Abstract
Rationale
Discriminative stimuli (DS) are cues that predict reward availability. DS are resistant to extinction and motivate drug seeking even after long periods of abstinence. Previous studies have demonstrated that sign-tracking (ST) and goal-tracking (GT) differences in Pavlovian approach predict distinct cue-modulated vulnerabilities to cocaine reinstatement. GT rats show heightened reinstatement to contextual and DS, while ST rats show heightened reinstatement to discrete stimuli. Here we examine whether DS modulate reinstatement after electric barrier-induced abstinence and whether tracking-related relapse vulnerabilities generalize to opioid relapse.
Objectives
We examine whether DS-modulated reinstatement to fentanyl seeking persists in the presence of reduced adverse consequences after electric barrier-induced abstinence. We also examine whether tracking differences predict the magnitude of DS-modulated reinstatement of fentanyl seeking after electric barrier-induced abstinence.
Methods
We used Pavlovian lever autoshaping (PLA) training to determine sign-, goal-, and intermediate tracking groups in male and female Sprague Dawley rats. We then trained rats in a DS model of intermittent fentanyl self-administration, and extinguished drug seeking by imposing an electric barrier of increasing intensity. We then measured the level of DS-modulated reinstatement in the presence of a reduced electric barrier intensity.
Results
We report that DS strongly modulate fentanyl seeking after electric barrier-induced abstinence. DS–modulation of fentanyl acquisition, electric barrier-induced abstinence, and reinstatement was similar for sign- and goal-tracking groups.
Conclusions
Discriminative stimuli powerfully motivate opioid seeking, despite continued aversive consequences. Pavlovian approach differences do not predict the level of DS-modulated reinstatement to fentanyl seeking after conflict-induced abstinence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conditioned stimuli (CS) motivate reward seeking behavior when animals learn their association with unconditioned stimuli (US) such as food or drugs. Individual reactivity to food-associated CS predicts drug seeking behaviors in models of addiction vulnerability (Flagel et al. 2010; Saunders and Robinson 2010). Sign-tracking (ST) and goal-tracking (GT) individual differences in Pavlovian CS approach behavior predict distinct relapse vulnerabilities for cocaine seeking (Saunders and Robinson 2010; Saunders et al. 2014), but this is not the case for remifentanil seeking (Chang et al. 2022). While reinstatement to contingent CS induced drug seeking persists despite negative consequences (Saunders et al. 2013), it is unclear if discriminative stimuli (DS), which predict US availability, also promote reinstatement despite negative consequences. Here, we determine whether DS-modulated relapse to fentanyl seeking persists following conflict-induced abstinence and whether ST and GT behaviors predict the magnitude of reinstatement effects.
The temporal relationships of CS relative to US delivery determine how stimuli affect behavior (Di Ciano and Everitt 2003). Discriminative stimuli (DS) predict when a reward seeking response will produce the US, whereas contingent CS are present only after a reward is earned. Both DS and contingent CS stimulate reward seeking, but they function through different mechanisms (Di Ciano and Everitt 2003; Namba et al. 2018). DS function similarly to contexts, informing when rewards are available, whereas contingent CS function as conditioned reinforcers. DS paired with cocaine resist extinction and promote drug seeking that escalates with abstinence (Weiss et al. 2001; Madangopal et al. 2019). In humans, DS may be important drivers of relapse to drug seeking, as exposure to cues predictive of drug availability occurs before relapse whereas contingent interoceptive and environmental stimuli associated with drug taking affect behavior after relapse has occurred. Therefore, defining the conditions that influence DS control of drug-seeking behavior will increase our understanding of drug relapse. The present study aims to determine whether DS predictive of opioid availability trigger relapse after negative consequences are imposed.
Sign- and goal-tracking individual differences in approach toward a food-associated CS correlate with differences in behaviors motivated by drug-associated CS and DS in relapse models (Saunders and Robinson 2010; Saunders et al. 2013, 2014). In Pavlovian lever autoshaping (PLA) procedures, a lever cue predicting food motivates either lever pressing, termed sign tracking (ST), or food-magazine exploration, termed goal tracking (GT). Previous work shows that individuals that preferentially exhibit ST to a food cue are more prone to both food (Yager and Robinson 2010) and cocaine (Saunders and Robinson 2010) reinstatement when a discrete CS is paired with delivery of reward during training, whereas GT individuals are more susceptible to contextual and DS-induced reinstatement of cocaine seeking (Saunders et al. 2014; Pitchers et al. 2017). These relationships support the hypothesis that individual differences in incentive salience attribution to CS predict reinstatement of reward seeking across diverse US (cocaine and food), and that the type of cue (predictive DS vs. contingent CS) may dictate whether ST or GT rats are more susceptible to reinstatement. However, it remains unknown whether patterns of reinstatement vulnerability to predictive cues (DS) are stable across other US types, such as for opioids.
The conflict aspect of the model we employ in this study uses a footshock barrier of escalating intensity to extinguish drug taking (Cooper et al. 2007), followed by reinstatement of DS-modulated drug seeking in the presence of reduced footshock barrier intensity. The conflict-induced abstinence phase is designed to model the aversive consequences of drug seeking and drug taking that fluctuate in humans. Here, we examine whether DS-modulated reinstatement to fentanyl seeking persists after conflict-induced abstinence and whether ST and GT predict sensitivity to DS-modulated relapse to opioid seeking despite conflict. Based on prior studies with psychostimulants (Pitchers et al. 2017), we predicted that GT individuals would exhibit greater DS-modulated reinstatement of fentanyl seeking even in the presence of the shock barrier.
Materials and methods
Subjects
Male and female Sprague–Dawley rats (Charles Rivers Laboratories, Wilmington, MA; 200–275 g upon arrival; n = 56 run as 2 separate cohorts) were 8 weeks old and triple housed with same-sex cagemates upon arrival. Rats were maintained on a 12-h reverse light/dark cycle (lights off at 10:00 am), and all behavioral training and testing was conducted during the dark phase of the cycle. Rats had ad libitum access to standard laboratory chow and water throughout all phases of the experiment. Rats were single housed after acclimation and prior to behavioral training. All behavioral experiments were performed in accordance to the “Guide for the Care and Use of Laboratory Animals” (8th edition, 2011, US National Research Council) and were approved by the University of Maryland, School of Medicine Institutional Animal Care and Use Committee (IACUC).
Catheterization surgery
After establishing tracking phenotype with Pavlovian lever autoshaping (see below), rats were anesthetized with isoflurane (5% induction, 1–3% maintenance) and catheters implanted into the right jugular vein. The catheter was made from Silastic tubing (cat#508–002, Dow Silicones Corp, Midland, MI, USA), subcutaneously inserted, and affixed to the 22-gauge guide stainless steel backmount cannula (PlasticsOne, Roanoke, VA, USA) that protruded through a small back incision. The non-steroid, anti-inflammatory drug, carprofen (5 mg/kg, Rimadyl ®) was subcutaneously administered prior to surgery and for three days after surgery. Rats were infused daily with 0.05 mL of anti-microbial, anti-bacterial, and anti-coagulant Taurolidine-Citrate (TCS) catheter lock solution i.v. (Cat# TCS-04, Access Technologies, IL, United States) to reduce biofilm and clot formation, to promote catheter patency, and to reduce the risk of microbial infection throughout the experiment. Catheter patency was checked periodically via i.v. injections of 0.1 mL of methohexital sodium (“Brevital”), and rats without a sudden loss of muscle tone were removed from the study (n = 8).
Drugs
Fentanyl citrate from Cayman Chemical was diluted in 0.9% sterile saline to 1 mg/mL before further diluting in 20 ml syringes to concentrations scaled to each rat’s weight for a dose of 1 μg/kg/injection.
Behavioral procedures
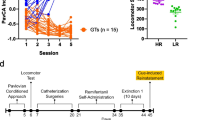
Experimental design is outlined in Fig. 1A. Behavioral experiments were conducted in identical behavioral chambers (25 × 27 × 30 cm; Med Associates) located in rooms different than the colony room. Each chamber was located in individual sound-attenuating cubicle with a ventilation fan. Each chamber had a red house light (6 W) located at the top of the wall opposite the experimental stimuli.
Pavlovian lever autoshaping
Apparatus
For Pavlovian lever autoshaping, the red house light was illuminated for the duration of each session. The opposite wall had a recessed food cup located 2 cm above the grid floor. The food cup had an attached programmed pellet dispenser to deliver 45 mg food pellets (catalog#1,811,155; Test Diet Purified Rodent Tablet (5TUL); protein 20.6%, fat 12.7%, carbohydrate 66.7%). A retractable lever was positioned on either side of the food cup 6 cm above the floor, and side was counterbalanced between subjects.
Behavioral procedure
Rats were habituated to the food pellets prior to training. Then, rats were trained for five daily, ~ 26 min Pavlovian lever autoshaping (PLA) sessions. Each session included 25 presentations of a lever presentation that served as the conditioned stimulus (CS) and occurred on a VI 60 s schedule (50–70 s). The lever was inserted for 10 s for each trial, retracted, and followed immediately with the delivery of two food pellets into the food cup. Food delivery occurred independent of lever or food cup approach or contact. After each training session, rats were transported back to the colony room.
Behavioral measurements
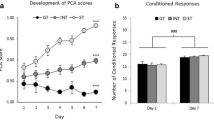
A Pavlovian Conditioned Approach (PCA) analysis (Meyer et al. 2012) was used to determine sign- and goal-tracking groups. PCA quantifies the continuum of lever-directed (sign tracking, ST) and food cup-directed (goal tracking, GT) behaviors. A PCA score is calculated for each rat and is the average of three difference score measures, including (1) preference score, (2) latency score, and (3) probability score, each ranging from –1.0 to + 1.0. For preference score, total number of contacts were recorded with the lever and food cup during the CS. The calculation of preference score was the number of lever contacts during the CS minus the number of food cup contacts during the CS, divided by the sum of these two measures. Latency to first contact to lever and food cup during the CS was recorded, and if contact did not occur, a latency of 10 s was recorded. The calculation of latency score was the average latency to make a food cup contact during the CS minus the latency to lever contact during the CS, divided by the duration of the CS (10 s). Lever and food cup probabilities were calculated by determining the number of trials that the response was made divided by total number of trials in the session. The calculation for the probability score was the probability of a lever contact minus the probability of a food cup contact throughout the session. PCA scores were averaged during session 5 to determine tracking groups. Sign-tracking PCA scores range from + 0.33 to + 1.0, goal-tracking PCA scores range from –0.33 to –1.0, and intermediate PCA scores range from –0.32 to + 0.32.
Fentanyl self-administration, discriminative training, conflict, and relapse
Apparatus
In a separate room from PLA training, rats were trained in self-administration chambers contained in sound attenuating cabinets (Med Associates) similar to PLA training, but stimuli were different from PLA to not confound actions between trainings. One wall contained two nose pokes located 5 cm above the grid floor with a white light located between them 10 cm above the grid floor. A red light was located at the top of the wall on the opposite side. The side of the active nosepokes were counterbalanced for each tracking group relative to the side of the lever during PLA training.
Self-administration acquisition and discriminative stimulus training
After a 7- to 12-day recovery from catheterization surgery, rats were trained in 5 sessions to self-administer fentanyl for 2 h per session. A nose poke into the active poke activated the syringe pump to deliver 1 ug/kg fentanyl in 28 μl over 1 s on a fixed ratio 1 schedule. A nose poke into the inactive poke was recorded, but no fentanyl was delivered. No stimuli were paired with nosepokes or drug infusions, and no lights were turned on during initial training. As in prior tracking studies (Saunders et al. 2013; Pitchers et al. 2017), an infusion maximum (IMax) capping the maximum number of infusions/session was imposed to limit differences in acquisition of self-administration between tracking groups. The IMax was 10 infusions/session for two sessions (IMax10), 20 for two sessions (IMax20), and 40 for one session (IMax40). Each rats’ session was concluded either when reaching IMax or at 2 h from session start, whichever came first. The IMax10 and IMax20 sessions used a 20-s timeout following each infusion during which active nosepokes were recorded but did not result in additional infusions. The IMax40 session and all subsequent discriminative stimulus (DS) sessions used a 1-s timeout corresponding to the length of the infusion.
After 5 IMax self-administration acquisition sessions, rats were trained in 10 sessions using an intermittent access (IntA) schedule with two distinct discriminative stimuli signaling drug availability (DS +) or non-availability (DS–). These sessions began with 2 min illumination of the red house light (DS–) followed by a 5 min illumination of a white light (DS +) located on the opposite wall between the two nose pokes. During the DS + , a response into the active nose poke resulted in delivery of fentanyl on a fixed ratio 1 schedule with 1-s timeout. A limit of seven infusions per 5 min DS + was imposed to avoid the potential for overdose. After the 5 min DS + , the white light turned off, and the DS– red light illuminated for 25 min signaling drug was unavailable. This pattern was repeated 3 more times before the session ended following a final DS + period. The total session length was 127 min, consisting of five, 5-min DS + periods, and four, 25-min DS– periods between them, in addition to the first, 2-min DS– period. All active and inactive nose pokes were recorded during the session.
Conflict
A conflict-induced abstinence model was used that introduced a negative consequence of increasing footshock intensity to decrease drug-seeking and taking behaviors while the reinforced DS schedule maintained. Sessions were similar to IntA sessions with DS + and DS–, except an electric current was constantly applied to the two-thirds of the grid floor closest to the nose pokes throughout the entire session. As a result, rats had to traverse the electric grid to nose poke and receive fentanyl infusions. Rats were trained in four daily sessions of conflict IntA sessions, with the footshock intensity set to either 0.15, 0.20, 0.25, or 0.30 mA using an aversive stimulator (Med Associates). All rats started at 0.15 mA, and if they received more than 5 infusions at this shock intensity, shock intensity was increased by 0.05 mA the following session. If they received less than 5 infusions, the same shock intensity was repeated the following session. Rats remained in their colony room for a week after the fourth day of conflict prior to reinstatement test.
Reinstatement test
After 1 week in their home cages with no testing, rats were tested in a reinstatement test under extinction (no drug available) conditions, although the animals were still tethered to a drug delivery line. Similar to conflict training, the two-thirds of the grid floor closest to the nose pokes were electrified, but to 50% of each rat’s maximum shock intensity reached during conflict training, consistent with a prior tracking study investigating CS-induced relapse after conflict-induced abstinence (Saunders et al. 2013). DS + and DS– periods were shorter in duration than during training (30 s and 150 s, respectively), but the ratio of DS + /DS– durations was identical. After an initial 2 min DS– to begin the session, the DS + was on for 30 s followed by the DS– for 150 s. The session ended after 21 DS–/DS + cycles (62.5 min total). Nose pokes into the active and inactive ports were recorded.
Statistical analysis
One- and two-way ANOVAs were employed where appropriate. Repeated measures were employed appropriately for mixed within-subject/between subject designs. Sphericity corrections were performed where appropriate with Greenhouse-Geiser corrections. Pearson’s correlation analysis was performed to correlate PCA scores with reinstatement behavior. Tests were performed in Prism (GraphPad). All data shown is that from rats that completed the entire study (n = 28). The remainder were either not surgerized following PLA testing (n = 12), lost catheter patency before finishing the study (n = 8), failed to reliably self-administer fentanyl (> 5 infusions/session; n = 3), or became sick and were removed the study (n = 5). A discrimination score was calculated to measure the response rate transitions between adjacent DS– and DS + bins by using the average response rates in the two bins surrounding all such transitions in a session:
Results
Acquisition of Pavlovian autoshaping
Prior to any drug experience, we screened male and female rats using PLA to classify their tracking phenotype as sign tracking (ST), goal tracking (GT), or intermediate (INT). Pavlovian conditioned approach (PCA) scores serve as a comprehensive index summarizing the number of contacts, latency to contact and probability to contact the lever or food cup across the session (Fig. 1A). We classified rats based on their performance on session 5 of training (see Methods for details). We analyzed the PCA scores over the five days using a mixed-design, repeated measures ANOVA, with a between-subject factor of tracking group (ST (n = 7), GT (n = 16), INT (n = 5)) and a within-subject factor of session (Fig. 1B). Based on session 5 characterization, we observed an interaction between tracking group and session (F(8,100) = 16.54, p < 0.0001), indicating that behavior motivated by a food-predictive lever stimulus developed differently between the assigned tracking groups, as expected (Fig. 1B). The differences in PCA scores are characterized by increases in pressing across sessions in STs, but not GTs (Fig. 1C), and an increase in pokes in GTs, but not STs (Fig. 1D).
Acquisition of fentanyl self-administration
After determination of tracking groups, we implanted intravenous jugular catheters in ST, GT, and INT rats. After recovery we trained rats to nosepoke for fentanyl infusions (1 μg/kg/infusion) in five 2-h sessions. To ensure there were no tracking-related differences in the initial acquisition of fentanyl self-administration, we imposed an infusion maximum (IMax) capping the total number of infusions/session to 10, 20, or 40. No lights (used later during DS training) were used during acquisition. No cues were explicitly paired with drug infusions, although the activation of the syringe pumps is audible. All rats included in the study reliably discriminated active from inactive nosepokes (Fig. 2A), as shown by a main effect of nosepoke (active vs inactive) (F(1,54) = 19, p < 0.0001) and an interaction between nosepoke and session (F(4,108) = 4.334, p = 0.0027). Active poking increased between session 1 and session 5 (p = 0.0007, Dunnett’s test), whereas inactive poking remained similar between session 1 and 5 (p = 0.9438, Dunnett’s test).
Fentanyl motivated behavior for all rats combined. A Fentanyl self-administration through acquisition (five sessions) and DS training phases (ten sessions). B Rates of responding in the DS + and DS– components of DS training sessions, binned by the first five and last five sessions of DS discrimination phase. C Day 15 of DS training. Data are binned into 1 min segments across the 25 min DS– component and 5 min DS + component, and the resulting data are averaged across all DS–/DS + cycles across the session. Inset shows the individual data for the transition between the last bin of the DS– component and the first bin of the DS + component (**p < 0.01, paired t-test). D Conflict extinction behavior in the presence of electrified floor barrier in front of nosepokes. E Poking rates (inactive/active) for the last conflict extinction test (left two columns) and reinstatement test (right two columns). F Poking rates (DS–/DS +) for the last conflict extinction test (left two columns) and reinstatement test (right two columns). G Reinstatement test poking rate data binned into 30 s segments across the 2.5 min DS– component and 30 s DS + component. Data are averaged across all DS–/DS + cycles in session. Inset shows the individual data for the transition between the last bin of the DS– component and the first (only) bin of the DS + component (***p < 0.001, paired t-test)
Discriminative stimuli training
After initial fentanyl self-administration training, we introduced discriminative stimuli to distinguish drug available (ON) from non-available (OFF) periods. The DS + (signaling ON periods) was a white light in between the two nosepokes, and the DS– (signaling OFF periods) was a red light on the back wall. Overall, rats maintained active and inactive nosepoke discrimination throughout DS training (main effect of Nosepoke: F(1,27) = 45.32, p < 0.0001), and increased the number of infusions received across DS sessions (one-way ANOVA; effect of session: F(4.67,126.2) = 10.56, p < 0.0001) (Fig. 2A). Importantly, rats reliably learned to discriminate DS + and DS– stimuli over the course of the ten DS sessions (Fig. 2B). This learning is partially captured by higher DS + active poking rates relative to DS– rates overall (main effect of stimulus (DS + , DS–): F(1,27) = 13.56, p = 0.001), as well as by a stimulus (DS + , DS–) × session interaction (F(1,27)=15.92, p < 0.001).
To further investigate how rats responded to the discriminative stimuli, we examined behavior on a finer timescale by separately binning DS– (25 min) and DS + (5 min) periods into 1-min bins and averaging across all DS–/DS + cycles within a DS session. In the first DS session (session 6), before rats have learned the stimuli, we observe a relatively uniform distribution of responding over time across the DS–/DS + cycle (Fig. S1A). In particular, we observe no difference between the last minute of responding during the DS– period and the first minute of responding during the DS + period (Fig. S1A, inset). These two minutes capture the transition from DS– OFF to DS + ON periods, when the white light turns on to signal fentanyl availability. In contrast, in the last DS session (session 15), responding slowly increases across the DS– period before suddenly increasing in the first minute of DS + periods (p = 0.003, paired t-test; Fig. 2C, inset). These data indicate that by the final DS session, fentanyl seeking behavior was under the control of the DS schedule.
Conflict-induced abstinence of fentanyl responding
Following ten sessions of DS training, we maintained the DS schedule of fentanyl reinforcement but imposed an electrified barrier in front of the nosepoke apparatus. We applied constant footshock to the front 2/3 of the floor grid, such that rats had to move across the electrified floor to reach the nosepokes. Consistent with a prior conflict relapse study investigating tracking differences (Saunders et al. 2013), we increased footshock intensity incrementally across 4 sessions (0.15, 0.20, 0.25, or 0.30 mA) if the number of infusions earned in the prior session did not drop below 5. If the number of infusions earned dropped below 5, shock intensity remained constant for the next session(s). We observed a marked decrease in infusions earned across the four sessions (RM one-way ANOVA, effect of session: F(2.64,71.3) = 24.43, p < 0.0001) as well as a decrease in all responses (main effect of Session: F(3,81) = 5.076, p = 0.0029) (Fig. 2D).
DS + modulated reinstatement of responding
Rats spent 1 week in their home cages with no testing prior to a DS reinstatement test. For this test, we used ½ maximum shock intensity reached for each rat during conflict-induced abstinence, consistent with prior CS conflict relapse study (Saunders et al. 2013). We tested rats under extinction conditions (no drug available). Overall, active responding rates dramatically increased during reinstatement testing compared with the final day of conflict-induced abstinence (Fig. 2E). We observed significant main effects of response (active vs. inactive) (F(1, 27) = 23.27, p < 0.0001) and session (conflict-induced abstinence vs. relapse test) (F(1,27) = 18.28, p = 0.002), as well as a significant interaction between response and session (F(1,27) = 21.50, p < 0.0001). While both active and inactive poking rates significantly increased during reinstatement relative to the last conflict session, the magnitude of the increase was higher for active rates (inactive Δrate: + 0.35 responses/min; active Δrate: + 2.05 responses/min, p < 0.0001, paired t-test) (Fig. S1B). During the reinstatement test, active poking rate during the DS + periods was significantly elevated relative to the final day of conflict-induced abstinence (Fig. 2F). We observed main effects of stimulus (DS + , DS–) (F(1,27) = 21.29, p < 0.0001) and session (F(1, 27) = 26.06, p < 0.001), as well as a stimulus × session interaction (F(1,27) = 19.99, p = 0.0001). While both DS + and DS– poking rates significantly increased during reinstatement relative to the last conflict session, the magnitude of the increase was higher for DS + rates (DS– Δrate: + 1.43 responses/min; active Δrate: + 5.13 responses/min, p = 0.0001, paired t-test) (Fig. S1B). We observed a > 50 fold increase in active nosepoking rate and > 65 fold increase DS + poking rate in the reinstatement vs. final conflict session (Fig. 2E–2F). These data indicate that the change in conditions during the reinstatement test (reduced shock, extinction conditions, and 1 week home cage abstinence) led to an increase in responses during the reinstatement test that was strongly modulated by the DS.
To further investigate the pattern of DS responding, we analyzed the reinstatement test data by binning DS– periods (150 s) and DS + periods (30 s) into 30-s bins, and averaging binned poking rates across all 21 equivalent DS–/DS + cycles (Fig. 2G). Similar to our Day 10 training data, we observed an increase in active pokes between the last DS– bin and the first DS + bin (p < 0.0001, paired t-test) (Fig. 2G, inset). These data further indicate that discriminative stimuli associated with fentanyl availability powerfully modulate drug seeking following conflict-induced abstinence in the face of attenuated but continued conflict.
Tracking and sex as factors
To assess tracking type and sex as factors during DS discrimination training, conflict-induced abstinence, and DS–modulated reinstatement of fentanyl seeking, we compared responding across all experimental phases, independently considering tracking type and sex as factors. The statistics for these comparisons are summarized in Table 1.
During acquisition and DS training, we found no differences in infusions earned between tracking groups and no tracking and session interactions (Fig. 3A). Similarly, we found no main effects of tracking or interactions between tracking and session on active poking rates during acquisition or during DS training (Fig. S1C) .
Fentanyl acquisition and DS training by tracking group and sex. A Fentanyl self-administration through acquisition (five sessions) and DS training phases (ten sessions) by tracking group. B Fentanyl self-administration through acquistion and DS training by sex. C Rates of responding in the DS + and DS– components of DS training sessions, binned by the first five and last five sessions of DS discrimination phase by tracking. D Rates of responding in the DS + and DS– components of DS training sessions binned by the first five and last five sessions of DS discrimination phase by sex
Collapsing across tracking groups to compare the sexes, we found no differences in acquisition response rates and infusions, nor in DS training response rates or infusions, with respect to sex (Fig. 3B, S2D). These data indicate that neither tracking nor sex was associated with differences in acquisition of fentanyl self-administration, which may in part be due to the IMax schedule we imposed. Furthermore, by the end of DS training, all tracking groups and both sexes were nearly identical in average number of infusions earned per session (Fig. 3A–B).
To understand potential differences between tracking groups in DS discrimination, we compared the ratio of DS + / DS– responding between early (sessions 6–10) and late (sessions 11–15) DS training (Fig. 3C). While we found a main effect of session, we found no main effects of tracking, nor a tracking by session interaction, indicating that all three groups’ relative ratios of DS + /DS– responding increased similarly across training (Fig. 3C). To understand potential differences between sexes in DS discrimination, we compared the ratio of DS + /DS– responding between early and late sessions and found no significant effects of sex nor any interaction between Sex and Session (Fig. 3D). Taking into account that the DS–:DS + transition is best captured by the rate of responding between the last DS– bin and first DS + bin of DS–/DS + cycles (see above), we compared these transitions between tracking types using a discrimination score (see the “Methods” section), and again found no significant differences in discrimination score between tracking groups or sexes on the last day of training (Fig. S1E), indicating all tracking groups expressed DS discrimination similarly.
As conflict training progressed, all tracking groups reduced their infusions earned (Fig. 3A) and active responding (Fig. 3B) across sessions and we found no main effects of tracking nor interactions of tracking with session during conflict-induced abstinence. We also compared the increases in shock intensities across tracking groups (Fig. 3C), and again found no main effects of Tracking, nor any significant interactions. We also compared across sexes for infusions (Fig. 3D), active responding (Fig. 3E), and shock intensities (Fig. 3F). Again, we found no main effects of sex, nor any interactions of sex with session for any of these three measures. These data indicate all tracking groups and both sexes were similarly sensitive to conflict-induced abstinence of fentanyl seeking (Fig. 4) .
Conflict extinction behavior by tracking group and sex. A Conflict extinction infusions by tracking. B Conflict extinction active pokes by tracking. C Average shock intensity used across sessions by tracking. D Conflict extinction infusions by sex. E Conflict extinction active pokes by sex. F Average shock intensity used across sessions by sex
In order to examine whether tracking group influenced responding after conflict-induced abstinence, we compared the active nosepoke response rates (Fig. 5A) and DS + response rates (Fig. 5B) between tracking groups and across the final conflict session and the reinstatement test session. Although we found a large main effect of session, we found no main effects of tracking, nor a session by tracking interaction (see Table 1 for statistics). We further examined whether there was any linear relationship between PCA score and active poking rate during reinstatement and found no significant relationship (R2 = 0.102, p = 0.0971) (Fig. 5C). The weak trend for a larger magnitude reinstatement effect in sign-tracking rats was mostly driven by a single rat (see Fig. 5C). We also compared active response rates (Fig. 5D) and DS + response rates (Fig. 5E) between the sexes and across the final conflict session and reinstatement session. Again, we found no main effects of sex, nor any sex by session interactions on these measures. Comparing tracking groups across the DS– periods, we binned data into 30-s bins across DS– periods (150 s) and DS + periods (30 s), and averaged binned rates across all 21 equivalent DS–/DS + cycles (Fig. S1F). We found no main effect of tracking group on active poking rate across the DS– period (F(2,25) = 2.109, p = 0.1424), nor a bin × tracking group interaction (F(8,100)=1.425, p = 0.1954). We also compared DS discrimination across transitions between tracking groups and sexes by calculating a discrimination score during reinstatement, and found no difference between tracking groups on this measure (Fig. 5F). We observed a modest decrease in discrimination score in females (p = 0.037, t-test) (Fig. 5F). These data suggest that although the DS + serves as a powerful fentanyl reinstatement stimulus in the face of continued but reduced conflict, this effect does not vary by tracking type, though it may be reduced in females.
Reinstatement behavior by tracking group and sex. A Active side poking rates during final conflict session (left) and reinstatement test (right) by tracking. B DS + poking rates during final conflict session (left) and reinstatement test (right) by tracking. C Linear correlation between PCA score and active poking rate (R2 = .1023, p = 0.0971). D Active side poking rates during final conflict session (left) and reinstatement test (right) by sex. E DS + poking rates during final conflict session (left) and reinstatement test (right) by sex. F Reinstatement DS scores by tracking (left, black bars) and by sex (right, gray bars) (‘*’ indicates p = 0.037, t-test)
Discussion
We first classified rats as ST, GT, or INT by their Pavlovian approach behaviors and then trained them to nosepoke to self-administer fentanyl. We then introduced an intermittent access schedule in which ON periods were signaled by a DS + , and OFF periods were scheduled by a DS–. Over the 10 days of DS training, rats learned the relationship between the DS and drug availability, as evidenced by a sudden ramping of drug seeking behavior at the onset of the light DS + . Under conditions of conflict between escalating shock intensity and continued fentanyl reinforcement, all rats reduced their drug seeking and intake to very low levels as shock intensity increased. In accordance with our predictions, following a week of homecage abstinence, we observed robust reinstatement under extinction conditions with reduced-intensity shock (1/2 maximum shock intensity), particularly during DS + periods. Additionally, we compared the development of DS-modulated drug taking, conflict-induced abstinence, and reinstatement across ST, GT, and INT rats. Throughout all stages of our experiments, and contrary to our expectations, we found no significant differences between rats across tracking groups. Males and females performed similarly throughout the experiments, with the exception that females exhibited a slightly smaller relative increase in response rates at the transition between DS– and DS + periods during the reinstatement test.
Our experimental conditions combine conflict-induced abstinence designs (Cooper et al. 2007; Saunders et al. 2013) that model adverse consequences associated with human drug use and DS designs, which model environmental cues signaling whether drugs are available. Our primary goal in employing this design was to determine if DS + stimuli produce robust modulation of opioid seeking following conflict-induced abstinence under conditions of continued, but reduced, conflict. Indeed, we observed very high rates of drug-seeking behavior during the reinstatement test, and this drug seeking was highly concentrated in the DS + periods.
Importantly, several factors change during the reinstatement test, including the introduction of reduced shock levels, extinction conditions, and a period of homecage abstinence. All of these are expected to increase responding during reinstatement, and we do observe increases in all response types (active, inactive, DS–, and DS +) during reinstatement, with DS + responses increasing the most. However, we cannot determine from these data the degree to which the above factors (extinction conditions, shock level, and abstinence time) interact with the DS to produce the response rates observed at reinstatement. These factors could override potential differences in DS– modulation of behavior that would otherwise arise between ST and GT using more traditional extinction/reinstatement study designs.
Many studies extinguish responding using extinction conditions prior to reinstatement, usually in the absence of paired cues that are reintroduced during reinstatement tests. In these relapse models, associations between responses, cues, and the US are altered prior to testing in a manner that likely diverges from reductions in human drug taking, in which extinction conditions are rarely introduced. To realize potential advantages of the current paradigm to model relapse following conflict-mediated reductions in drug-taking, further studies should determine the contribution of the relevant parameters discussed above to reinstatement behavior.
Many studies have shown that DS + stimuli modulate reward seeking responses (e.g. Dinsmoor 1950; McFarland and Ettenberg 1997). Drug-associated DS in particular motivate behavior that is resistant to extinction (Martin-Fardon and Weiss 2017) and increases with the passage of time (Madangopal et al. 2019). Our results here add to previous results by showing that DS + associated with opioid availability motivate vigorous drug seeking behavior in the face of continued conflict.
In addition to establishing robust DS + reinstatement of fentanyl seeking under conflict, we sought to compare and correlate the intensity of reinstatement across individuals with differences in PCA behavior. Numerous studies have found that sign- and goal-tracking behaviors in response to a food cue predict the intensity of cue-induced reinstatement of drug taking – in a cue-dependent fashion (Robinson et al. 2014). While sign tracking correlates with discrete contingent cue-induced reinstatement (Saunders and Robinson 2010), goal tracking has been associated with contextual reinstatement (Saunders et al. 2014) and DS + reinstatement (Pitchers et al. 2017). Directly relevant to our study, recent work has demonstrated a significantly greater DS + mediated reinstatement of cocaine seeking in goal tracking relative to sign-tracking rats (Pitchers et al. 2017). Therefore, the lack of difference between tracking groups in our work is surprising and may be due to one or a combination several differences in the studies, which we explore below.
Importantly, our study used the opioid agonist, fentanyl, as the US whereas the previous study (Pitchers et al. 2017) used the psychostimulant, cocaine. Many studies to date linking PCA behaviors to differential drug-related behaviors have used cocaine as a reinforcer. In addition to established relationships of PCA behavior to cocaine reinstatement (Saunders and Robinson 2010; Saunders et al. 2014; Pitchers et al. 2017), sign tracking predicts choice of cocaine over food (Tunstall and Kearns 2015), sensitivity to cocaine psychomotor sensitization (Flagel et al. 2008), and acquisition of cocaine self-administration (Beckmann et al. 2011). Additionally, sign tracking predicts contingent CS-induced reinstatement to methamphetamine (Everett et al. 2020) and nicotine (Versaggi et al. 2016). However, a recent study using the opioid remifentanil found no difference in contingent CS-induced reinstatement of drug seeking between ST and GT rats (Chang et al. 2022). These results are consistent with the present findings for DS-modulated relapse to opioid seeking. When considered together, these studies suggest that drug class (psychostimulant versus opioid) may be an important factor in determining cue-induced reinstatement across tracking groups.
Neither our results nor the results of Chang et al. (2022) were predicted, as heroin (Peters and De Vries 2014), remifentanil (Yager et al. 2015), and cocaine (Yager and Robinson 2013) similarly support approach to Pavlovian drug-paired cues. Pavlovian drug-associated cue approach is magnified in individuals that ST to a food-predictive lever (Yager and Robinson 2013; Yager et al. 2015). However, the inability of PCA behavior to predict DS and CS mediated reinstatement suggests that Pavlovian sign tracking and reinstatement of opioid seeking in an operant context are unrelated processes (Chang et al. 2022). Interestingly, when using a lever as the Pavlovian cue predictive of drug reward, cocaine supports lever approach (Uslaner et al. 2006), but only heroin supports significant lever pressing (i.e. sign tracking) (Madsen and Ahmed 2015). We further note that PCA behavior to food cues may not correlate in every case to PCA behavior using different US, as previous work showed that sign tracking for liquid sucrose showed no relationship to sign tracking for food (Patitucci et al. 2016), and that sign tracking for food was not related to visual nicotine cue approach (Yager and Robinson 2015). Clearly, further investigation into the importance of the US in the development of PCA behavior is warranted to understand the relationship between PCA behavior and reinstatement using diverse US.
In addition to the US used, our study also differs methodologically with earlier work. To extinguish drug-seeking responses, we used conflict conditions in the presence of continued drug availability under the DS schedule. In contrast, the previous DS study removed the drug and DS stimuli during classic extinction procedures in which drug was not available (Pitchers et al. 2017). While in both cases, drug seeking is similarly decreased, the associative processes underlying decreased responding are fundamentally different. Therefore, it is conceivable that differences in reinstatement between tracking groups emerged due to the number or nature of extinction sessions. However, there were no differences in behavior reduction during extinction between tracking groups in either study (Pitchers et al. 2017). Importantly, as discussed above, several factors changed during our reinstatement tests (extinction conditions, reduced shock, and passage of time) that could override potential differences in DS– modulation of behavior that would otherwise arise between ST and GT using more traditional extinction/reinstatement paradigms. Alternatively, different sensitivities between tracking groups to shock might explain our result that ST and GT do not differ with respect to DS– modulation of reinstatement behavior, but we observed no group differences in sensitivity to shock during conflict training, consistent with earlier work using this procedure (Saunders et al. 2013). However, others have found greater resistance to punishment in sign-tracking animals (Pohořalá et al. 2021).
Our study also employed different conditions for DS training (fewer and shorter sessions) and reinstatement testing than similar previous DS work with cocaine (Pitchers et al. 2017). Perhaps, the most notable difference is our DS + periods during reinstatement were 30 s, followed by an ITI of 150 s; their DS + presentations were shorter: 4 s followed by an average ITI of 30 s. We observed a very large increase in drug seeking rates across all tracking groups during DS + vs. DS– periods relative to the more modest DS + rate increases observed in the prior study (Pitchers et al. 2017). This may potentially be due to the length of our DS + , but the continued presence of conflict during the reinstatement test may also have discouraged responding during DS– periods in our study. Overall, because the discrimination between DS + and DS– was both robust and equivalent between tracking groups in training and reinstatement in our study, it is difficult to reconcile the marked discrepancy in tracking effects between studies based on differences in DS training and testing parameters.
In the current work, intermittent access to fentanyl was available for many (19) sessions prior to the reinstatement test. We would expect this schedule to considerably increase economic demand for fentanyl over baseline levels (Martin et al. 2020), conceivably strengthening the DS-US relationship across all tracking types as the drug becomes more valuable. Supporting this idea, sign-tracking individuals exhibit higher cocaine demand early in training, but extended cocaine experience equalizes this difference, as well as equalizing early differences in cue-induced reinstatement (Kawa et al. 2016). Furthermore, a recent study using 45 sessions found that a composite addiction measure combining persistence of cocaine seeking, motivation for cocaine taking, and resistance to punishment did not correlate with PCA behavior (Pohořalá et al. 2021). Further work exploring the importance of the drug and amount drug experience in modifying cue-motivated behaviors across different drugs and stimulus types is necessary to understand the complex relationships between traits and experience that drive drug seeking behaviors.
We find no significant differences between sexes in the experiments, with the exception of a smaller response rate increase at DS–:DS + transitions in females during reinstatement, but not training. A recent review of sex differences in opioid reinstatement models found no consistent evidence that the sexes are differentially susceptible to cue or context-induced reinstatement to opioid seeking (Nicolas et al. 2022). While some studies found increased seeking in females (Vazquez et al. 2020), others observed decreased seeking or no change (Malone et al. 2021). A recent study found no difference in context reinstatement to heroin or oxycodone seeking after conflict using long-access conditions (Bossert et al. 2022). Previous work measuring fentanyl reinforcement found females exhibited higher reinforcing effects in economic demand analysis, but males exhibited higher drug choice relative to a non-drug reinforcer (Townsend et al. 2019). Others found similar overall levels of unconstrained economic demand (Q0) between sexes for the short-acting opioid remifentanil, however female Q0 varied across estrous cycle (Lacy et al. 2020). Estrous cycle-associated behavioral differences could potentially lead to variance in the female data we observe, but we did not evaluate this possibility.
In summary, we report that discriminative stimuli signaling the availability of fentanyl powerfully motivate drug seeking even in the presence of conflict, and the strength of this drug seeking does not correlate with Pavlovian tracking phenotypes. This work adds to literature delineating the complex relationship between sign- and goal-tracking behaviors and addiction models.
References
Beckmann JS, Marusich JA, Gipson CD, Bardo MT (2011) Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res 216:159–165. https://doi.org/10.1016/j.bbr.2010.07.022
Bossert JM, Townsend EA, Altidor LK-P et al (2022) Sex differences in the effect of chronic delivery of the buprenorphine analogue BU08028 on heroin relapse and choice in a rat model of opioid maintenance. Br J Pharmacol 179:227–241. https://doi.org/10.1111/bph.15679
Chang SE, Krueger LD, Flagel SB (2022) Investigating individual differences in opioid-taking and opioid-seeking behavior in male rats. Psychopharmacology. https://doi.org/10.1007/s00213-021-06023-2
Cooper A, Barnea-Ygael N, Levy D et al (2007) A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology 194:117–125. https://doi.org/10.1007/s00213-007-0827-7
Di Ciano P, Everitt BJ (2003) Differential control over drug-seeking behavior by drug-associated conditioned reinforcers and discriminative stimuli predictive of drug availability. Behav Neurosci 117:952–960. https://doi.org/10.1037/0735-7044.117.5.952
Dinsmoor JA (1950) A quantitative comparison of the discriminative and reinforcing functions of a stimulus. J Exp Psychol 40:458–472. https://doi.org/10.1037/h0056266
Everett NA, Carey HA, Cornish JL, Baracz SJ (2020) Sign tracking predicts cue-induced but not drug-primed reinstatement to methamphetamine seeking in rats: effects of oxytocin treatment. J Psychopharmacol 34:1271–1279. https://doi.org/10.1177/0269881120954052
Flagel SB, Robinson TE, Clark JJ et al (2010) An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology 35:388–400. https://doi.org/10.1038/npp.2009.142
Flagel SB, Watson SJ, Akil H, Robinson TE (2008) Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res 186:48–56. https://doi.org/10.1016/j.bbr.2007.07.022
Kawa AB, Bentzley BS, Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233:3587–3602. https://doi.org/10.1007/s00213-016-4393-8
Lacy RT, Austin BP, Strickland JC (2020) The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addict Biol 25:e12716. https://doi.org/10.1111/adb.12716
Madangopal R, Tunstall BJ, Komer LE et al (2019) Discriminative stimuli are sufficient for incubation of cocaine craving. Elife 8:e44427. https://doi.org/10.7554/eLife.44427
Madsen HB, Ahmed SH (2015) Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol 20:433–444. https://doi.org/10.1111/adb.12134
Malone SG, Keller PS, Hammerslag LR, Bardo MT (2021) Escalation and reinstatement of fentanyl self-administration in male and female rats. Psychopharmacology 238:2261–2273. https://doi.org/10.1007/s00213-021-05850-7
Martin DA, Gyawali U, Calu DJ (2021) Effects of 5-HT2A Receptor stimulation on economic demand for fentanyl after intermittent and continuous access self-administration in male rats. Addict Biol 26:e12926. https://doi.org/10.1111/adb.12926
Martin-Fardon R, Weiss F (2017) Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict Biol 22:923–932. https://doi.org/10.1111/adb.12374
McFarland K, Ettenberg A (1997) Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology 131:86–92. https://doi.org/10.1007/s002130050269
Meyer PJ, Lovic V, Saunders BT et al (2012) Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE 7:e38987. https://doi.org/10.1371/journal.pone.0038987
Namba MD, Tomek SE, Olive MF et al (2018) The winding road to relapse: forging a new understanding of cue-induced reinstatement models and their associated neural mechanisms. Front Behav Neurosci 12:17. https://doi.org/10.3389/fnbeh.2018.00017
Nicolas C, Zlebnik NE, Farokhnia M et al (2022) Sex differences in opioid and psychostimulant craving and relapse: a critical review. Pharmacol Rev 74:119–140. https://doi.org/10.1124/pharmrev.121.000367
Patitucci E, Nelson AJD, Dwyer DM, Honey RC (2016) The origins of individual differences in how learning is expressed in rats: a general-process perspective. J Exp Psychol Anim Learn Cogn 42:313. https://doi.org/10.1037/xan0000116
Peters J, De Vries TJ (2014) Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology 231:447–453. https://doi.org/10.1007/s00213-013-3258-7
Pitchers KK, Phillips KB, Jones JL et al (2017) Diverse roads to relapse: a discriminative cue signaling cocaine availability is more effective in renewing cocaine seeking in goal trackers than sign trackers and depends on basal forebrain cholinergic activity. J Neurosci 37:7198–7208. https://doi.org/10.1523/JNEUROSCI.0990-17.2017
Pohořalá V, Enkel T, Bartsch D et al (2021) Sign- and goal-tracking score does not correlate with addiction-like behavior following prolonged cocaine self-administration. Psychopharmacology 238:2335–2346. https://doi.org/10.1007/s00213-021-05858-z
Robinson TE, Yager LM, Cogan ES, Saunders BT (2014) On the motivational properties of reward cues: Individual differences. Neuropharmacology 76 Pt B:450–459. https://doi.org/10.1016/j.neuropharm.2013.05.040
Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE (2014) A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology 39:2816–2823
Saunders BT, Robinson TE (2010) A cocaine cue acts as an incentive stimulus in some, but not others: implications for addiction. Biol Psychiatry 67:730–736. https://doi.org/10.1016/j.biopsych.2009.11.015
Saunders BT, Yager LM, Robinson TE (2013) Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci 33:13989–14000. https://doi.org/10.1523/JNEUROSCI.0450-13.2013
Townsend EA, Negus SS, Caine SB et al (2019) Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacol 44:2022–2029. https://doi.org/10.1038/s41386-019-0356-1
Tunstall BJ, Kearns DN (2015) Sign-tracking predicts increased choice of cocaine over food in rats. Behav Brain Res 281:222–228. https://doi.org/10.1016/j.bbr.2014.12.034
Uslaner JM, Acerbo MJ, Jones SA, Robinson TE (2006) The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res 169:320–324. https://doi.org/10.1016/j.bbr.2006.02.001
Vazquez M, Frazier JH, Reichel CM, Peters J (2020) Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking. Learn Mem 27:6–11. https://doi.org/10.1101/lm.050187.119
Versaggi CL, King CP, Meyer PJ (2016) The tendency to sign-track predicts cue-induced reinstatement during nicotine self-administration, and is enhanced by nicotine but not ethanol. Psychopharmacology 233:2985–2997. https://doi.org/10.1007/s00213-016-4341-7
Weiss F, Martin-Fardon R, Ciccocioppo R et al (2001) Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacol 25:361–372. https://doi.org/10.1016/S0893-133X(01)00238-X
Yager LM, Pitchers KK, Flagel SB, Robinson TE (2015) Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacol 40:1269–1277. https://doi.org/10.1038/npp.2014.314
Yager LM, Robinson TE (2010) Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res 214:30–34. https://doi.org/10.1016/j.bbr.2010.04.021
Yager LM, Robinson TE (2013) A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology 226:217–228. https://doi.org/10.1007/s00213-012-2890-y
Yager LM, Robinson TE (2015) Individual variation in the motivational properties of a nicotine cue: sign-trackers vs. goal-trackers. Psychopharmacology 232:3149–3160. https://doi.org/10.1007/s00213-015-3962-6
Funding
This work was funded through National Institute on Drug Abuse (NIDA) RO1 grant # RO1DA043533 awarded to Donna Calu.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martin, D.A., Keefer, S.E. & Calu, D.J. Investigating discriminative stimulus modulation of opioid seeking after conflict-induced abstinence in sign- and goal-tracking rats. Psychopharmacology 239, 3223–3236 (2022). https://doi.org/10.1007/s00213-022-06204-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06204-7