Abstract
Rationale
P-glycoprotein (P-gp) is a drug efflux pump expressed, amongst others, on the luminal surface of the cerebral endothelial cells forming the blood–brain barrier. Studies in rodents have demonstrated that antihistamines that are substrates of the P-gp transporter display no or minor central nervous system (CNS) effects as compared to antihistamines that are not P-gp transporter substrates.
Objectives
The present study explored whether P-gp contributes in similar ways to the occurrence of sedative effects of antihistamines in humans.
Methods
An fMRI study was conducted according to a double-blind, randomized, placebo-controlled, cross-over design in 13 healthy volunteers. Participants received cetirizine 15 mg (an antihistamine), verapamil 120 mg (a P-gp blocker), a combination of cetirizine + verapamil, and a placebo. Brain activity was assessed while conducting the attention network test (ANT) in a 3T magnetic resonance scanner. The ANT measures three independent attention domains: i.e., alerting, orienting, and executive attention. It was expected that the combined treatment of cetirizine with verapamil would prevent efflux of cetirizine from the CNS, thus increasing attentional impairment, as compared to cetirizine administered alone.
Results
The present study provides evidence that the P-gp transporter is involved in central antihistamine effects in humans. Participants were less alert during the combined treatment of cetirizine and verapamil as indicated by longer reaction times and decreased blood oxygen level-dependent response in the right superior temporal gyrus.
Conclusion
It is concluded that the affinity for the P-gp transporter may contribute to the lower incidence of CNS side effects of certain antihistamines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sedation is a frequently occurring side effect of antihistamines that can produce attentional impairments in day to day operations such as driving a car and working (Abbas et al. 2004; Jauregui et al. 2006; Montoro et al. 2006; Ramaekers and O’Hanlon 1994; Tashiro et al. 2008; Theunissen et al. 2006b; Van Ruitenbeek et al. 2008; Verster and Volkerts 2004). The magnitude of sedation varies between individual antihistamines but is more prominent in first-generation antihistamines as compared to second-generation antihistamines (Kay 2000; Tashiro et al. 2005; Theunissen et al. 2006b; Vuurman et al. 2004). It is thought that the lipophilicity of first-generation antihistamines enables them to cross the blood–brain barrier and bind to central histamine-1 receptors (H1Rs) whereby they reduce the wake-promoting effects of centrally acting histamine. Second-generation antihistamines are less lipophilic and therefore produce less sedation (Kay 2000).
Besides lipophilicity, the presence and/or magnitude of sedation may also be determined by the P-glycoprotein (P-gp) transporter (Bartra et al. 2006; Chen et al. 2003; Mahar Doan et al. 2004; Mimura et al. 2008; Obradovic et al. 2007). P-gp is an efflux transporter that is expressed on the luminal surface of the cerebral endothelial cells forming the blood–brain barrier. P-gp and other transporters actively pump substrates out of the brain, thereby limiting their effects in the central nervous system (CNS) (Bartra et al. 2006; Chen et al. 2003; Schinkel 1999; Sun et al. 2003; Zhou et al. 2009).
Studies in experimental animal models have demonstrated that the P-gp transporter may reduce CNS effects of second-generation antihistamines. When comparing wild-type mice with P-gp knockout mice, the latter showed significantly higher brain distribution of nonsedative second-generation antihistamines such as cetirizine, loratadine, and desloratadine. On the other hand, brain penetration of sedative first-generation antihistamines hydroxyzine, diphenhydramine, and triprolidine did not differ between wild-type and P-gp knockout mice (Chen et al. 2003; Polli et al. 2003). This apparent difference between second- and first-generation antihistamines is in keeping with the knowledge that only the former are P-gp substrates (Obradovic et al. 2007).
P-gp is also expressed in the small intestine, liver, and kidney, and studies in humans have revealed that P-gp affects the pharmacokinetics of antihistamines. For example, P-gp inhibition has been shown to increase blood plasma concentrations of the second-generation antihistamine fexofenadine (Kim et al. 2009; Sakugawa et al. 2009; Yasui-Furukori et al. 2005). However, to our knowledge, no human studies have been conducted to study the effects of the P-gp transporter on pharmacodynamic effect caused by antihistamines, such as CNS sedation. Data from animal research may serve as a translational model for potential effects in humans, but these results need to be confirmed and validated in human subjects.
This project focused on the role of the P-gp transporter in the occurrence of CNS sedation after administration of cetirizine, a second-generation antihistamine, in healthy volunteers. Blocking the P-gp transporter was expected to increase the distribution of cetirizine and to produce more CNS sedation due to enhanced H1R binding in the brain. It was hypothesized that sedation after cetirizine would be apparent in combination with a P-gp blocker, but not when administered alone.
Impairing effects of antihistamines have often been demonstrated in attention tasks (Conen et al. 2011b; Theunissen et al. 2004; Verster et al. 2003; Vuurman et al. 2004). In the present proposal, we selected the attention network test (ANT) to assess the sedative effects of cetirizine with and without P-gp blockade, in three independent attention domains: i.e., alerting, orienting, and executive attention. The ANT was conducted during functional MRI in order to assess drug-induced changes in the neural networks underlying performance in the three attention domains. Previous research has shown that fMRI can be effectively used to examine drug-induced changes in CNS functions (Jenkins et al. 2003; Leslie and James 2000).
Materials and methods
Subjects
Sixteen volunteers (eight female) were recruited through poster advertisement. However, four participants could not complete all sessions. Hence, data will be reported on 13 participants for the behavioral data (7 male, 6 female; age range 19 to 33 years; mean ± SD: 22.31 ± 3.6 years) and 12 participants for the imaging data (7 male, 5 female; age range 19 to 33 years; mean ± SD: 22.42 ± 3.8 years). Participants were screened using a medical questionnaire and a physical examination. The latter included a 12-lead ECG evaluation, blood chemistry, and hematology, urinalysis, and drug and pregnancy screening.
Volunteers with a history of medical or psychiatric illness, any major medical, endocrine and/or neurological condition, hypertension (diastolic >10, systolic >170), hypersensitivity to H1 antihistamines, and/or seasonal allergic rhinitis or urticaria treated by antihistamines were excluded from the study. Additional exclusion criteria were alcohol consumption of >21 units per week, smoking, caffeine consumption of >6 cups per day, history of drug abuse or addiction, treatment with atropine or atropine-like drugs, and treatment with CNS depressants. Female volunteers could not participate when pregnant or breast-feeding.
The study was approved by the standing medical ethics committee of Maastricht University and was carried out in compliance with the code of ethics on human experimentation established by the Declaration of Helsinki (1964), amended in Seoul (2008).
Design and treatments
The study was conducted according to a randomized, double-blind, four-way cross-over design with treatments consisting of cetirizine 15 mg, verapamil 120 mg, cetirizine 15 mg and verapamil 120 mg, and placebo. Cetirizine is a H1R antagonist for the treatment of allergic rhinitis and urticaria (Polli et al. 2003; Theunissen et al. 2006b). Peak plasma concentrations are reached between 1 and 1.5 h after oral ingestion. Its half-life varies between 7 and 11 h (del Cuvillo et al. 2006).
Verapamil is used in order to treat tachycardia, arterial fibrillation, and hypertensions. It is also known as a P-gp inhibitor (Dadashzadeh et al. 2006). Peak plasma concentrations are reached between 0.5 and 1.5 h after oral ingestion. Its half-life varies from 7 and 15 h (Choi et al. 2010; Dadashzadeh et al. 2006). Because this was, to our knowledge, the first study to investigate the interaction between verapamil and cetirizine in human volunteers, it was decided to use a standard dosage of verapamil 120 mg and not any higher dosage.
Participants visited the facility on two separate test days. On each test day, participants received two treatments and were scanned twice. The participants received either (a) placebo before the first scan session and verapamil 120 mg before the second scan session, or (b) cetirizine 15 mg before the first scan session and verapamil 120 mg before the second scan session. Because of the relative long half-life of cetirizine (7–11 h), during the second scan session on day (b), the combination of cetirizine with verapamil 120 mg was measured instead of only verapamil 120 mg. Test days were separated by a washout period of at least 7 days. All treatments were administered as identical encapsulated tablets to ensure blinding, and test day order was randomized and counterbalanced across participants.
Procedure
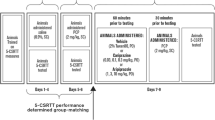
Participants received a training of the tests and a habituation session before the first test day. Drug screens and pregnancy tests were conducted upon arrival on the study site. Participants received a light standardized breakfast 2 h prior to medication intake. Participants were asked not to consume any caffeine-containing beverages during the test days and not to drink any grapefruit juice during their entire participation in the study. Approximately one and a half hour after medication intake, blood samples were taken and questionnaires filled out after which participants performed the ANT while being scanned. The second medication intake was at the end of the first scan session, after filling out another questionnaire. The further procedure was similar to the first half of the test day. A flowchart of the test days is given in Fig. 1.
Flowchart of the test days. Pla, placebo; Cet, cetirizine; Ver, verapamil. The asterisk indicates that because of the relative long half-life (7–11 h) of cetirizine, during the second scan session, the combination of cetirizine 15 mg with verapamil 120 mg was measured on the day that cetirizine was administered before test session 1
Assessments
Pharmacokinetic assessment
Blood samples (10 ml) for drug monitoring were collected before each scan session, i.e. 65 min post drug administration. The blood samples were centrifuged to separate plasma and frozen at −20 °C. Plasma samples were analyzed after liquid–liquid extraction using a triple quadrupole LC/MS system in MRM mode. The lower limits of quantification were 0.002 mg/l for verapamil and 0.003 mg/l for cetirizine.
Subjective evaluations
The Groningen Subjective Quality of Sleep Questionnaire (GSQS) was filled out at the beginning of a test day in order to assess sleep quality and sleep duration of the previous night (Mulder-Hajonides 1981). This questionnaire ranges from 0 (good sleep) to 14 (bad sleep).
Before the scan sessions, subjective evaluations of alertness, contentedness, and calmness were rated with the Bond and Lader questionnaire using visual analog scales (Bond and Lader 1974). Subjective feelings of daytime sleepiness were rated with the Karolinska Sleepiness Scale (KSS) which provides scores ranging from 1 (“extremely alert”) to 9 (“very sleepy”, “fighting sleep”) (Akerstedt and Gillberg 1990).
The Rating Scale Mental Effort (Zijlstra 1993) was used in order to rate the effort it took participants to perform the scan session. The scale is a visual analog scale (150 mm) with labels ranging from “extremely demanding” to “not demanding at all”.
Attention network test
The attention network test measures three independent attention networks: the alerting network, the orienting network, and the executive control network (Fan et al. 2002, 2005). During the ANT, participants are asked to determine whether a central arrow (the target display) points to the left or to the right. The arrow appears above or below a fixation point and is accompanied by four arrows that either point in the same direction (congruent flankers) or in the opposite direction (incongruent flankers). Each trial begins with the presentation of a fixation cross, and before the display of the arrows, a warning cue may appear in the center (center-cue condition), above or below the fixation cross (spatial-cue condition), or not at all (no-cue condition) for 200 ms. After a variable duration (300–11,800 ms), the arrows appear and remain visible on the screen until a response is made (pressing a key indicating the direction of the target arrow) (Fan et al. 2007). The time between the onset of the target and the start of the next trial varies between 3,000–15,000 ms. There are 6 runs with 38 trials in which combinations of the flankers and cues are randomly presented. Each run lasted approximately 6 min. Participants performed three runs consecutively after which they had a break for approximately 9 min. After the break, the second half (i.e., three runs) of the test was conducted.
In the present study, we used the same event-related design and the same timing parameters as in a previous research conducted by Fan et al. (2005) to study the change in activations of the attention networks after medication intake. In order to separate brain activity to the cues and targets, time intervals between cue and target and between target and the next trial were jittered, similarly to the study of Fan et al. (2005).
Also in line with the study conducted by Fan et al. (2005), efficiency of the alerting network was defined as the difference in brain activity and reaction time (RT) between center-cue (the warning cue) and no-cue conditions. Efficiency of the orienting network was defined as the difference in brain activity and RT between center- and spatial-cue conditions. Efficiency of the executive network was defined as the difference in brain activity and RT between incongruent and congruent flankers (Fan et al. 2007).
Image acquisition
MRI data were acquired using a Siemens MAGNETOM Allegra 3T head scanner with a single channel quadrature transceiver head coil. All participants completed four experimental runs. A total of 32 axial slices covering the whole brain were obtained using a T2*-weighted gradient echo planner pulse sequence (repetition time (TR) = 2,000 ms; echo time (TE) = 30 ms; field-of-view (FoV) = 224; slice thickness = 3.5 mm; matrix size = 64 × 64; flip angle = 90°). Voxel size was 3.5 mm3. Slice scanning order was interleaved. A high-resolution T1-weighted sequence was used to collect structural scans (TR = 2250 ms, TE = 2.6 ms, FoV = 256, slice thickness = 1 mm, matrix size = 256 × 256, flip angle = 90°, number of slices = 192).
Image preprocessing
Image preprocessing was conducted using SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). The first two volumes were discarded because of the T1 saturation effect and images were corrected for slice scan time differences. The functional data from all treatment sessions were aligned to the anatomical data from the first session, using unwrapped fieldmaps. When translational movement of more than 3.5 mm from the first volume and/or rotational movement of more than 2.3 degrees were apparent in more than three runs, those runs were removed from the dataset together with all other data from that participant. When movement of more than 3.5 mm from the first volume was apparent in fewer than three runs, movement parameters across treatment conditions were compared in order to evaluate whether there were significant differences between treatment conditions. In case of significant differences, the data from that participant were also removed. Each participant’s anatomical image was linearly co-registered to a standard template from the Montreal Neurological Institute (MNI), and its functional data co-registered with this co-registered anatomical data. Next, the anatomical data were segmented into CSF, white matter, and gray matter, and in the same procedure, the nonlinear normalization parameters were estimated. These parameters were used to nonlinearly normalize the anatomical and functional data to the MNI template. Finally, functional data were spatially smoothed using a Gaussian filter (8 mm FWHM).
Statistical analyses
All subjective parameters and behavioral data were analyzed using paired sample t-test. More precisely, comparison between the first scan sessions was conducted to reveal differences between placebo and cetirizine 15 mg. Comparison between the second scan sessions was conducted to reveal differences between verapamil 120 mg and verapamil + cetirizine.
Secondly, to determine whether verapamil modulates cetirizine effects, change scores were calculated for the first scan sessions (cetirizine 15 mg minus placebo), and the second scan sessions (verapamil 120 mg and cetirizine combined minus verapamil 120 mg) separately, and then compared by testing for significance using paired sample t-tests.
Imaging analysis
Statistical analysis using general linear modeling was conducted in order to analyze the imaging data. At the first level, data in the four treatment conditions were analyzed separately. For each participant and treatment conditions, regressors were created that represented the separate trial events, i.e., fixation with no cue, center cue, spatial cue, and targets with congruent flankers and targets with incongruent flankers. Six realignment parameters were used as covariates. Contrast images consisting of t-maps (center cue minus no cue for alerting, spatial cue minus center cue for orienting, and incongruent minus congruent for conflict) from each subject were calculated.
At a second level of analysis, the contrast images were used in order to analyze the effects of treatment (cetirizine vs. placebo and verapamil vs. cetirizine + verapamil) on alerting, orienting, and conflict with voxel-wise paired samples t-tests. The regions activated for alerting, orienting, and executive attention that were observed by Fan et al. (2005) were used as anatomical regions of interest (ROI) and defined a priori in the WFU Pickatlas (Wake Forest University, Winston-Salem, NC). For the alerting network, these regions were the superior temporal gyrus, superior colliculus, thalamus, inferior parietal lobe, fusiform gyrus, inferior frontal gyrus, and the superior parietal lobe, while for the orienting network, the fusiform gyrus, precentral gyrus, superior parietal lobe, superior frontal gyrus, postcentral and precentral gyrus. For the executive attention network, the regions included the thalamus, superior frontal gyrus, inferior frontal gyrus, fusiform gyrus, middle frontal gyrus, and the anterior cingulated gyrus. Comparisons were thresholded at p < 0.05, and familywise error (FWE) rate was corrected with a minimum cluster of 30 voxels. If no significant results were found, a less conservative threshold of false discovery rate, p < 0.05 was applied.
Results and discussion
A total of 16 right-handed participants entered the study. However, only 13 participants (7 male, 6 female) were included in the analyses of the behavioral data, because 2 participants experienced the scanning session as uncomfortable resulting in an early termination of the session, and one subject missed the sessions due to personal matters.
Pharmacokinetic assessment
Mean (±SE) cetirizine and verapamil concentrations in the four treatment conditions are presented in Table 1. t-tests revealed that mean cetirizine concentration when given alone did not significantly differ from that when given in combination with verapamil. Likewise, verapamil concentrations did not significantly differ when given alone as compared to when given in combination with cetirizine.
Subjective measurements
Sleep quality and sleep duration as assessed with the GSQS did not differ between nights preceding both test days. Treatments did not affect visual analog scale measures, KSS, and mental effort ratings (see Table 2).
Attention network test
The combination of cetirizine and verapamil increased the difference in RT between center cue and no cue (alerting) as compared to verapamil alone (see Fig. 2a) (t 12 = 3.11, p = 0.009). Alerting, orienting, and executive attention were not affected by cetirizine alone as compared to placebo. Comparison of change scores from scan session 1 (cetirizine 15 mg minus placebo) and scan session 2 (verapamil 120 mg + cetirizine minus verapamil 120 mg) further confirmed a significant difference in the effect of cetirizine on alerting with and without P-gp blockade (t 12 = −2.88, p = 0.014).
a Mean (±SE) changes in RT in the attention network test, divided into placebo and cetirizine with and without P-gp blockade. The asterisk indicates significant difference from placebo, indicating an increased difference in RT between no-cue and center-cue conditions (i.e., alerting), p < 0.05. b Mean (±SE) RT between no-cue and center-cue conditions (i.e., alerting) of the attention network test. The single asterisk indicates significant differences between no-cue and center-cue conditions, indicating that in all conditions the task manipulation was as expected, p < 0.01. The double asterisks indicate significant difference between placebo and cetirizine after P-gp blockade in the no-cue condition, indicating that the significant result in a is mostly caused by the no-cue and not the center-cue (i.e., warning cue) condition, p < 0.005
Additional analyses were conducted to identify the effect of cetirizine in two separate cue conditions that contribute to the alerting effect (i.e., center cue and no cue). This analysis revealed that the significant difference between placebo and cetirizine after P-gp blockade was caused by an increased reaction time in the no-cue condition (t 12 = 3.54, p = 0.004), and not in the (alerting) center-cue condition (t 12 = 0.87, p = 0.466) (Fig. 2b). Mean (±SE) RT during the alerting, orienting, and executive trials are shown in Table 2.
Taken together, the combination of verapamil and cetirizine increased the difference in RT between center cue and no cue. Further analyses revealed that blocking P-gp affected responsiveness (i.e., increased RT) when no warning cue was apparent, whereas it did not affect responsiveness when a prior cue indicated that a target would appear.
Imaging data
Data from one participant were removed from the dataset due to excessive head movement. Therefore, 12 subjects (7 male, 5 female) were included in the analyses of the imaging data.
Task activation
The aforementioned regions associated with the alerting network (center cue minus no cue), the orienting network (spatial cue minus center cue), and the executive attention network (incongruent minus congruent) were used as ROI (Fan et al. 2005). Within these areas, we detected significant task activation of the alerting network in the left inferior parietal lobe (t 47 = 4.97, p = 0.009, FWE corrected), left inferior frontal gyrus (t 47 = 6.35, p = 0.002, FWE corrected), and right superior temporal gyrus (t 47 = 6.35, p = 0.002, uncorrected). See Table 3 for details. No significant task activation was detected for orienting and executive attention; therefore, effects of treatments on orienting and executive attention were not further analyzed.
Treatment effects
The combination of cetirizine and verapamil increased the difference in blood oxygen level-dependent (BOLD) activation associated with alerting in the right superior temporal gyrus as compared to verapamil alone. More precisely, the difference in brain activity between center-cue and no-cue (i.e., the alerting effect) was larger in the cetirizine/P-gp blockade combination as compared with the placebo/P-gp blockade combination (t 11 = 8.39, p = 0.05, FWE corrected). No significant differences between cetirizine alone and placebo were detected.
Treatment effects in center-cue and no-cue conditions
As the behavioral data revealed that the difference between placebo and cetirizine after P-gp blockade was primarily located in the no-cue condition, BOLD effects in center-cue and no-cue conditions were analyzed separately to determine if a similar pattern occurs at the neural level. These analyses also revealed that the effect of cetirizine was prominent in the no-cue condition, but not in de center-cue condition. Cetirizine + verapamil decreased the BOLD response relative to verapamil alone in the no-cue condition, which approached significance in the right superior temporal gyrus (t 11 = 7.68, p = 0.06, FWE corrected). The comparison between verapamil and cetirizine + verapamil is depicted in Fig. 3b and shows a positive difference of verapamil over cetirizine + verapamil.
a fMRI results for the alerting network of the attention network test, showing significant activation in the left inferior parietal lobe, left inferior frontal gyrus, and right superior temporal gyrus. b Significant treatment effects in the alerting network, showing activation in the right superior temporal gyrus when compared with cetirizine + verapamil versus verapamil. c Treatment effect in the no-cue condition, showing activation in the right superior temporal gyrus when compared with verapamil versus cetirizine + verapamil
Discussion
The present study was conducted in order to investigate the effect of the P-gp transporter on the central effects of antihistamines in healthy volunteers. It was expected that blocking P-gp with verapamil would increase the distribution of the second-generation antihistamine cetirizine in the CNS, which would result in increased H1R binding, and promote CNS sedation. The present study supports this hypothesis both at the behavioral and the neural level.
At the behavioral level, cetirizine combined with verapamil reduced alertness in the ANT, when compared to verapamil alone and when compared to cetirizine alone. This impairing effect of cetirizine was apparent in the alerting network, but not in the orienting and executive attention network. Moreover, the influence of cetirizine was most apparent in the no-cue condition, rather than in the center-cue condition. This implies that participants were still able to profit from the alerting cue in the center-cue condition in every treatment but were slow in their response during cetirizine in the absence of an alerting cue, indicating reduced sustained attention/top-down control (Sturm and Willmes 2001). The locus of the cetirizine effect contrasts with that in a previous work showing that drug- or sleep-induced changes in alertness are mostly reflected in the center-cue condition. For example, caffeine decreased RT in the center-cue condition while leaving RT in the no-cue condition unaffected (Brunye et al. 2010). Likewise, sleep deprivation increased RT in the center-cue conditions, but not in the no-cue condition (Martella et al. 2011). The present data seem to indicate that central cetirizine affected intrinsic alertness (i.e., vigilance) more than readiness to a target/bottom-up activation of alertness (Mottaghy et al. 2006).
Neural effects of cetirizine on alerting were apparent in the right superior temporal gyrus. Bold activations in this area were larger after cetirizine and verapamil combined in comparison to verapamil alone. Further analyses of treatment effects in the center-cue and no-cue condition separately revealed that brain activations were less in the no-cue condition after the combination of cetirizine and verapamil. This decrement in brain activation coincides with the increment in RT that was measured at the behavioral level during combined treatment in the no-cue condition. The neural data in the present study support the conclusion from behavioral data showing that cetirizine decreases alertness when given in combination with verapamil.
It should be considered that verapamil is not a specific modulator of P-gp. For instance, verapamil is known to inhibit the metabolism of co-administered cytochrome P450 (CYT P450) substrates as well (e.g. Wang et al. 2004). However, cetirizine does not undergo liver metabolization and does not interact with other drug substances via CYT P450, or more precisely CYP3A (Bartra et al. 2006; Renwick 1999). It is therefore assumed that both behavioral and neural data support the hypothesis that P-gp affinity plays an important role in the occurrence of CNS sedation associated with antihistamines. Cetirizine is a second-generation antihistamine that is known to produce no or only mild sedation and has often been portrayed as a “nonsedating” antihistamine (Kay 2000). In the present study, cetirizine did not affect performance or brain activation in the ANT task when given alone. However, when given in combination with a P-gp blocker, CNS sedation became apparent. This finding demonstrates that CNS effects of antihistamines depend on the affinity of these drugs for the P-gp transporter. In case of high affinities, as in second-generation histamines, CNS side effects can be low. In case of no affinity, as in first-generation antihistamines, CNS side effects can be high.
The current finding may also have implications for other CNS drugs that have affinity for the P-gp transporter. P-gp plays a role in the distribution of many drugs besides antihistamines, such as antipsychotics, antidepressants, and anticancer treatments (Bartra et al. 2006; Boulton et al. 2002; de Klerk et al. 2009; Schinkel 1998). Concerning those treatments, P-glycoprotein may play a role in treatment resistance. Research has shown that, for instance, antipsychotics appear to be P-gp substrates, which makes them less effective in the treatment of schizophrenia as P-gp reduces their distribution in the CNS and consequently their effectiveness (El Ela et al. 2004; Wang et al. 2009). In that case, the combined use of a P-gp blocker with an antipsychotic may promote or prolong therapeutic efficacy of certain medicinal drugs. Also, it has been shown that genetic variations in P-gp activity can change responsiveness to antipsychotic treatment (Xing et al. 2006). More knowledge about genetic variations in P-gp activity might be of importance, as these can explain interindividual variations in treatment response and may support the development and improvement of tailor-made treatment procedures.
Task activations in the present study partly confirmed previous definitions of brain networks involved in ANT performance (Fan et al. 2005). Alerting effects were prominent in a number of predefined ROIs such as the left inferior parietal lobe, the left inferior frontal gyrus, and the right superior temporal gyrus, but not in the other predefined ROIs that have also been associated with the alerting network. Cue and target conditions priming the orienting and the executive attention network, respectively, did not produce any significant fMRI task activation. The lack of task activation during orienting and executive attention may have caused by the lower number of volunteers in the present study, relative to the study by Fan et al. (2005).
Several other studies confirm the involvement of parietal and frontal sites in alerting (Raz and Buhle 2006; Xu et al. 2010). In addition, research has shown that activation of the left hemisphere can be associated with phasic alertness and right hemisphere activation with intrinsic (or tonic) alertness (Coull et al. 2001; Raz and Buhle 2006; Sturm et al. 2006). As the no-cue condition is associated with intrinsic alertness, the finding of no-cue-related activation in the superior temporal gyrus in the right hemisphere is therefore in line with these findings.
In general, the finding that decrements in alertness occur after administration of cetirizine is in line with that of a previous work demonstrating that blockade of H1R decreases wakefulness (Conen et al. 2011a; Kay 2000; Passani et al. 2007; Schwartz and Arrang 2002). However, the impact of sedative actions on attention networks may actually differ between antihistamines. Our group demonstrated in a previous study that the sedative actions of hydroxyzine as measured with the ANT caused impairment in the executive network, but not in the alerting and orienting network (Conen et al. 2011b). Differential effects of antihistamines on attention networks may be caused by differences in binding affinities for H1R or may be due to differences in drug pharmacology. Cetirizine is the major metabolite of hydroxyzine and displays selective binding affinity for H1 receptors. Hydroxyzine, in addition, also binds to cholinergic and dopaminergic receptors that are known to produce sedation when blocked (Snowman and Snyder 1990). Dopamine plays an important modulating role in the control of executive attention (Fan et al. 2005; Fan and Posner 2004; Posner and Rothbart 2007) which may explain why antihistamines with dopaminergic action affect executive attention.
Cetirizine concentrations with and without verapamil did not significantly differ from each other. Yet, mean cetirizine levels still increased by 40 % after P-gp inhibition. As P-gp is also expressed in the small intestine, liver, and kidney (Fromm 2004), and this may cause P-gp inhibition to alter plasma concentrations of P-gp substrates. Blood samples were taken at Tmax (i.e., 1 h after drug administration) during cetirizine alone, and in addition at 4 h after cetirizine administration, and after verapamil was added. Cetirizine has a relatively long half-life of 7–11 h, but nonetheless, a decline in cetirizine plasma levels would be expected at 4 h after administration. The fact that this decline was not present can be interpreted as indicative of P-gp inhibition by verapamil. Indeed, increments in drug plasma concentration have previously been reported after combined administration of fexofenadine, a second-generation antihistamine, and a P-gp inhibitor (Kim et al. 2009; Sakugawa et al. 2009; Yasui-Furukori et al. 2005).
Cetirizine did not affect subjective rating of alertness, sleepiness, or mental effort, before and after P-gp blockade. This suggests that subjects did not subjectively experience the sedative effect of combined cetirizine and verapamil use that was apparent in objective measures of CNS sedation. Such discrepancy between subjective and objective measures of CNS effects of antihistamines has been reported before in studies assessing antihistamine effects on cognition and driving (Theunissen et al. 2006a; Vermeeren et al. 2002). It underscores the notion that users of sedating antihistamines should receive explicit warnings that these drugs may cause deleterious effects on cognition that may go unnoticed.
Because verapamil has multiple pharmacodynamic effects, it cannot be excluded that the combination of verapamil and cetirizine resulted in synergistic effects. It is therefore suggested to investigate the role of P-gp on central cetirizine effects by comparing effects of P-gp between individuals with lower expression and function of P-gp and higher expression of P-gp. Furthermore, because of the relatively small effects, it is suggested that future research is necessary investigating the effects of P-g with cetirizine interactions in patient populations, for instance, after a histamine challenge. Also, in order to confirm that verapamil increases the penetration of drugs through the blood–brain barrier, and subsequently that the effects in the present study were caused by increased availability of cetirizine in the brain, it is suggested to investigate this further using PET imaging.
In conclusion, alertness was sensitive to the combined treatment of cetirizine with a P-gp blocker, and both plasma levels and brain activation underpinned this finding. These results suggest that P-gp blockade may exacerbate the effects of second-generation antihistamines. The affinity for the P-gp transporter may therefore contribute to the lower incidence of CNS side effects.
References
Abbas MN, Abdel Fattah AA, Zahran E (2004) A novel membrane sensor for histamine H1-receptor antagonist “fexofenadine”. Anal Sci 20:1137–1142
Bartra J, Valero AL, del Cuvillo A, Davila I, Jauregui I, Montoro J, Mullol J, Sastre J (2006) Interactions of the H1 antihistamines. J Investig Allergol Clin Immunol 16(Suppl 1):29–36
Boulton DW, DeVane CL, Liston HL, Markowitz JS (2002) In vitro P-glycoprotein affinity for atypical and conventional antipsychotics. Life Sci 71:163–169
Brunye TT, Mahoney CR, Lieberman HR, Taylor HA (2010) Caffeine modulates attention network function. Brain Cogn 72:181–188
Chen C, Hanson E, Watson JW, Lee JS (2003) P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug Metab Dispos 31:312–318
Choi DH, Chung JH, Choi JS (2010) Pharmacokinetic interaction between oral lovastatin and verapamil in healthy subjects: role of P-glycoprotein inhibition by lovastatin. Eur J Clin Pharmacol 66:285–290
Conen S, Theunissen EL, Van Oers AC, Valiente R, Ramaekers JG (2011a) Acute and subchronic effects of bilastine (20 and 40 mg) and hydroxyzine (50 mg) on actual driving performance in healthy volunteers. J Psychopharmacol 25:1517–1523
Conen S, Theunissen EL, Vermeeren A, Ramaekers JG (2011b) Short-term effects of morning versus evening dose of hydroxyzine 50 mg on cognition in healthy volunteers. J Clin Psychopharmacol 31:294–301
Coull JT, Nobre AC, Frith CD (2001) The noradrenergic alpha2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cereb Cortex 11:73–84
Dadashzadeh S, Javadian B, Sadeghian S (2006) The effect of gender on the pharmacokinetics of verapamil and norverapamil in human. Biopharm Drug Dispos 27:329–334
de Klerk OL, Willemsen AT, Roosink M, Bartels AL, Hendrikse NH, Bosker FJ, den Boer JA (2009) Locally increased P-glycoprotein function in major depression: a PET study with [11C]verapamil as a probe for P-glycoprotein function in the blood–brain barrier. Int J Neuropsychopharmacol 12:895–904
del Cuvillo A, Mullol J, Bartra J, Davila I, Jauregui I, Montoro J, Sastre J, Valero AL (2006) Comparative pharmacology of the H1 antihistamines. J Investig Allergol Clin Immunol 16(Suppl 1):3–12
El Ela AA, Hartter S, Schmitt U, Hiemke C, Spahn-Langguth H, Langguth P (2004) Identification of P-glycoprotein substrates and inhibitors among psychoactive compounds—implications for pharmacokinetics of selected substrates. J Pharm Pharmacol 56:967–975
Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI (2007) The relation of brain oscillations to attentional networks. J Neurosci 27:6197–6206
Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005) The activation of attentional networks. Neuroimage 26:471–479
Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002) Testing the efficiency and independence of attentional networks. J Cognitive Neurosci 14:340–347
Fan J, Posner M (2004) Human attentional networks. Psychiatr Prax 31(Suppl 2):S210–S214
Fromm MF (2004) Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci 25:423–429
Jauregui I, Mullol J, Bartra J, del Cuvillo A, Davila I, Montoro J, Sastre J, Valero AL (2006) H1 antihistamines: psychomotor performance and driving. J Investigation Allergol Clin Immunol 16(Suppl 1):37–44
Jenkins BG, Chen Y-CI, Mandeville JB (2003) Pharmacological magnetic resonance imaging (phMRI). In: van Bruggen N, Roberts T (eds) Biomedical imaging in experimental neuroscience. CRC, New York, pp 155–209
Kay GG (2000) The effects of antihistamines on cognition and performance. J Allerg Clin Immunol 105:S622–S627
Kim KA, Park PW, Park JY (2009) Short-term effect of quercetin on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein, in healthy volunteers. Eur J Clin Pharmacol 65:609–614
Leslie RA, James MF (2000) Pharmacological magnetic resonance imaging: a new application for functional MRI. Trends Pharmacol Sci 21:314–318
Mahar Doan KM, Wring SA, Shampine LJ, Jordan KH, Bishop JP, Kratz J, Yang E, Serabjit-Singh CJ, Adkison KK, Polli JW (2004) Steady-state brain concentrations of antihistamines in rats: interplay of membrane permeability, P-glycoprotein efflux and plasma protein binding. Pharmacology 72:92–98
Martella D, Casagrande M, Lupianez J (2011) Alerting, orienting and executive control: the effects of sleep deprivation on attentional networks. Exp Brain Res 210:81–89
Mimura N, Nagata Y, Kuwabara T, Kubo N, Fuse E (2008) P-glycoprotein limits the brain penetration of olopatadine hydrochloride, H1-receptor antagonist. Drug Metab Pharmacokinet 23:106–114
Montoro J, Sastre J, Bartra J, del Cuvillo A, Davila I, Jauregui I, Mullol J, Valero AL (2006) Effect of H1 antihistamines upon the central nervous system. J Investigational Allergol Clin Immunol 16(Suppl 1):24–28
Mottaghy FM, Willmes K, Horwitz B, Muller HW, Krause BJ, Sturm W (2006) Systems level modeling of a neuronal network subserving intrinsic alertness. Neuroimage 29:225–233
Obradovic T, Dobson GG, Shingaki T, Kungu T, Hidalgo IJ (2007) Assessment of the first and second generation antihistamines brain penetration and role of P-glycoprotein. Pharm Res 24:318–327
Passani MB, Giannoni P, Bucherelli C, Baldi E, Blandina P (2007) Histamine in the brain: Beyond sleep and memory. Biochem Pharmacol 73:1113–1122
Polli JW, Baughman TM, Humphreys JE, Jordan KH, Mote AL, Salisbury JA, Tippin TK, Serabjit-Singh CJ (2003) P-glycoprotein influences the brain concentrations of cetirizine (Zyrtec), a second-generation non-sedating antihistamine. J Pharm Sci 92:2082–2089
Posner MI, Rothbart MK (2007) Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol 58:1–23
Ramaekers JG, O’Hanlon JF (1994) Acrivastine, terfenadine and diphenhydramine effects on driving performance as a function of dose and time after dosing. Eur J Clin Pharmacol 47:261–266
Raz A, Buhle J (2006) Typologies of attentional networks. Nat Rev Neurosci 7:367–379
Renwick AG (1999) The metabolism of antihistamines and drug interactions: the role of cytochrome P450 enzymes. Clin Exp Allergy 29(Suppl 3):116–124
Sakugawa T, Miura M, Hokama N, Suzuki T, Tateishi T, Uno T (2009) Enantioselective disposition of fexofenadine with the P-glycoprotein inhibitor verapamil. Br J Clin Pharmacol 67:535–540
Schinkel AH (1998) Pharmacological insights from P-glycoprotein knockout mice. Int J Clin Pharmacol Ther 36:9–13
Schinkel AH (1999) P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Deliv Rev 36:179–194
Schwartz JC, Arrang JM (2002) Histamine. In: Davis KL, Charney D, Coyle JT, Nemeroff C (eds) Neuropsychopharmacology: the fifth generation of progress. Lippincott Williams & Wilkins, Philadelphia, pp 179–190
Snowman AM, Snyder SH (1990) Cetirizine: actions on neurotransmitter receptors. J Allergy Clin Immunol 86:1025–1028
Sturm W, Schmenk B, Fimm B, Specht K, Weis S, Thron A, Willmes K (2006) Spatial attention: more than intrinsic alerting? Exp Brain Res 171:16–25
Sturm W, Willmes K (2001) On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 14:S76–S84
Sun H, Dai H, Shaik N, Elmquist WF (2003) Drug efflux transporters in the CNS. Adv Drug Deliv Rev 55:83–105
Tashiro M, Duan X, Kato M, Miyake M, Watanuki S, Ishikawa Y, Funaki Y, Iwata R, Itoh M, Yanai K (2008) Brain histamine H1 receptor occupancy of orally administered antihistamines, bepotastine and diphenhydramine, measured by PET with 11C-doxepin. Br J Clin Pharmacol 65:811–821
Tashiro M, Horikawa E, Mochizuki H, Sakurada Y, Kato M, Inokuchi T, Ridout F, Hindmarch I, Yanai K (2005) Effects of fexofenadine and hydroxyzine on brake reaction time during car-driving with cellular phone use. Human Psychopharmacol 20:501–509
Theunissen EL, Jonkman LM, Kuypers KP, Ramaekers JG (2006a) A combined neurophysiological and behavioural study into the stimulating effects of fexofenadine on performance. J Psychopharmacol 20:496–505
Theunissen EL, Vermeeren A, Ramaekers JG (2006b) Repeated-dose effects of mequitazine, cetirizine and dexchlorpheniramine on driving and psychomotor performance. Br J Clin Pharmacol 61:79–86
Theunissen EL, Vermeeren A, van Oers AC, van Maris I, Ramaekers JG (2004) A dose-ranging study of the effects of mequitazine on actual driving, memory and psychomotor performance as compared to dexchlorpheniramine, cetirizine and placebo. Clin Experimental Allerg 34:250–258
Van Ruitenbeek P, Vermeeren A, Riedel WJ (2008) Histamine H1-receptor blockade in humans affects psychomotor performance but not memory. J Psychopharmacol 22:663–672
Vermeeren A, Ramaekers JG, O’Hanlon JF (2002) Effects of emedastine and cetirizine, alone and with alcohol, on actual driving of males and females. J Psychopharmacol 16:57–64
Verster JC, Volkerts ER (2004) Antihistamines and driving ability: evidence from on-the-road driving studies during normal traffic. Annals Allerg, Asthma Immunol 92:294–303, quiz 303–5, 355
Verster JC, Volkerts ER, van Oosterwijck AW, Aarab M, Bijtjes SI, De Weert AM, Eijken EJ, Verbaten MN (2003) Acute and subchronic effects of levocetirizine and diphenhydramine on memory functioning, psychomotor performance, and mood. J Allerg Clin Immunol 111:623–627
Vuurman EF, Rikken GH, Muntjewerff ND, de Halleux F, Ramaekers JG (2004) Effects of desloratadine, diphenhydramine, and placebo on driving performance and psychomotor performance measurements. Eur J Clin Pharmacol 60:307–313
Wang LJ, Ree SC, Chen CK (2009) Courses of aripiprazole-associated tardive dyskinesia: report of two cases. Prog Neuropsychopharmacol Biol Psychiatry 33:743–744
Wang YH, Jones DR, Hall SD (2004) Prediction of cytochrome P450 3A inhibition by verapamil enantiomers and their metabolites. Drug Metab Dispos 32:259–266
Xing Q, Gao R, Li H, Feng G, Xu M, Duan S, Meng J, Zhang A, Qin S, He L (2006) Polymorphisms of the ABCB1 gene are associated with the therapeutic response to risperidone in Chinese schizophrenia patients. Pharmacogenomics 7:987–993
Xu GQ, Lan Y, Huang DF, Rao DZ, Pei Z, Chen L, Zeng JS (2010) Visuospatial attention deficit in patients with local brain lesions. Brain Res 1322:153–159
Yasui-Furukori N, Uno T, Sugawara K, Tateishi T (2005) Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin Pharmacol Ther 77:17–23
Zhou SF, Liu JP, Chowbay B (2009) Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 41:89–295
Acknowledgments
We would like to thank Cees van Leeuwen for the medical supervision, Lotty Huijboom for the advice concerning the MRI measurements, and Ilja Croijmans, Emma Kremer, and Melinda Ravlic for their assistance in data collection.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This trial has been registered at www.trialregister.nl as NTR2609, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2609.
Rights and permissions
About this article
Cite this article
Conen, S., Theunissen, E.L., Vermeeren, A. et al. The role of P-glycoprotein in CNS antihistamine effects. Psychopharmacology 229, 9–19 (2013). https://doi.org/10.1007/s00213-013-3075-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3075-z