Abstract
Rationale
Novel stimuli paired with exposure to addictive drugs can elicit approach through Pavlovian learning. While such approach behavior, or sign tracking, has been documented for cocaine and alcohol, it has not been shown to occur with opiate drugs like heroin. Most Pavlovian conditioned approach paradigms use an operandum as the sign, so that sign tracking can be easily automated.
Objectives
We were interested in assessing whether approach behavior occurs to an audiovisual cue paired with an intravenous heroin infusion. If so, would this behavior exhibit characteristics of other Pavlovian conditioned behaviors, such as extinction and spontaneous recovery?
Methods
Rats were repeatedly exposed to an audiovisual cue, similar to that used in standard self-administration models, along with an intravenous heroin infusion. Sign tracking was measured in an automated fashion by analyzing motion pixels within the cue zone during each cue presentation.
Results
We were able to observe significant sign tracking after only five pairings of the conditioned stimulus (CS) with the unconditioned stimulus (US). This behavior rapidly extinguished over 2 days, but exhibited pronounced spontaneous recovery 3 weeks later.
Conclusions
We conclude that sign tracking measured by these methods exhibits all the characteristics of a classically conditioned behavior. This model can be used to examine the Pavlovian component of drug memories, alone, or in combination with self-administration methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relapse is a recurring problem in drug addicts, and may be more likely to occur when an addict encounters certain cues in the environment that act as reminders of the drug's reward and thereby precipitate craving and subsequent relapse. The evidence for this in humans is largely anecdotal (Drummond 2000; Sinha and Li 2007), but is supported by evidence from rodent self-administration models where drug-associated cues induce relapse (Fuchs et al. 2008). In these models, experimenters frequently use audiovisual cues, and these cues are often delivered simultaneously with the drug infusion when the animal makes a drug-seeking response. Such response-contingent cues are arguably not predictive of drug reward, but still effectively reinstate drug seeking, even more so than predictive cues, by virtue of their ability to act as conditioned reinforcers (Di Ciano and Everitt 2003; Fuchs et al. 2008). It is hypothesized that predictive cues initiate relapse, whereas response-contingent cues maintain responding (Di Ciano and Everitt 2003; Fuchs et al. 2008). Though the magnitude of reinstatement elicited by predictive cues is smaller, owing to their limited role in the initiation of relapse, such cues impart greater face validity to the animal model, and relapse therapies should logically be targeted toward preventing the initiation of relapse, as opposed to its maintenance. Furthermore, response-contingent cues make it difficult to separate the operant learning component from Pavlovian cue learning, and experimental manipulations that effectively inhibit cue-induced reinstatement in these models may work on one or both processes to elicit therapeutic effects.
Extinction-based behavioral therapy has been used to diminish cue responses in addicts (Childress et al. 1986; McClernon et al. 2007; Price et al. 2010). Identifying novel therapeutics to enhance the success of behavioral therapy in the clinic relies on the identification of neurobiological substrates controlling extinction memory. Given that the Pavlovian cue memory is more easily targeted in the clinic, and the operant (seeking) memory must be targeted indirectly through this cue memory, addiction models are lacking that isolate the former component. Sanchez and colleagues have developed a model that isolates Pavlovian cue extinction after operant training with response-contingent cues (Sanchez et al. 2010; Torregrossa et al. 2010), which has moved the field forward in this respect. However, it is unclear how or why response-contingent cues act as conditioned reinforcers to maintain relapse rates in self-administration models (Di Ciano and Everitt 2004), and whether this form of relapse depends on a Pavlovian cue memory at all has recently been called to question given the finding that novel cues will reinstate drug seeking as effectively as conditioned cues (Bastle et al. 2012).
Pavlovian conditioned approach, or "sign tracking," reflects learning about drug-associated cues and is a basic demonstration of Pavlovian cue memory. For some time, it was thought that sign tracking did not occur in response to drug-associated cues, though it was readily observed for cues paired with food reward (Kearns and Weiss 2004). In recent years, however, sign tracking has been clearly demonstrated for a number of addictive substances including cocaine and alcohol (Cunningham and Patel 2007; Krank 2003; Krank et al. 2008; Uslaner et al. 2006; Yager and Robinson 2013). In these models, the cue (e.g., the sign) is presented prior to non-contingent reward delivery, and sign tracking is indicated by the amount of interaction with the cue during the "cue on" period, which predicts impending reward. The sign being tracked is often a lever operandum, which makes the approach response easily amenable to automated methods of measurement (e.g., each lever press indicates an approach response). As a lever is often used to measure the drug seeking response in the standard self-administration model of relapse, it is difficult to compare results between traditional sign tracking versus self-administration models, or to measure effects of the sign on drug seeking within the same model.
Recently, Yager and Robinson (2013) observed sign tracking in response to the presentation of a visual cue repeatedly paired with an intravenous cocaine infusion. They additionally showed that these Pavlovian conditioned cues could elicit reinstatement in animals that self-administered cocaine in the absence of such cues, using a hybrid sign tracking–self-administration model. We set out to extend these findings to another major class of drugs, for which sign tracking has yet to be demonstrated, namely opiates. We further wanted to assess whether this sign tracking is amenable to extinction and spontaneous recovery, as with Pavlovian conditioned fear (Quirk 2002). We chose to use a predictive audiovisual cue in order to enhance the validity of the model, measure sign tracking, and enhance comparison to Pavlovian conditioned fear models.
Methods
Subjects
Twenty-four male, Sprague–Dawley rats (wild types bred in house with sires that were heterozygous transgenics carrying a c-fos-lacZ gene) weighing 350–500 g at the time of surgery were housed in a temperature- and humidity-controlled vivarium on a 12-h reverse light–dark cycle (7:00 am lights off). Food and water were available ad libitum throughout the experiment. Behavioral sessions were conducted during the dark phase, at the same time of day each day. All procedures were approved by the animal care committee at the VU University of Amsterdam.
Experimental chamber
Standard operant chambers for rats (Med-Associates) measuring 24 × 30 × 29 cm (w × l × h) were equipped with a lever located 6 cm above the floor and a triple stimulus light panel above the lever (10 cm above the floor). For this study, the lever was always retracted (never used) and all three stimulus lights were turned on and off simultaneously for each cue presentation. The sonalert used to deliver the 4-kHz pure tone presented in conjunction with the triple stimulus lights was located on the same panel as the lights near the ceiling of the chamber. The house light was positioned on the opposing wall near the ceiling and was always turned on when animals were in the chamber. A spring tethering line was used to connect the external backpack apparatus (e.g., the intravenous catheter) and entered through a hole in the ceiling of the chamber. The line permitted free-range movement of the rat within the chamber, while a counterbalancing weight prevented entanglement of the line. A pump was positioned outside the sound-attenuating cubicle, and was used to deliver non-contingent infusions of heroin. A camera was positioned on the back wall of the sound-attenuating cubicle, to record video from each behavioral session.
Surgery for intravenous catheter implants
One week after transport from the breeding facility to the behavioral facility, rats were placed in a stereotaxic device under gas anesthesia (isoflurane) for the implantation of intravenous jugular catheters. Silicon tubing was fitted to the ventral surface of a vascular access harness (Med-Associates) with an aseptic port on the dorsal surface for connection to the spring tether. This catheter apparatus resembles a backpack, and is continually worn by the animal at all times. The unattached end of the silicon tubing is inserted into the right jugular vein and runs over the right shoulder, exiting between the shoulder blades. The backpack is fitted snuggly over each forearm of the animal and adjusted periodically as the animal gains weight. Ketofen (5 mg/kg, s.c.) and Baytril (2.5 %, 0.15 ml, s.c.) were administered at the end of surgery to relieve pain and prevent infection, respectively. Animals were allowed to recover for 5 to 7 days before behavioral procedures commenced. Four rats removed their backpacks within 48 h after surgery (n = 2), or before the end of conditioning (n = 2), and were excluded from the study.
Heroin infusion
Heroin was dissolved in saline (0.82 mg/ml) and filtered prior to use. The dose of heroin was chosen based on previous self-administration studies from our group indicating that, at the beginning of the session, animals will "load up" with about three to four infusions before maintaining steady successive responding for heroin. These infusions range from 2 to 3 s, depending on the animal's body weight, to achieve a dose of 100 μg/kg/inf. Here, we used the same concentration of heroin, but with a longer infusion duration of 10 s, for a dose of 175 μg/inf (roughly equivalent to 3.7 infusions of our lower, self-administration dose). A previous study suggests that higher doses (approximately 4-fold) of noncontingently administered i.v. heroin are necessary to achieve neurophysiological responses in nucleus accumbens neurons compared to self-administered doses (Lee et al. 1999), presumably owing to the lack of expectation effects on accumbal dopamine (Lecca et al. 2007). Additionally, we wanted to ensure that animals experienced a substantial rewarding effect, even with a single infusion, so that each pairing of the conditioned stimulus (CS) with the unconditioned stimulus (US) would induce learning, and so that each conditioning session could be conducted with a single CS–US pairing. Because the effects of a single infusion of heroin can be long-lasting (e.g., 1 to 4 h), the intertrial interval to ensure isolated CS–US learning would be too long for multi-trial conditioning sessions.
Conditioning
An overview of all behavioral procedures is shown in Fig. 1. After recovery from surgery, conditioning was conducted in five, daily 11-min sessions. Rats were connected to the spring tether and placed in the experimental chamber. Five minutes later, the triple stimulus light and sonalert were turned on. The compound cue presentation lasted a total of 30 s, and the heroin infusion was initiated 4 s prior to cue offset, continuing for an additional 6 s after cue offset. Neurophysiological responses in nucleus accumbens neurons are observed rapidly (within seconds) after intravenous infusion of a non-contingent bolus of heroin at doses comparable to ours (Lee et al. 1999). Thus, we attempted to coincide the CS offset with the initial sensation of reward. Five minutes after the infusion, rats were returned to their home cage.
Experimental design. Conditioning consisted of five 11-min sessions, once daily for 5 days. In the middle of each session, a single (1×) 30-s audiovisual cue was presented, followed by a non-contingent intravenous infusion of heroin. On day 6, extinction began, and heroin was no longer administered. Extinction sessions consisted of 20 cue presentations (20×) over a 63-min session (with a 2.5-min intertrial interval). Extinction recall was tested on day 7 under identical procedures, then rats were returned to their home cages for 3 weeks. On day 28, a final extinction session was administered to test for spontaneous recovery of sign tracking
Extinction
Twenty-four hours after the last conditioning session, extinction training took place. Rats were connected to the spring tether and placed in the experimental chamber, where they received 20 tone + light cue presentations over the course of a 63-min session. The first cue presentation occurred 5 min into the session, with all subsequent cue presentations on a 2.5-min intertrial interval. At the end of the session, rats were returned to their home cage. The next day, they underwent an identical extinction session in a test for extinction recall. After this session, backpacks were removed, and animals were returned to their home cage.
Spontaneous recovery
After 3 weeks of home cage abstinence, rats were returned to the experimental chambers for a test of spontaneous recovery. The session parameters were identical to the previous extinction sessions. As backpacks had been removed, animals were not tethered.
Measuring sign tracking
Pavlovian conditioned approach to the triple stimulus display was measured in an automated fashion using FreezeScan (Clever Systems). A zone of interest for the cue zone (7.5 × 4 cm) was drawn around the triple stimulus display of each analysis arena, and the total number of motion pixels in that zone was scored during each cue presentation. A control zone of the same dimensions was drawn at the same height on the opposing wall, and motion pixels within this zone were scored during each cue presentation as well. Figure 2b depicts the arena and zones of interest. A dummy triple stimulus panel positioned outside the experimental chamber was used to embed the video with a light change that could be detected by FreezeScan as an "event" and used to signal the cue presentations for automated scoring. With a single camera system, as we used here, the camera must be positioned on the wall closest to the zones of interest, roughly perpendicular to the zone, to minimize "noise" from non-sign-tracking zone entries (see Supplemental Video and Fig. 2a for reference).
Automated analysis of sign tracking. Sign tracking was scored using FreezeScan software (Clever Sys, Inc.). A still frame of the Supplemental Video is shown in panel a, and the chamber in the top right quadrant is enlarged in panel b to illustrate how the analysis was performed. An irregular polygon (blue) was drawn around the borders of the chamber to define the arena where the animal can be located. A rectangle (red) was drawn around the triple stimulus cue lights where sign tracking occurs. Another rectangle (green) of the same dimensions was positioned on the opposing wall and served as a control zone, where little to no sign tracking occurs. The software detects the number of motion pixels in each zone during defined "cue on" periods, which serves as a measure of sign tracking
Data analysis and statistics
Data are depicted as number of motion pixels within each zone during cue presentations. To determine whether there was an initial preference for one zone, a planned comparison t-test was conducted between the control zone and cue zone for the first cue presentation (before the first heroin infusion). To determine whether activity in the cue zone was higher than the control zone during conditioning, extinction, and spontaneous recovery, data were analyzed using two-way repeated-measures analysis of variance (ANOVA) with time as the within-subjects factor, and zone as the between-subjects factor. All five conditioning trials were included in the analysis, while extinction and spontaneous recovery were analyzed across five blocks of four trials (20 cue presentations/session). For between-session comparisons, two-way repeated-measures ANOVAs were conducted over the first four-trial block of each session, as indicated in the Results section. Post-hoc comparisons were conducted only when there was an interaction between time and zone. All 20 rats were included in each analysis. All analyses were conducted using GraphPad Prizm software.
Results
Rats exhibit rapid conditioning to the heroin-paired cue
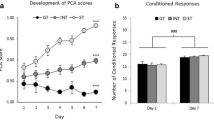
Each day for 5 days, rats were placed in the experimental chambers, and in the middle of the 11-min session, they received a single 30-s light-tone presentation that predicted a 10-s heroin infusion. Motion pixels in the cue zone versus the control zone were measured, and rats were said to exhibit sign tracking behavior if motion in the cue zone significantly exceeded that in the control zone. A planned comparison t-test conducted on the first cue trial revealed no differences between the cue zone and the control zone (p > 0.62), indicating that the rats did not show an initial preference for one zone over the other. A two-way repeated-measures ANOVA conducted over the five conditioning days/trials revealed a main effect of zone [F(1,152) = 12.69, p < 0.01], but no main effect of time and no interaction (p > 0.27). Thus, rats began sign tracking the heroin-paired cue light with less than five CS–US pairings (Fig. 3, COND).
Sign tracking to a heroin-paired audiovisual cue. Total numbers of motion pixels in the cue zone containing the cue lights and a control zone on the opposing wall were analyzed during "cue on" periods as a measure of sign tracking. Conditioning (COND) consisted of five trials (1 trial/day) where the cue was paired with a heroin infusion (175 μg/inf). Extinction training (EXT) occurred during a single session, consisting of 20 cue presentations, without heroin availability. The test for extinction recall (EXT TEST) was conducted 24 h later. After 3 weeks of home cage abstinence, rats underwent a test for spontaneous recovery (SR TEST). *p < 0.05, **p < 0.01 compared to control zone
Sign tracking to the heroin-paired cue extinguishes when heroin is withheld
The day after the last conditioning session, rats were placed in the experimental chamber for a 63-min extinction session consisting of 20 light-tone presentations in the absence of any heroin infusions. A two-way repeated-measures ANOVA conducted over the five 4-trial blocks indicated a significant main effect of time [F(4,152) = 7.17, p < 0.001], zone [F(1,152) = 14.82, p < 0.001], and a significant interaction [F(4,152) = 3.72, p < 0.01]. Bonferroni post-hoc indicated that rats showed significant sign tracking early in the session, during the first two blocks (Fig. 3, EXT). Thus, within-session extinction of sign tracking can be observed under these parameters. The next day, rats underwent a second extinction session identical to the first, to test for extinction recall. A two-way repeated-measures ANOVA conducted over the five 4-trial blocks indicated a significant main effect of zone [F(1,152) = 14.82, p < 0.001], but no main effect of time and no interaction (p > 0.57). Hence, rats were still exhibiting substantial sign tracking behavior after 2 days of extinction (Fig. 3, EXT TEST). As a stringent test to determine whether they remembered their extinction training from the first day, a two-way repeated-measures ANOVA was conducted over the first four-trial block from each extinction session. As expected, this confirmed that significant sign tracking occurred, indicated by a main effect of zone [F(1,38) = 14.08, p < 0.001]. In addition, a main effect of time [F(1,38) = 8.43, p < 0.01] indicated successful extinction recall (Fig. 4).
Sign tracking early in the session shows extinction and recovery. Total numbers of motion pixels in the cue zone versus control zone during the first block of four trials from each extinction phase are depicted in a cumulative bar graph. Examining responses made early in the session is a stringent test for memory retrieval as it eliminates the contribution of within-session extinction over repeated trials. Rats showed evidence of successful extinction memory retrieval based on reduced sign tracking on EXT TEST relative to EXT. They also showed substantial spontaneous recovery after abstinence, as indicated by the rebound on the SR TEST relative to EXT TEST. **p < 0.01
Sign tracking to the heroin-paired cue exhibits spontaneous recovery
After the second extinction session, rats were returned to their home cages for 3 weeks of forced abstinence from heroin. They were then returned to the experimental chambers for a test of spontaneous recovery. The session was identical to the extinction sessions conducted 3 weeks earlier. A two-way repeated-measures ANOVA conducted over the five 4-trial blocks indicated a significant main effect of time [F(4,136) = 4.66, p < 0.01] and zone [F(1,136) = 15.65, p < 0.001], but no interaction (p > 0.18). Hence, rats were still exhibiting substantial sign tracking behavior after 3 weeks of abstinence (Fig. 3, SR TEST). To determine whether this sign tracking was greater than that observed on the extinction recall test, a two-way repeated measures ANOVA was conducted over the first four-trial block from the extinction recall test and the spontaneous recovery test. As expected, this confirmed that significant sign tracking occurred, indicated by a main effect of zone [F(1,34) = 13.44, p < 0.001]. In addition, a main effect of time [F(1,34) = 9.87, p < 0.01] indicated significant spontaneous recovery (Fig. 4). A comparable analysis conducted on the first extinction session and the spontaneous recovery test also confirmed a main effect of zone [F(1,34) = 14.91, p < 0.001], but no main effect of time and no interaction (p > 0.15), thus indicating that sign tracking spontaneously recovered to pre-extinction levels, without additional "incubation."
Discussion
The present study demonstrates, for the first time, that sign tracking occurs to heroin-associated cues, particularly an audiovisual cue similar to the type used in standard self-administration models. While Pavlovian conditioned approach in the form of conditioned place preference (CPP) has been demonstrated repeatedly for opiates (Bardo and Neisewander 1986; Shippenberg and Elmer 1998; Wise 1989), CPP is distinguishable from sign tracking in that it occurs in response to contextual cues, as opposed to discrete cues, and the underlying neurobiology is distinct (Le Merrer et al. 2012). Although they did not use an explicit sign-tracking paradigm, Su and colleagues (2011) have previously demonstrated that animals will run an alley to obtain heroin reward denoted by an olfactory cue. Our study adds to a growing body of literature confirming the once-disputed notion that sign tracking develops to drug-associated cues, as with food-associated cues (Kearns and Weiss 2004). With our design, sign tracking of the heroin-associated cue occurs very rapidly, with as few as five CS–US pairings. This may be owed, at least in part, to the high dose of i.v. heroin used in this study, although future studies using other doses of heroin will be necessary to confirm this hypothesis.
While there are many reports of sign tracking to reward-associated cues, almost none of these studies attempt to examine extinction of the sign-tracking behavior and its reinstatement after challenge conditions. The closest example of such an undertaking was reported by Kearns and Weiss (2011), who observed extinction of sign tracking for food in context B in rats trained to sign track in context A. Interestingly, the authors did not observe significant renewal when the rats were returned to context A (the day after the last extinction session). The reasons for this are unclear, and may simply be due to an underpowered sample. It is interesting to speculate, however, that extinction of sign tracking for reward-associated cues may transfer across contexts, at least in the short-term, and raises the intriguing possibility that the 3 weeks of forced abstinence imposed in our model may be integral to observing relapse of sign-tracking behavior. It should be noted, however, that in the aforemetioned study, the sign was a lever (with sign tracking measured as the number of lever presses), whereas our study employed an audiovisual cue. Thus, further studies are necessary to determine whether it is appropriate to generalize between these sign tracking models, not to mention the different types of reward (food pellet vs. i.v. heroin).
In our novel sign-tracking model, a single cue extinction session was sufficient to observe successful extinction recall the following day, making this protocol ideal for single pharmacological interventions targeting Pavlovian cue memory for intravenous-heroin reward. Furthermore, the spontaneous recovery we observed after 3 weeks of abstinence was robust. Because spontaneous recovery is thought to reflect, at least in part, extinction failure (Rescorla 2004), this endpoint is an ideal readout for pharmacological interventions aimed at strengthening extinction memory on day 6 of the model. Alternatively the number of cue exposure trials (here 20) could be shortened (i.e., to eight) in order to produce partial extinction, thus increasing the ability to observe pharmacological enhancement of extinction recall on day 7. That we were capable of observing both extinction and spontaneous recovery of this sign-tracking behavior suggests that the Pavlovian cue memory can successfully be studied in isolation from operant learning and memory, which is often superimposed on Pavlovian processes in standard self-administration models of addiction.
Based on the data presented here, we conclude that heroin, like cocaine and alcohol, is another addictive substance capable of eliciting sign-tracking behavior. Like other cued conditioned behaviors, this sign tracking underwent extinction when heroin was withheld and exhibited spontaneous recovery after subsequent abstinence (Quirk 2002). This novel model permits the automated measurement of sign-tracking behavior and is amenable to use in standard self-administration protocols, with some basic considerations about the cue position within the chamber. In theory, this design makes possible the simultaneous measurement of Pavlovian memory and operant memory, using sign tracking (to the audiovisual cue) as a read-out of the former and lever pressing (drug seeking) as a read-out of the latter, within the same paradigm. Future studies will be directed toward understanding how the Pavlovian cue memory intersects with the operant memory to drive cue-induced relapse.
The clinical significance of these findings are twofold: (1) a Pavlovian memory develops very rapidly to discrete cues paired with intravenous heroin delivery and (2) this memory is long-lasting, and recovers after extinction therapy is ceased. Importantly, this memory can be formed independent of any action required to obtain the heroin infusion. Studies in human heroin abusers have suggested that attentional bias to heroin-related cues predicts susceptibility to relapse, and that this attentional bias can be reduced by extinction and other forms of cognitive behavioral therapy (Marissen et al. 2006). Unfortunately, these therapies have not proven effective at protecting against relapse (Marissen et al. 2007). Our study suggests this may be related to the spontaneous recovery of the Pavlovian cue memory when extinction therapy is ceased, or alternatively, to an inability of the Pavlovian extinction memory to efficiently access the neural circuits controlling drug seeking. This underscores the need for pharmacological treatments that can enhance the permanence of extinction memory for opiate cues and/or link the Pavlovian extinction memory trace to seeking circuits.
References
Bardo MT, Neisewander JL (1986) Single-trial conditioned place preference using intravenous morphine. Pharmacol Biochem Behav 25:1101–5
Bastle RM, Kufahl PR, Turk MN, Weber SM, Pentkowski NS, Thiel KJ, Neisewander JL (2012) Novel cues reinstate cocaine-seeking behavior and induce Fos protein expression as effectively as conditioned cues. Neuropsychopharmacology 37:2109–20
Childress AR, McLellan AT, O'Brien CP (1986) Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict 81:655–60
Cunningham CL, Patel P (2007) Rapid induction of Pavlovian approach to an ethanol-paired visual cue in mice. Psychopharmacology (Berl) 192:231–41
Di Ciano P, Everitt BJ (2003) Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci 117:952–60
Di Ciano P, Everitt BJ (2004) Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology 47(Suppl 1):202–13
Drummond DC (2000) What does cue-reactivity have to offer clinical research? Addiction 95(Suppl 2):S129–44
Fuchs RA, Lasseter HC, Ramirez DR, Xie X (2008) Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models 5:251–258
Kearns DN, Weiss SJ (2011) A comparison of explicitly unpaired treatment and extinction: recovery of sign-tracking within a context renewal design. Behav Processes 86:364–7
Kearns DN, Weiss SJ (2004) Sign-tracking (autoshaping) in rats: a comparison of cocaine and food as unconditioned stimuli. Learn Behav 32:463–76
Krank MD (2003) Pavlovian conditioning with ethanol: sign-tracking (autoshaping), conditioned incentive, and ethanol self-administration. Alcohol Clin Exp Res 27:1592–8
Krank MD, O'Neill S, Squarey K, Jacob J (2008) Goal- and signal-directed incentive: conditioned approach, seeking, and consumption established with unsweetened alcohol in rats. Psychopharmacology (Berl) 196:397–405
Le Merrer J, Faget L, Matifas A, Kieffer BL (2012) Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology (Berl) 223:99–106
Lecca D, Valentini V, Cacciapaglia F, Acquas E, Di Chiara G (2007) Reciprocal effects of response contingent and noncontingent intravenous heroin on in vivo nucleus accumbens shell versus core dopamine in the rat: a repeated sampling microdialysis study. Psychopharmacology (Berl) 194:103–16
Lee RS, Criado JR, Koob GF, Henriksen SJ (1999) Cellular responses of nucleus accumbens neurons to opiate-seeking behavior: I. Sustained responding during heroin self-administration Synapse 33:49–58
Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM (2007) Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychother Psychosom 76:97–105
Marissen MA, Franken IH, Waters AJ, Blanken P, van den Brink W, Hendriks VM (2006) Attentional bias predicts heroin relapse following treatment. Addiction 101:1306–12
McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE (2007) Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol 12:503–12
Price KL, Saladin ME, Baker NL, Tolliver BK, DeSantis SM, McRae-Clark AL, Brady KT (2010) Extinction of drug cue reactivity in methamphetamine-dependent individuals. Behav Res Ther 48:860–5
Quirk GJ (2002) Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem 9:402–7
Rescorla RA (2004) Spontaneous recovery. Learn Mem 11:501–9
Sanchez H, Quinn JJ, Torregrossa MM, Taylor JR (2010) Reconsolidation of a cocaine-associated stimulus requires amygdalar protein kinase A. J Neurosci 30:4401–7
Shippenberg TS, Elmer GI (1998) The neurobiology of opiate reinforcement. Crit Rev Neurobiol 12:267–303
Sinha R, Li CS (2007) Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev 26:25–31
Su ZI, Wenzel J, Baird R, Ettenberg A (2011) Comparison of self-administration behavior and responsiveness to drug-paired cues in rats running an alley for intravenous heroin and cocaine. Psychopharmacology (Berl) 214:769–78
Torregrossa MM, Sanchez H, Taylor JR (2010) D-cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J Neurosci 30:10526–33
Uslaner JM, Acerbo MJ, Jones SA, Robinson TE (2006) The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res 169:320–4
Wise RA (1989) Opiate reward: sites and substrates. Neurosci Biobehav Rev 13:129–33
Yager LM, Robinson TE (2013) A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 226:217–28
Acknowledgments
The authors thank Jasper A. Heinsbroek for editing the Supplemental Video and Dr. Matthew W. Feltenstein for helpful advice on the use of the backpack catheters.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
This video illustrates the acquisition of sign-tracking behavior from day 1 of Conditioning to day 5. The video has been edited to show only the "cue on" periods ±10 s before and after the cue. Sign tracking is absent on day 1, but evident in all four rats from this quad unit on day 5. Bouts of sign tracking are indicated by white arrows. (MP4 31427 kb)
Rights and permissions
About this article
Cite this article
Peters, J., De Vries, T.J. Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology 231, 447–453 (2014). https://doi.org/10.1007/s00213-013-3258-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3258-7