Abstract
Attentional deficits are prominent in schizophrenia, affecting nearly all cognitive functions. Human attention comprises three essential components: alerting, orienting and executive control. For the assessment of these functions, the attention network test (ANT) has been proposed and used in healthy controls and patients. In schizophrenia, the ANT has revealed behavioral deficits; however, the corresponding neural correlates have not been examined. In the present study, neural correlates of attention were investigated in 17 schizophrenia patients and 17 healthy controls using the ANT with fMRI. Behavioral deficits emerged in the alertness system with a reduced efficiency for temporal cues. In fMRI, changes were observed for all three domains–alerting, orienting and conflict–and revealed hyper- as well as hypoactivation in patients. Affected regions during alerting comprised a broad fronto-temporo-parieto-occipito-cerebellar network, while differences during orienting mainly tapped fronto-parietal regions and during conflict processing a thalamo-frontal-temporal occipital network including the postcentral regions. In general, hyperactivations were positively correlated with more severe psychopathologial symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairments are broad and devastating in schizophrenia and affect all cognitive domains. Attentional components are certainly core functions affecting other domains such as memory, executive and perceptual functions, resulting in the inherent problem of separating attentional dysfunctions from other cognitive disturbances. Hence, the impact of attentional dysfunctions on the cognitive profile of schizophrenia is difficult to assess specifically.
Concepts of human attention have been proposed, e.g., by Corbetta [2] and Posner [15] and their colleagues. The latter postulate that three main components make up human attention: alerting, orienting and executive control. Alertness represents the state in which a response follows a signal cue. Selection of information from a sensory input indicates the orienting response, and executive control encompasses conflict monitoring, planning, decision, error detection, habit change, etc. For the assessment of these components, the attention network test (ANT; [15]), a combined cue-target and flanker test, has been applied [19].
Posner and Petersen [15] proposed separate underlying cerebral networks and neurotransmitter systems. Existing neuroimaging evidence largely confirmed this model but produced mixed results for some of the assumptions [5]. In the executive control network, the central role of the dorsal anterior cingulate gyrus (dACC) and the lateral prefrontal cortex has been repeatedly demonstrated [4], while the dorsolateral prefrontal cortex has been shown to be predominantly involved in conflict solving [10]. Neural correlates of intrinsic alertness comprise the fronto-parietal network [17] with a strong dominance of the right hemisphere. Brain regions associated with orienting are the bilateral superior posterior parietal lobes and the left temporoparietal junction, the frontal eye field and the posterior lateral thalamus [15].
Applying the ANT to patients with schizophrenia, Wang and colleagues [21] found a marked deficit in the executive control system, which was less pronounced in the orienting network. Executive dysfunctions have been investigated with STROOP or flanker task. Opgen-Rhein et al. [13] confirmed an impaired executive control system in schizophrenia; however, in contrast to Wang [21], they reported a reduced time for conflict resolving in patients. Reduced interference effects were supported and extended by alertness impairments for the ANT in schizophrenia [11]. No study exists on neural correlates using the ANT in schizophrenia.
Here, we applied the ANT to investigate the neural correlates in schizophrenia. On the basis of the previous findings, we expected impairments in all attentional domains, although mostly in the executive system. We hypothesized the involvement of the lower ACC and lateral prefrontal cortex during conflict processing and reduced orienting effects in the superior parietal lobes and temporo-parietal areas.
Method
Participants
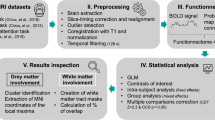
17 schizophrenia (SCID [22]) patients (men, women) and 17 healthy control subjects (HC) of matching age, gender and parental education participated. The local ethics committee approved the protocol, and participants gave informed consent. The study was in accordance with the Declaration of Helsinki (for details, see online resource 1).
Procedure
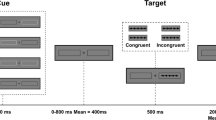
The subjects performed a modified version of the ANT ([6] for details, see online resource 1). Subjects indicated by button press if a target arrow pointed to the left or right. The target arrow was surrounded by four flankers (two above, two below), which had either the same (congruent) or the opposite direction (incongruent). These stimuli were presented to the left or right of a central fixation cross, with a visual angle of approximately 7–8°. Participants’ gaze was not constrained (overt orienting).
Items were either preceded by a central cue (replacing the fixation cross) to stimulate alertness or by a valid spatial cue to elicit orientation. Control trials were not cued at all (fixation cross-present). For each combination of factor levels, we presented twenty trials, resulting in a total of 240 stimuli.
Behavioral data analysis
We set up a mixed-model ANOVA with 2 (diagnosis) × 3 (cue: no, central, spatial) × 2 (target: congruent, incongruent) factors for fixed effects and the random effect factor subject in order to account for dependent observations within subjects. Statistical analyses were computed using R [18], and the ANOVA was implemented as a mixed effect model (NLME [14]).
Attention network scores were calculated based on reaction time (RT) differences for the different conditions (for correct trials). The alerting effect was assessed by the difference between the RTs for no cue and central cue; for the orienting, we subtracted the RTs for the spatial cue from those of the central cue. The conflict effect was the difference between RTs for incongruent and congruent trials. Effect ratios resulted by referring network scores to the individual mean RT (for details, see online resource 1).
Results
Behavioral data
The results for the ANOVA on reaction times yielded significant main effects for the three factors, conflict (F(1,160) = 273.75, P < 0.0001), cue (F(2, 160) = 70.54, P < 0.0001) and group (F(1,32) = 10.98, P = 0.002), indicating slower responses for incongruent trials, for no cue conditions, and in patients. Only one significant interaction emerged between conflict and cue (F(2,160) = 6.01, P = 0.003). The three-way interaction revealed a trend (F(2,160) = 2.77, P = .066). For the three attentional network scores [4] and additional results, see online resource 2.
The comparison of correct rates yielded no significant group differences (t(20.7) = 1.74, P = .10).
fMRI data
Results for the single group (one-sample t-tests) are displayed in the online resource 3. Group differences are described below (see Fig. 1).
Alertness
Alertness demonstrated that only two regions were more strongly activated in controls, namely the precuneus and the inferior parietal cortex. The reverse contrast revealed hyperactivated regions in patients in the middle and superior frontal gyri, the cuneus, the inferior temporal gyrus, the cerebellum and the occipital gyrus.
Orienting
In group comparisons, patients had reduced activity for orienting in several regions, namely the thalamus, the middle frontal gyrus, the occipital cortex and the superior frontal gyrus. Patients, on the other hand, exhibited hyperactivations in the middle and transverse temporal gyri, the temporal pole and the postcentral gyrus.
Executive system
In patients, hypoactivations emerged only in the superior temporal gyrus, extending into the inferior parietal cortex. Hyperactivations could be observed in the supramarginal gyrus, the middle frontal and temporal gyri and the superior medial gyrus.
Corollary analyses
In each group, corollary analyses demonstrated positive correlations for the alerting contrast in healthy controls in the dorsal frontal gyrus (for details, see online resource 3), bilaterally in the central parietal lobe and the thalamus. The middle frontal gyrus correlated negatively with behavioral performance. The orienting response correlated positively with the precuneus and negatively with the cingulate gyrus, the conflict response correlating only negatively with the superior frontal gyrus and the inferior parietal lobe.
The corresponding positive correlations in schizophrenia patients revealed alertness activation in the middle frontal gyrus. Negative correlations were also found in the middle frontal gyrus, the precentral gyrus and the superior temporal gyrus. The orienting response correlated positively with the brainstem and the middle temporal gyrus and negatively with the precuneus. Finally, the conflict contrast showed positive correlations with the insula, the middle frontal gyrus, the superior parietal lobe and the postcentral gyrus. Negative correlations emerged in the inferior parietal lobe and the cingulate gyrus.
Medication in CPZ equivalents correlated mainly with the alerting contrast, positively with the precuneus, the bilateral inferior parietal gyrus and the middle frontal gyrus and negatively with the hippocampus and the midcingulate gyrus. No correlations were found for the orienting contrast and only negative correlations for the conflict contrast in the precuneus and the inferior parietal cortex.
Psychopathology in patients revealed only positive correlations for the alerting contrast (PANSS positive: posterior cingulate; PANSS negative: bilateral inferior parietal cortex; PANSS general: cerebellum; inferior occipital gyrus).
For the orienting contrast also, only positive correlations emerged for positive and negative psychopathology (PANSS positive: precentral gyrus, inferior parietal, midcingulate gyrus, postcentral gyrus; PANSS negative: postcentral gyrus and precentral gyrus).
The conflict contrast revealed positive correlations between the following regions and scores (PANSS positive: parahippocampal gyrus, inferior parietal cortex, middle frontal gyrus; PANSS negative: bilateral inferior parietal). Negative correlations were also observed (PANSS positive: superior temporal gyrus and middle occipital gyrus; PANSS general: insula).
Discussion
The ANT has been proven a useful tool to reveal the neural correlates of attentional components. Our results point to behavioral impairments in alerting and neural dysfunctions in all components of attention.
Behavioral results
As expected, patients reacted more slowly but their correct response rate matched that of their healthy counterparts. In alerting, patients profited much less from a cue, causing a smaller reaction time difference between trials with and without a cue. In orienting and conflict, no group differences were found, which can be interpreted as a failure to use the cue. Based on our results, and in line with studies by Nestor et al. [11] but in contrast to those by Urbanek [20], we hypothesize that schizophrenia patients are unable to benefit from cues to speed up their responses. Depending on the specific ANT version, impairments in orienting and, most frequently, deficits in the executive control of attention [21] are found. Hence, our results indicate that impairments in schizophrenia may comprise deficits in alerting, extending beyond the most frequently reported deficits in the executive system, and that the sort and extent of dysfunctions may be strongly modulated by ANT sample characteristics and task modifications.
Neural correlates of attentional dysfunctions in alerting and orienting
Our brain activation results revealed differences between patients and controls in all three attentional components, suggesting a rather global attentional deficit.
For alerting, the fMRI parameter estimates (see online resource 3) in healthy controls show a facilitating effect for cueing. Central cues elicit reduced activation compared to no cue in the superior and middle frontal gyri, the cuneus, the inferior temporal gyrus, the cerebellum and the occipital gyrus in controls. Furthermore, less activation in the middle frontal region was correlated with a stronger behavioral alerting effect.
In contrast, patients showed an increase in the frontal regions for the cued condition. The reverse pattern was found in the precuneus and angular gyrus with higher cue activation in controls and reduced activation in patients. The involved frontal regions were mostly right lateralized, supporting a dominant role of the right hemisphere in alertness. Previous studies have reported the involvement of the temporal and occipital regions as well as the cerebellum and precuneus during alerting [4]. However, in our study, the parietal cortex failed to show group differences. This might indicate that alerting deficits in schizophrenia are mostly confined to the frontal lobe.
Our observation of increased frontal activation in cue conditions in patients revealed heightened activation in the superior frontal and inferior and medial frontal areas during sustained attention [12], which might reflect a stronger recruitment of these areas for cues and suggest a more phasic than tonic attentional demand in patients.
The extent of psychopathology also seems to influence the observed dysfunctions [3]. Correspondingly, we found positive correlations during the alerting contrast between the secondary visual areas and PANSS general psychopathology, in the posterior cingulate with PANSS negative and positive and in the precuneus with PANSS positive symptomatology. However, the latter also correlated with medication, which may indicate that these impairments are not only disease related. Medication effects can never be excluded completely. However, there is only this single overlap; therefore, it is highly implausible that group differences are only due to medication.
Summarizing, it seems that the cue poses a higher processing demand in schizophrenia patients, recruits more regions and causes higher activations, whereas healthy controls show a facilitating effect with less activations in the involved network. The altered activation pattern in patients, in general, indicates an imbalance in adjusting the adequate amount of activation. Correlations with psychopathology even corroborate this conclusion as higher activations emerged in parallel with a more severe disorder.
For the orienting response, group differences with higher responses of controls to spatial cues and the reverse pattern in patients were observed in the thalamus, middle and superior frontal and occipital gyri, while reduced responses to spatial cues were found in the temporal and postcentral regions of controls with the patients demonstrating the reverse response pattern. The postcentral gyrus (PCG), among other regions, mediates the reorientation of attention and seems to be specifically involved in arrow-cued orienting [7].
Hence, in orienting, an enhanced activation seemingly reflects higher efforts in regions necessary to constitute and orient attentional processes. Correspondingly, patients reveal insufficient activation in the thalamus and occipital cortex [15]. Such task-specific dysfunctions may be indicative of disrupted inter-regional brain integration in schizophrenia as already demonstrated for attentional requirements [8] in the cortico-cerebellar-thalamo-cortical circuit. Again, we observed higher activations with more psychopathology mainly in the postcentral gyrus (PANSS positive and negative psychopathology), but no influence of medication.
Neural correlates of executive dysfunctions
The conflict condition revealed more activation in patients for incongruent compared to congruent trials. These activations were located predominantly right-sided in the middle and superior frontal and middle temporal regions as well as the inferior parietal cortex. These findings may point to compensatory processes in the fronto-temporo-parietal network subsisting conflict processing and solution and implicated in the dorsal attentional system. Again, those compensatory processes were more pronounced in patients with a more severe psychopathology, with higher activations in the inferior parietal cortex for higher negative as well as positive psychopathology and in the middle frontal area for patients with more positive symptoms.
In schizophrenia, the prominent role of the lateral prefrontal cortex [15] and the temporal cortex [16] has been repeatedly underlined. However, a major role in the executive and cognitive control of conflict as well as conflict resolution is ascribed to the dorsal anterior cingulate cortex [1]. In our study, no differences were found in this region, although many reports exist on dysfunctions in the ACC during conflict processing in schizophrenia (e.g., [23]). Perhaps, our design was inadequate as even in controls ACC activation underlying the ANT executive system was rather weak and did not reach significance on a group level [9]. This lack of involvement of the ACC might also be explained by our rather small schizophrenia sample and modifications of the ANT structure.
In conclusion, using the ANT, we found dysfunctions in all components of attention, the alerting, orienting and executive systems, while behavioral deficits only became obvious in the alerting efficiency. Specifically, schizophrenia patients could not profit from additional temporal contextual cue information. The changes in the observed activation patterns point to cerebral dysfunctions in the cerebellar-thalamic and thalamo-cortical networks during attention, which may reflect deficits in schizophrenia and explain the general slowing of responses.
References
Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546
Corbetta M (1998) Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci USA 95:831–838
Erkwoh R, Sabri O, Schreckenberger M, Setani K, Assfalg S, Sturz L, Fehler S, Plessmann S (2002) Cerebral correlates of selective attention in schizophrenic patients with formal thought disorder: a controlled h2 15o-pet study. Psychiatry Res 115:137–153
Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005) The activation of attentional networks. Neuroimage 26:471–479
Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002) Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14:340–347
Fossella J, Posner MI, Fan J, Swanson JM, Pfaff DW (2002) Attentional phenotypes for the analysis of higher mental function. ScientificWorldJournal 2:217–223
Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hamalainen H (2006) Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage 33:406–413
Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC (2005) Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain 128:2597–2611
Kellermann T, Reske M, Jansen A, Satrapi P, Shah NJ, Schneider F, Habel U (2011) Latencies in bold response during visual attention processes. Brain Res 1386:127–138
MacDonald AW III, Cohen JD, Stenger VA, Carter CS (2000) Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288:1835–1838
Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME (2007) Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res 90:308–315
Ojeda N, Ortuno F, Arbizu J, Lopez P, Marti-Climent JM, Penuelas I, Cervera-Enguix S (2002) Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp 17:116–130
Opgen-Rhein C, Neuhaus AH, Urbanek C, Hahn E, Sander T, Dettling M (2008) Executive attention in schizophrenic males and the impact of comt val108/158met genotype on performance on the attention network test. Schizophr Bull 34:1231–1239
Pinheiro JC, Bates DM (2000) Mixed-effects models in s and s-plus. Springer, Heidelberg
Posner MI, Petersen SE (1990) The attention system of the human brain. Annu Rev Neurosci 13:25–42
Rusnakova S, Daniel P, Chladek J, Jurak P, Rektor I (2011) The executive functions in frontal and temporal lobes: a flanker task intracerebral recording study. J Clin Neurophysiol 28:30–35
Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, Herzog H, Tellmann L, Muller-Gartner HW, Willmes K (1999) Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia 37:797–805
Team RDC (2006) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Thienel R, Voss B, Kellermann T, Reske M, Halfter S, Sheldrick AJ, Radenbach K, Habel U, Shah NJ, Schall U, Kircher T (2009) Nicotinic antagonist effects on functional attention networks. Int J Neuropsychoph 12:1295–1305
Urbanek C, Neuhaus AH, Opgen-Rhein C, Strathmann S, Wieseke N, Schaub R, Hahn E, Dettling M (2009) Attention network test (ant) reveals gender-specific alterations of executive function in schizophrenia. Psychiatry Res 168:102–109
Wang K, Fan J, Dong Y, Wang CQ, Lee TM, Posner MI (2005) Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res 78:235–241
Wittchen HU, Zaudig M, Fydrich T (1997) Strukturiertes klinisches interview für dsm-iv: Skid [structured clinical interview for dsmiv: Scid]. Hogrefe, Göttingen
Yucel M, Pantelis C, Stuart GW, Wood SJ, Maruff P, Velakoulis D, Pipingas A, Crowe SF, Tochon-Danguy HJ, Egan GF (2002) Anterior cingulate activation during stroop task performance: a pet to mri coregistration study of individual patients with schizophrenia. Am J Psychiatry 159:251–254
Acknowledgments
This work was supported by the German Research Foundation KFO 112/2-1 and 2-2, DFG HA 3202/3-1, the Helmholtz Alliance `Mental Health in an Ageing Society′ funded by the Initiative and Networking Fund of the Helmholtz Association (HelMA, 016W0751), The IZKF of the Medical School of the RWTH Aachen (VV N68-j; N4-4).
Conflict of interest
The authors declare that they have no conflicts of interest.
This supplement was not sponsored by outside commercial interests. It was funded by the German Association for Psychiatry and Psychotherapy (DGPPN).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Backes, V., Kellermann, T., Voss, B. et al. Neural correlates of the attention network test in schizophrenia. Eur Arch Psychiatry Clin Neurosci 261 (Suppl 2), 155 (2011). https://doi.org/10.1007/s00406-011-0264-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-011-0264-9