < 0.01
Effects of modafinil on BOLD signal changes in the left middle and inferior occipital gyri
Further, we focused on the left middle and inferior occipital gyri as ROIs for analyses of BOLD signal changes, as these regions showed significant differences in BOLD activation between the placebo and modafinil treatments in the alerting network in whole-brain analysis. Correspondingly, these regions have been reported to be involved in the alerting network in several previous studies (Backes et al. 2011; Muto et al. 2012; supplementary Table 1). The percent BOLD signal changes were larger in the modafinil than in the placebo treatments in the left middle occipital gyrus (p = 0.006) and left inferior occipital gyrus (p = 0.006) in the alerting network (Fig. 5b).
Correlations between RT and the percent BOLD signal changes in the left middle or inferior occipital gyri were calculated in the alerting network in both placebo and modafinil treatments. As shown in the results in Fig. 6, a greater number of data points were distributed in an area with higher signal changes and shorter reaction time in the modafinil treatment as compared to the placebo treatment. Further, we found negative correlations between RT and BOLD activations in the left middle occipital gyrus (r = −0.423, p = 0.003, Fig. 6a) and left inferior occipital gyrus (r = −0.387, p = 0.008, Fig. 6b). Thus, more intense activations in the left middle and inferior occipital gyri were associated with faster reaction time in the alerting network.
Discussion
In this study, we employed an fMRI study combined with the ANT task and investigated the components of attention that are affected by modafinil and the neural substrates that are responsible for the drug effects. Our main findings were that modafinil distinctly enhances alerting performance and mood in the attention networks and that BOLD responses in the left middle and inferior occipital gyri are significantly increased in the alerting network following modafinil treatment in association with enhanced alerting performance. On the other hand, there were no differences in regional brain activation between the placebo and modafinil treatments in the orienting and executive control contrasts in fMRI. Consistently, previous fMRI studies using the ANT task showed differential effects on individual components of the attentional network, although these discrepancies could not be fully explained. Passive hyperthermia impaired executive function but did not change alerting and orienting in behavior, while it altered three networks in brain activity (Liu et al. 2013). Carriers of an alpha 1C subunit of the L-type voltage-gated calcium channel (CACNA1C) with the risk allele for psychosis showed impaired behavioral performance in alerting and orienting, while neural activities in fMRI were reduced in the right inferior parietal lobule during orienting and in the medial frontal gyrus during executive control of attention (Thimm et al. 2011). Thus, brain activity in each network is independently affected by external and endogenous factors.
Neurotransmitter systems responsible for enhanced alerting by modafinil remain elusive. The mechanism of action of modafinil is unique and complex (Mereu et al. 2013; Minzenberg and Carter 2008). Modafinil acts not only through dopaminergic and noradrenergic mechanisms similar to traditional psychostimulants (Wisor 2013) but it also affects other neurotransmitter systems including glutamate, GABA, histamine, and serotonin (Ferraro et al. 1999, 2000; Ishizuka et al. 2010). Pharmacological studies have shown that noradrenaline affects the efficiency of alerting, while orienting and executive control are affected by acetylcholine and dopamine, respectively (Green et al. 2008). The α2-adrenoreptor agonist clonidine impaired behavioral measures and attenuated the activity of the alerting effect (Coull et al. 2001). Noradrenaline-containing fibers originate from the locus coeruleus and innervate a broad range of cortical and subcortical regions including the occipital cortex (Berridge and Waterhouse 2003). Extracellular noradrenaline in the rat occipital cortex was increased by electrical stimulation of the locus coeruleus and administration of the α2-adrenoreptor antagonist mirtazapine or the NET inhibitors reboxetine and desipramine (Devoto et al. 2004; Devoto and Flore 2006; Valentini et al. 2006). NET is distributed in the occipital cortex in human brain (Schou et al. 2007), and modafinil actually binds to NET (Madras et al. 2006). In contrast, the density of DAT is very low in the occipital cortices (Shih et al. 2006). Thus, the enhanced alerting by modafinil may be mediated at least partly by increased noradrenergic activity in the occipital cortex through the inhibition of noradrenaline reuptake.
Our results showed increases in BOLD responses in the left middle and inferior occipital gyri following modafinil treatment. Further, the BOLD signal changes in the occipital gyri were negatively correlated with reaction time during alerting. A previous fMRI study reported that alerting effects were observed in the occipital gyrus, suggesting that increased alertness results in a top-down modulation of activity in visual processing areas (Thiel et al. 2004). Top-down attention involves voluntary allocation of attention to locations or features mediated by dorsal frontoparietal areas (Vossel et al. 2014), which can modulate the activity of occipital areas (Corbetta and Shulman 2002; Vossel et al. 2014). In the present study, however, dorsal frontoparietal activity during alerting was not detected in either placebo or modafinil treatment. It is noteworthy that, according to a previous report, sleep deprivation reduced BOLD responses in occipital areas without changes of dorsal frontoparietal areas, suggesting that wakefulness could modulate occipital activity in the alerting network without top-down regulation (Muto et al. 2012). Further research would confirm whether modafinil induced the occipital activity through top-down modulation of the dorsal frontoparietal area by analyzing effective connectivity between these areas.
The enhancing effect of modafinil on alerting was significant among the three attention networks. Alerting is defined as achieving and maintaining a state of high sensitivity to incoming stimuli, consisting of intrinsic (tonic) and phasic alertness (Sturm and Willmes 2001). Intrinsic alertness refers to an internal control of arousal in the absence of an external cue, whereas phasic alertness represents an ability to increase response for a short time period following an external warning stimulus. In the present study, modafinil treatment showed significantly faster reaction times than placebo treatment in both no-cue and center-cue conditions, suggesting that modafinil enhances both intrinsic and phasic alertness.
Attentional impairment is observed in a variety of neuropsychiatric disorders such as ADHD and schizophrenia (Millan et al. 2012). Cognitive impairment in sustained attention has been shown to be an endophenotype in ADHD (Pironti et al. 2014). Imaging studies on patients with ADHD or schizophrenia showed alterations in the anatomy and function of the occipital lobe (Schneider et al. 2006; Tohid et al. 2015). These patients also demonstrated behavioral deficits in alerting when the ANT task was applied (Johnson et al. 2008; Backes et al. 2011). Although modafinil has been reported to be effective in improving attention in patients with ADHD (Turner 2006) and schizophrenia (Turner et al. 2004), the underlying mechanism has not been clarified. The present result may provide insight into the clinical availability of modafinil and the pathogenesis of attentional impairment in neuropsychiatric disorders. Methylphenidate has also been used for the treatment of ADHD, and it was reported that it improved poor attentional performance in both ADHD and control groups, irrespective of diagnosis (del Campo et al. 2013). In contrast to modafinil, methylphenidate improves the performance of executive function, which depends on dopaminergic transmission (Kratz et al. 2012). Taken together, modafinil and methylphenidate may preferentially act on the noradrenergic and dopaminergic systems, respectively, although DAT occupancy and extracellular dopamine level under modafinil and methylphenidate treatments at clinical doses have appeared similar in positron emission tomography (PET) studies (Kim et al. 2014; Volkow et al. 2009). It is known that patients with ADHD often show impairment of alerting and executive functions (Oberlin et al. 2005), but some suffer more severely from either function. Given the characteristics of the drugs, modafinil and methylphenidate could be differentially used according to the major clinical conditions of the patients.
The present study has limitations in terms of interpreting the results. Because the pharmacological actions of modafinil are complicated, the possibility of the involvement of other neurotransmitters cannot be ruled out. Some studies using the ANT task in the alerting network reported that an antihistamine caused less alerting as indicated by longer reaction time and decreased BOLD response, and that a single nucleotide polymorphism in GRIN2B encoding the NR2B subunit of NMDA receptors showed faster reaction time (Conen et al. 2013; Schulz et al. 2012). Further studies are needed to identify which neurotransmitter systems are substantially responsible for the effects of modafinil on the alerting network in combination with other measures such as use of receptor antagonists and PET imaging studies for responsible neurotransmitter receptors. In addition, methodological limitations, including the single-blind study and the volunteer number, and the single-dose administration study, may affect the behavioral and fMRI analyses. Especially, single-dose treatment may not be effective enough to identify the modafinil effects on orienting and executive control in fMRI data analysis. In fact, in previous human studies on task performance, chronic administration with modafinil showed different effects on attentional measures from those with single-dose administration (Minzenberg and Carter 2008; Repantis et al. 2010).
In conclusion, healthy subjects showed enhanced performance and increased activation in the occipital lobe by a single dose of modafinil, especially in the alerting network possibly relevant to noradrenergic activity during the ANT task. The fMRI study combined with the ANT task could provide a rationale for characterizing the cognitive effects of central-acting drugs and select appropriate drugs for individual patients with distinct symptoms of impaired attention.
References
Abdullaev Y, Posner MI, Nunnally R, Dishion TJ (2010) Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behav Brain Res 215:45–57. doi:10.1016/j.bbr.2010.06.023
Backes V, Kellermann T, Voss B, Krӓmer J, Depner C, Schneider F, Habel U (2011) Neural correlates of the attention network test in schizophrenia. Eur Arch Psychiatry Clin Neurosci 261(Supple 2):S155–S160. doi:10.1007/s00406-011-0264-9
Beck AT, Steer RA, Brown GK (1996) Manual for the Beck Depression Inventory, 2nd edn. Pearson, Texas
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84. doi:10.1016/S0165-0173(03)00143-7
Bond A, Lader M (1974) The use of analogue scales in rating subjective feelings. Br J Med Psychol 47:211–218. doi:10.1111/j.2044-8341.1974.tb02285.x
Caviola L, Faber NS (2015) Pills or push-ups? Effectiveness and public perception of pharmacological and non-pharmacological cognitive enhancement. Front Psychol 6:1852. doi:10.3389/fpsyg.2015.01852
Conen S, Theunissen EL, Vermeeren A, van Ruitenbeek P, Stiers P, Mehta MA, Toennes SW, Ramaekers JG (2013) The role of P-glycoprotein in CNS antihistamine effects. Psychopharmacology 229:9–19. doi:10.1007/s00213-013-3075-z
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215. doi:10.1038/nrn755
Coull JT, Nobre AC, Frith CD (2001) The noradrenergic α2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex 11:73–84. doi:10.1093/cercor/11.1.73
del Campo N, Fryer TD, Hong YT, Smith R, Brichard L, Acosta-Cabronero J, Chamberlain SR, Tait R, Izquierdo D, Regenthal R, Dowson J, Suckling J, Baron JC, Aigbirhio FI, Robbins TW, Sahakian BJ, Müller U (2013) A positron emission tomography study of nigro-striatal dopaminergic mechanisms underlying attention: implications for ADHD and its treatment. Brain 136:3252–3270. doi:10.1093/brain/awt263
Devoto P, Flore G (2006) On the origin of cortical dopamine: is it a co-transmitter in noradrenergic neurons? Curr Neuropharmacol 4:115–125. doi:10.2174/157015906776359559
Devoto P, Flore G, Pira L, Longu G, Gessa GL (2004) Mirtazapine-induced corelease of dopamine and noradrenaline from noradrenergic neurons in the medial prefrontal and occipital cortex. Eur J Pharmacol 487:105–111. doi:10.1016/j.ejphar.2004.01.018
Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002) Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14:340–347. doi:10.1162/089892902317361886
Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005) The activation of attentional networks. NeuroImage 26:471–479. doi:10.1016/j.neuroimage.2005.02.004
Ferraro L, Antonelli T, Tanganelli S, O’Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K (1999) The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology 20:346–356. doi:10.1016/S0893-133X(98)00085-2
Ferraro L, Fuxe K, Tanganelli S, Fernandez M, Rambert FA, Antonelli T (2000) Amplification of cortical serotonin release: a further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology 39:1974–1983. doi:10.1016/S0028-3908(00)00019-8
Green AE, Munafò MR, DeYoung CG, Fossella JA, Fan J, Gray JR (2008) Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat Rev Neurosci 9:710–720. doi:10.1038/nrn2461
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. doi:10.1111/j.2044-8341.1959.tb00467.x
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. doi:10.1136/jnnp.23.1.56
Hart CL, Haney M, Vosburg SK, Comer SD, Gunderson E, Foltin RW (2006) Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology 31:1526–1536. doi:10.1038/sj.npp.1300991
Ikeda Y, Koeda M, Kim W, Tateno A, Okubo Y, Suzuki H (2012) Caffeine’s effects on attentional networks in healthy subjects: a pharmacological functional magnetic resonance imaging study. J Nippon Med Sch 79:318–319. doi:10.1272/jnms.79.318
Ishizuka T, Murotani T, Yamatodani A (2010) Modafinil activates the histaminergic system through the orexinergic neurons. Neurosci Lett 483:193–196. doi:10.1016/j.neulet.2010.08.005
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Johnson KA, Robertson IH, Barry E, Mulligan A, Dáibhis A, Daly M, Watchorn A, Gill M, Bellgrove MA (2008) Impaired conflict resolution and alerting in children with ADHD: evidence from the attention network test (ANT). J Child Psychol Psychiatry 49:1339–1347. doi:10.1111/j.1469-7610.2008.01936.x
Kim W, Tateno A, Arakawa R, Sakayori T, Ikeda Y, Suzuki H, Okubo Y (2014) In vivo activity of modafinil on dopamine transporter measured with positron emission tomography and [18F]FE-PE2I. Int J Neuropsychopharmacol 17:697–703. doi:10.1017/S1461145713001612
Kratz O, Studer P, Baack J, Malcherek S, Erbe K, Moll GH, Heinrich H (2012) Differential effects of methylphenidate and atomoxetine on attentional processes in children with ADHD: an event-related potential study using the attention network test. Prog Neuro-Psychopharmacol Biol Psychiatry 37:81–89. doi:10.1016/j.pnpbp.2011.12.008
Lin JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M (1992) Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res 591:319–326. doi:10.1016/0006-8993(92)91713-O
Liu K, Sun G, Li B, Jiang Q, Yang X, Li M, Li L, Qian S, Zhao L, Zhou Z, von Deneen KM, Liu Y (2013) The impact of passive hyperthermia on human attention networks: an fMRI study. Behav Brain Res 243:220–230. doi:10.1016/j.bbr.2013.01.013
Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miler GM, Fischman AJ (2006) Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther 319:561–569. doi:10.1124/jpet.106.106583
Marchant NL, Kamel F, Echlin K, Grice J, Lewis M, Rusted JM (2009) Modafinil improves rapid shifts of attention. Psychopharmacology 202:487–495. doi:10.1007/s00213-008-1395-1
McNair DM, Lorr M, Droppleman LF (1971) Manual for the Profile of Mood States. Educational and Industrial Testing Service, California
Mereu M, Bonci A, Newman AH, Tanda G (2013) The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology 229:415–434. doi:10.1007/s00213-013-3232-4
Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B, Geyer MA, Goodwin GM, Gorwood P, Jay TM, Joëls M, Mansuy IM, Meyer-Lindenberg A, Murphy D, Rolls E, Saletu B, Spedding M, Sweeney J, Whittington M, Young LJ (2012) Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11:141–168. doi:10.1038/nrd3628
Minzenberg MJ, Carter CS (2008) Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 33:1477–1502. doi:10.1038/sj.npp.1301534
Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS (2008) Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science 322:1700–1702. doi:10.1126/science.1164908
Murillo-Rodríguez E, Haro R, Palomero-Rivero M, Millán-Aldaco D, Drucker-Colín R (2007) Modafinil enhances extracellular levels of dopamine in the nucleus accumbens and increased wakefulness in rats. Behav Brain Res 176:353–357. doi:10.1016/j.bbr.2006.10.016
Muto V, Shaffii-Le Bourdiec A, Matarazzo L, Foret A, Mascetti L, Jaspar M, Vandewalle G, Phillips C, Degueldre C, Balteau E, Luxen A, Collette F, Maquet P (2012) Influence of acute sleep loss on the neural correlates of alerting, orientating and executive attention components. J Sleep Res 21:648–658. doi:10.1111/j.1365-2869.2012.01020.x
Oberlin BG, Alford JL, Marrocco RT (2005) Normal attention orienting but abnormal stimulus alerting and conflict effect in combined subtype of ADHD. Behav Brain Res 165:1–11. doi:10.1016/j.bbr.2005.06.041
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. doi:10.1016/0028-3932(71)90067-4
Peñaloza RA, Sarkar U, Claman DM, Omachi TA (2013) Trends in on-label and off-label modafinil use in a nationally representative sample. JAMA Intern Med 173:704–706. doi:10.1001/jamainternmed.2013.2807
Pironti VA, Lai MC, Müller U, Dodds CM, Suckling J, Bullmore ET, Sahakian BJ (2014) Neuroanatomical abnormalities and cognitive impairments are shared by adults with attention-deficit/hyperactivity disorder and their unaffected first-degree relatives. Biol Psychiatry 76:639–647. doi:10.1016/j.biopsych.2013.09.025
Posner MI, Petersen SE (1990) The attention system of the human brain. Annu Rev Neurosci 13:25–42. doi:10.1146/annurev.neuro.13.1.25
Posner MI, Rothbart MK (2007) Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol 58:1–23. doi:10.1146/annurev.psych.58.110405.085516
Pringle A, Browning M, Parsons E, Cowen PJ, Harmer CJ (2013) Early markers of cognitive enhancement: developing an implicit measure of cognitive performance. Psychopharmacology 230:631–638. doi:10.1007/s00213-013-3186-6
Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y (2008) Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci 28:8462–8469. doi:10.1523/JNEUROSCI.1819-08.2008
Randall DC, Shneerson JM, Plaha KK, File SE (2003) Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol 18:163–173. doi:10.1002/hup.456
Randall DC, Shneerson JM, File SE (2005) Cognitive effects of modafinil in student volunteers may depend on IQ. Pharmacol Biochem Behav 82:133–139. doi:10.1016/j.pbb.2005.07.019
Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, Arrillaga-Romany IC, Goldberg TE, Callicott JH, Apud JA, Weinberger DR (2010) Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology 35:2101–2109. doi:10.1038/npp.2010.83
Repantis D, Schlattmann P, Laisney O, Heuser I (2010) Modafinil and methylphenidate for neuroenhancement in healthy individuals: a systematic review. Pharmacol Res 62:187–206. doi:10.1016/j.phrs.2010.04.002
Robertson P Jr, Hellriegel ET (2003) Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet 42:123–137. doi:10.2165/00003088-200342020-00002
Rugino T (2007) A review of modafinil film-coated tablets for attention-deficit/hyperactivity disorder in children and adolescents. Neuropsychiatr Dis Treat 3:293–301
Sarter M, Givens B, Bruno JP (2001) The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev 35:146–160. doi:10.1016/S0165-0173(01)00044-3
Schneider M, Retz W, Coogan A, Thome J, Rösler M (2006) Anatomical and functional brain imaging in adult attention-deficit/hyperactivity disorder (ADHD)—a neurological view. Eur Arch Psychiatry Clin Neurosci 256:i32–i41. doi:10.1007/s00406-006-1005-3
Schou M, Pike VW, Sóvágó J, Gulyás B, Gallagher PT, Dobson DR, Walter MW, Rudyk H, Farde L, Halldin C (2007) Synthesis of 11C-labelled (R)-OHDMI and CFMME and their evaluation as candidate radioligands for imaging central norepinephrine transporters with PET. Bioorg Med Chem 15:616–625. doi:10.1016/j.bmc.2006.10.065
Schulz S, Arning L, Pinnow M, Epplen JT, Beste C (2012) N-methyl-d-aspartate receptor 2B subunit (GRIN2B) gene variation is associated with alerting, but not with orienting and conflicting in the attention network test. Neuropharmacology 63:259–265. doi:10.1016/j.neuropharm.2012.02.024
Shih MC, Hoexter MQ, de Andrade LAF, Bressan RA (2006) Parkinson’s disease and dopamine transporter neuroimaging—a critical review. Sao Paulo Med J 124:168–175. doi:10.1590/S1516-31802006000300014
Spielberger CD (1983) Manual for the State-Trait Anxiety Inventory, STAI-form Y. Consulting Psychologists Press, California
Sturm W, Willmes K (2001) On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage 14:S76–S84. doi:10.1006/nimg.2001.0839
Theunissen EL, Elvira Jde L, van den Bergh D, Ramaekers JG (2009) Comparing the stimulant effects of the H1-antagonist fexofenadine with 2 psychostimulants, modafinil and methylphenidate. J Clin Psychopharmacol 29:439–443. doi:10.1097/JCP.0b013e3181b3b5f3
Thiel CM, Zilles K, Fink GR (2004) Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. NeuroImage 21:318–328. doi:10.1016/j.neuroimage.2003.08.044
Thienel R, Voss B, Kellermann T, Reske M, Halfter S, Sheldrick AJ, Radenbach K, Habel U, Shah NJ, Schall U, Kircher T (2009) Nicotinic antagonist effects on functional attention networks. Int J Neuropsychopharmacol 12:1295–1305. doi:10.1017/S1461145709990551
Thimm M, Kircher T, Kellermann T, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stöcker T, Shah NJ, Nöthen MM, Rietschel M, Witt SH, Mathiak K, Krug A (2011) Effects of a CACNA1C genotype on attention networks in healthy individuals. Psychol Med 41:1551–1561. doi:10.1017/S0033291710002217
Tohid H, Faizan M, Faizan U (2015) Alterations of the occipital lobe in schizophrenia. Neurosciences (Riyadh) 20:213–224. doi:10.17712/nsj.2015.3.20140757
Turner D (2006) A review of the use of modafinil for attention-deficit hyperactivity disorder. Expert Rev Neurother 6:455–468. doi:10.1586/14737175.6.4.455
Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ (2004) Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology 29:1363–1373. doi:10.1038/sj.npp.1300457
Valentini V, Cacciapaglia F, Frau R, Di Chiara G (2006) Differential α2-mediated inhibition of dopamine and noradrenaline release in the parietal and occipital cortex following noradrenaline transporter blockade. J Neurochem 98:113–121. doi:10.1111/j.1471-4159.2006.03851.x
Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K (2009) Effects of modafinil on dopamine an dopamine transporters in the male human brain: clinical implications. JAMA 301:1148–1154. doi:10.1001/jama.2009.351
Vossel S, Geng JJ, Fink GR (2014) Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20:150–159. doi:10.1177/1073858413494269
Wisor J (2013) Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions. Front Neurol 4:139. doi:10.3389/fneur.2013.00139
Zheng J, Qin B, Dang C, Ye W, Chen Z, Yu L (2012) Alertness network in patients with temporal love epilepsy: a fMRI study. Epilepsy Res 100:67–73. doi:10.1016/j.eplepsyres.2012.01.006
Acknowledgements
The authors are entirely responsible for the scientific content of this paper. We are thankful to the Clinical Imaging Center for Healthcare, Nippon Medical School, for their support. In particular, we thank Koji Nagaya, Megumi Hongo, Koji Kanaya, Masaya Suda, and Minoru Sakurai for their technical assistance with the MRI examinations and Michiyo Tamura for research assistance. We also thank Chieko Kishi and Yumiko Fukano for help as clinical research coordinators and Arndt Gerz for his English editing of the manuscript. This work was partially supported by a Grant-in-Aid for Scientific Research (B) (24791237 to Y.I.) from the Japan Society for the Promotion of Science, Japan, and a Ministry of Education, Culture, Sports, Science and Technology-Supported Program for the Strategic Research Foundation at Private Universities, 2008–2012, Japan (S0801035 to H.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests.
Electronic supplementary material
ESM 1
(PDF 103 kb)
Rights and permissions
About this article
Cite this article
Ikeda, Y., Funayama, T., Tateno, A. et al. Modafinil enhances alerting-related brain activity in attention networks. Psychopharmacology 234, 2077–2089 (2017). https://doi.org/10.1007/s00213-017-4614-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4614-9