Abstract
Rationale
The exact role of delta opioid receptors in drug-induced conditioned place preference (CPP) remains debated. Under classical experimental conditions, morphine-induced CPP is decreased in mice lacking delta opioid receptors (Oprd1 −/−). Morphine self-administration, however, is maintained, suggesting that drug-context association rather than drug reward is deficient in these animals.
Objectives
This study further examined the role of delta opioid receptors in mediating drug-cue associations, which are necessary for the expression of morphine-induced CPP.
Methods
We first identified experimental conditions under which Oprd1 −/− mice are able to express CPP to morphine (5, 10 or 20 mg/kg) in a drug-free state and observed that, in this paradigm, CPP was dependent on circadian time conditions. We then took advantage of this particularity to assess the ability of various cues (internal or discrete), predicting either drug or food reward, to restore CPP induced by morphine (10 mg/kg) in Oprd1 −/− mice in conditions under which they normally fail to express CPP.
Results
We found that presentation of circadian, drug or auditory cues, predicting morphine or food reward, restored morphine CPP in Oprd1 −/− mice, which then performed as well as control mice.
Conclusions
This study reveals that, in contrast to spatial cues, internal or discrete morphine-predicting stimuli permit full expression of morphine CPP in Oprd1 −/− mice. Delta receptors, therefore, appear to play a crucial role in modulating spatial contextual cue-related responses. This activity may be critical when context gains control over behavior, as is the case for context-induced relapse in drug abuse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The conditioned place preference (CPP) paradigm is commonly used to evaluate the appetitive properties of abused drugs in rodents (Cunningham et al. 2011; Sanchis-Segura and Spanagel 2006; Tzschentke 2007). In this paradigm, based on Pavlovian conditioning, animals develop preference for a specific context (conditioned stimulus) in which exposure to an appetitive drug (unconditioned stimulus) is repeatedly experienced, over another context where the drug stimulus is never experienced. CPP generated by opiates as well as a variety of non-opioid drugs has been shown to depend critically on mu opioid receptors (Le Merrer et al. 2009; McBride et al. 1999; Tzschentke 1998, 2007). The exact contribution of delta opioid receptors to drug-induced CPP remains, however, a matter of debate, and elicits sustained interest in the literature.
A wealth of evidence from pharmacological studies supports a major role of delta receptors in drug-induced CPP. Delta opioid receptor agonists have been shown to elicit the development of CPP (Longoni et al. 1998; Morales et al. 2001; Shippenberg et al. 1987; Suzuki et al. 1997); but see (Kotlinska et al. 2010) and also reinstate CPP for cocaine (Kotlinska et al. 2010). Conversely, delta antagonists attenuate CPP to cocaine, methamphetamine, or morphine and prevent morphine-induced contextual sensitization of morphine CPP (Chefer and Shippenberg 2009; Menkens et al. 1992; Moron et al. 2010; Suzuki et al. 1994). These observations have been proposed to reflect a role of delta receptors in mediating hedonic properties of drugs of abuse. However, a major concern when using pharmacological approaches is the possible cross-reactivity of delta agonists at mu opioid receptors (Hutcheson et al. 2001; Scherrer et al. 2004), which play a key role in reward processes (Le Merrer et al. 2009). Genetic deletion of the delta opioid receptors thus represents a unique tool to address the role of these receptors in vivo. In agreement with pharmacological data, mice lacking delta opioid receptors (Oprd1 −/−) fail to express morphine-induced CPP when tested in a drug-free state (Chefer and Shippenberg 2009; Le Merrer et al. 2011). However, these animals display intact morphine self-administration as compared to their wild-type (WT) counterparts, indicating preserved reinforcing properties of morphine (David et al. 2008; Le Merrer et al. 2011). Interestingly, we showed that the expression of both lithium-induced conditioned place aversion and morphine-induced CPP was impaired in Oprd1 −/− mice, suggesting a general drug-context association deficit in these animals. Further, this deficit was restored when animals were tested under the effects of lithium and morphine, respectively, indicating that impaired CPP displayed in Oprd1 −/− mice is state-dependent, and that internal drug cues are able to restore place conditioning in these mice. These data suggest that mice lacking the delta opioid receptors show a specific disruption of drug association with the context (Le Merrer et al. 2011), but that other types of associations with the drug remain intact.
The present study sought to further investigate the role of delta opioid receptors in mediating association of the drug with a number of cues, apart the context, using a paradigm of morphine-induced CPP. For this, we first identified experimental conditions under which Oprd1 −/− mice displayed morphine-induced CPP comparable to wild type animals when tested in a drug-free state. This was possible by performing two conditioning sessions on the same day, 7 h apart, to increase the contrast between drug- and saline-pairings as compared to alternate day paradigms (Chefer and Shippenberg 2009; Le Merrer et al. 2011). Interestingly, under those conditions, expression of CPP was circadian time-state dependent in mutant but not control mice, suggesting that Oprd1 −/− mice were able to use circadian cues to retrieve a place preference. We then took advantage of this time-state dependency to assess the ability of various cues (internal or discrete) predicting either drug or food reward, to restore morphine-induced CPP in Oprd1 −/− mice in conditions under which they normally fail to express CPP.
Methods and materials
Animals
Male and female Oprd1 −/− mice (Filliol et al. 2000) and their wild-type (WT) controls were bred in-house on a hybrid 50 % 129SVPas–50 % C57BL/6J background and were aged 8–14 weeks at the beginning of experiments. Animals were housed in groups (2–5 mice per cage) on a 12 h light/dark cycle (lights on at 7:00 AM) at controlled temperature (22 ± 1 °C). Food and water were available ad libitum throughout all experiments, unless otherwise stated. Experimental procedures were conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and approved by the Comité régional d'éthique en matière d'expérimentation animale de Strasbourg (CREMEAS, 2003-10-08-[1]-58).
Conditioned place preference
Apparatus
Place conditioning experiments were performed in unbiased computerized boxes (Imetronic, Pessac, France) formed by two Plexiglas chambers (15.5 × 16.5 × 20 cm) separated by a central alley (6 × 16.5 × 20 cm). Two sliding doors (3 × 20 cm) connected the alley with the chambers. Two triangular prisms of transparent polycarbonate were arranged in one chamber, and one rectangular prism in the other to form different shape patterns (covering the same surface). Distinct textured removable floors made of translucent polycarbonate provided additional contextual cues. The activity and location of mice were recorded using five photocells located throughout the apparatus. Behavioral data were collected by an interface connected to a PC. Light intensity in the chambers was set at 30 lx.
Experimental protocols
Morphine conditioning consisted of three phases. On day 1, naïve mice were placed in the central alley and allowed to freely explore the apparatus for 20 min. Based on the individuals' spontaneous preference during this pretest phase, the drug-paired chambers were assigned in such a way that saline and morphine groups were counterbalanced and unbiased towards contextual cues. Conditioning phase lasted 3 days. Mice underwent two conditioning, vehicle and drug-paired, sessions daily, 7 h apart. During vehicle and drug-conditioning sessions, animals were confined immediately after s.c. injection of morphine or vehicle for 45 min in the appropriate drug or vehicle-paired chamber. The testing phase was conducted on day 5, at the same time of the day as the pretest session. The animals, in a drug-free state (unless otherwise stated: see “Experiment 2”), were placed in the neutral central alley and permitted to explore the apparatus for 20 min with the two sliding doors opened. The time spent in each chamber was recorded. All the animals tested in the following experiments were naïve when conditioning started.
Experiment 1
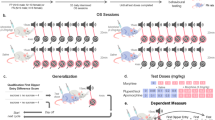
Animals were randomly assigned to one of four groups (see Fig. 1). Each of these groups included drug-treated animals (receiving morphine during drug pairings) and control animals (receiving saline during drug pairings). All the animals received saline injections during vehicle pairings.
Scheme showing the different place conditioning paradigms on a timeline. During drug pairings, control animals received a saline injection and experimental mice received a morphine (5, 10, or 20 mg/kg) injection. During vehicle pairings, all animals received a saline injection. Please note that in the AM–AM and PM–PM paradigms, pretest and test sessions were conducted at the same circadian time as drug pairings (matching time conditions); in the AM–PM and PM–AM paradigms, mice were pretested and tested at a different time of day from drug pairings (non-matching time conditions)
In the AM–AM paradigm, pretest session took place in the morning (9.00 AM) on day 1. During conditioning phase, drug pairings were performed in the morning. The animals were injected with either morphine (5, 10, or 20 mg/kg, s.c.) or saline (controls) before being confined in the “drug-paired” chamber. Vehicle-pairings were performed in the afternoon (4:00 AM). All the animals received an injection of saline and were confined in the vehicle-paired compartment. On day 5, the testing phase was conducted in the morning. In the AM–PM paradigm, drug (morphine at 10 mg/kg, s.c. or saline) and vehicle pairings were conducted in the morning. Pretest and test sessions, however, were performed in the afternoon. In the PM–PM paradigm, drug (morphine at 10 mg/kg, s.c. or saline) pairings were conducted in the afternoon and vehicle pairings in the morning. Pretest and test sessions were conducted in the afternoon. Finally, in the PM–AM paradigm, drug (morphine at 10 mg/kg, s.c. or saline) pairings were conducted in the afternoon and vehicle-pairings in the morning. Pretest and test sessions took place in the morning. This experimental design allows the circadian time of test session to match or not the time of previous morphine conditioning sessions, thereby making possible to assess potential effect of circadian cues on the expression of CPP.
Experiment 2
In this experiment, the circadian time of drug (morphine at 10 mg/kg, s.c. or saline) and vehicle pairings as well as testing session was the same as for the non-matching AM–PM condition. In a first group, mice were conditioned in the AM–PM condition. State dependency was assessed on test day by injecting all the animals (drug-treated and controls) with morphine (10 mg/kg, s.c.) immediately before the session. In a second group, a white noise (Alita® linear air pump, model AL-80P, 39 db) was emitted during each drug-pairing (but not vehicle-pairing) session. Control morphine-treated animals (10 mg/kg, s.c.) were exposed for 45 min to white noise independently from morphine conditioning sessions (starting time between 12.00 AM and 2.00 PM) in a dedicated separate room. The influence of auditory cues paired/not paired with morphine exposure on the expression of a morphine-induced CPP was explored by testing the animals in the presence of the white noise.
Experiment 3
Animals were maintained under food restriction (90 ± 2 % of ad libitum body weight). The circadian time of drug (morphine at 10 mg/kg, s.c. or saline) and vehicle pairings as well as test session was the same as for the AM–PM condition. In a first group, mice were fed (lab chow) once a day at 5.30 PM for 2 weeks before the pretest phase. During the conditioning phase, the animals received food when back in the home cage immediately after vehicle pairing (at 5.30 PM). Control morphine-treated animals (10 mg/kg, s.c.) were food-deprived for 2 weeks before the pretest phase and during the week of conditioning. They were fed daily at a random time (between 8.00 AM and 6.00 PM) to prevent circadian cues from becoming predictive of feeding. The day before test session, control animals were fed at 5.30 PM. The influence of food reward anticipation was assessed during a normal test session performed between 4.30 and 5.30 PM. In a second group, mice were fed once a day at a random time between 8:00 AM and 6.00 PM (to avoid providing predictive circadian cues) for 2 weeks before pretest phase and during the week of conditioning. Feeding took place in a dedicated room where a white noise (Alita® linear air pump, model AL-80P, 39 db) was emitted during 15 min before food (lab chow) was provided and for another 20 min when food was available. Then, the animals were returned to the animal house. The influence of food-predicting auditory cues on the expression of a morphine-induced CPP was explored by testing the animals in the presence of the white noise. The last pairing between food presentation and white noise took place in the morning before test session (8.30 PM).
Drugs
Morphine hydrochloride (Francopia, Sanofi Synthelabo Laboratories, Paris, France) and naloxone hydrochloride (Natick, MA, USA) were dissolved in sterile isotonic saline (NaCl 0.9 %). Both drugs were administered in a volume of 10 ml/kg. Doses refer to salt weight.
Data analyses and statistics
Place conditioning data were expressed as percentage of time spent in the drug-paired compartment. Four-way ANOVA was performed with gender, genotype, and treatment (or dose when multiple doses were tested) as between-group factors and conditioning (pretest versus test session) as a within-group factor. Post-hoc analyses were performed for test session using a one-way ANOVA (treatment effect) followed by Dunnett's test where appropriate, to compare to the saline group. Effect of pairing white noise with morphine conditioning was assessed in morphine-treated animals using four-way ANOVA with gender, genotype and pairing (paired versus unpaired) as between-group factors and conditioning (pretest versus test session) as a within-group factor. Post hoc analysis was processed for test session using a one-way ANOVA (pairing effect) followed by Newman–Keul's test. Statistical significance was set at p < 0.05 for all tests.
Results
Experiment 1: circadian cues restored a morphine-induced CPP in Oprd1 −/− mice
The animals underwent two conditioning sessions daily, 7 h apart, to facilitate the contrast between drug- and saline-pairings as compared to alternate day paradigms (Chefer and Shippenberg 2009; Le Merrer et al. 2011). When test session took place at the same time as morphine conditioning sessions (see Fig. 2a and c), WT and mutant mice similarly displayed a marked preference for the morphine-paired chamber (AM–AM paradigm—gender effect: F 1,48 < 1; genotype effect: F 1,48 < 1; dose effect: F 3,48 = 2.79, p = 0.05; conditioning effect: F 1,48 = 27.49, p < 0.0001; PM–PM paradigm—gender effect: F 1,24 = 1.15, NS; genotype effect: F 1,24 = 1.62, p = NS; dose effect: F 1,24 = 97.48, <0.0001; conditioning effect: F 1,24 = 119.35, p < 0.0001). In contrast, when the time of test session matched the time of vehicle conditioning sessions (see Fig. 2b, d), WT but not mutant mice exhibited morphine-induced CPP (AM–PM paradigm—gender effect: F 1,24 < 1; genotype effect: F 1,24 = 8.41, p < 0.01; treatment effect: F 1,24 = 1.29, NS; conditioning effect: F 1,24 = 2.05, NS; genotype × conditioning interaction: F 1,24 = 12.84, p < 0.01; genotype × conditioning × treatment interaction: F 1,24 = 14.25, p < 0.001; PM–PM paradigm—gender effect: F 1,24 < 1; genotype effect: F 1,24 < 1; treatment effect: F 1,24 = 5.18, p < 0.05; conditioning effect: F 1,24 = 5.67, p < 0.05; genotype × conditioning interaction: F 1,24 = 5.87, p < 0.05). Place preference in Oprd1 −/− mice was thus circadian time-state-dependent under these conditions.
Place preference to morphine is circadian time-state dependent in mice lacking the delta opioid receptor (Oprd1 −/−) as compared to their wild-type (WT) counterparts. a and c Under matching time conditions (AM–AM and PM–PM paradigms), WT and Oprd1 −/− mice displayed similar place preference to morphine. b and d Under non-matching time conditions (AM–PM and PM–AM paradigms), WT but not Oprd1 −/− animals exhibited a significant preference for the morphine-paired compartment. Place preference data show mean (±SEM) time spent in the drug-paired compartment (expressed as a percentage of time spent in the two compartments) during the 20-min pre- and post-conditioning sessions (n = 3–5 per gender, genotype and dose). Solid star: comparison to vehicle group (one-way analysis of variance); asterisk: comparison between genotypes (four-way analysis of variance); open star: comparison to vehicle group (one-way analysis of variance in the WT group). One symbol: p < 0.05; two symbols: p < 0.01; three symbols: p < 0.001
Experiment 2: internal or auditory cues predicting morphine restored morphine-induced CPP in Oprd1 −/− mice
We then used the AM–PM non-matching time condition, where mutant animals are unable to express morphine CPP, to evaluate the effects of drug (internal) or auditory (external non-contextual) cues predicting morphine on the expression of a morphine-induced CPP. When tested under the effect of morphine (10 mg/kg), both WT and Oprd1 −/− mice displayed significant place preference for the drug-paired compartment (gender effect: F 1,24 = 1.08, NS; genotype effect: F 1,24 = 1.81; NS; treatment effect F 1,24 = 43.17, p < 0.0001; conditioning effect: F 1,24 = 35.19, p < 0.0001; treatment × conditioning interaction: F 1,24 = 31.51, p < 0.0001; Fig. 3a, and see Fig. 2b for comparison). Moreover, WT and mutant animals also showed a similar marked preference for the morphine-paired compartment when a white noise paired with morphine was emitted during the test session (gender effect: F 1,24 < 1; genotype effect: F 1,24 = 1.11; NS; treatment effect F 1,24 = 45.30, p < 0.0001; conditioning effect: F 1,24 = 23.08, p < 0.0001; treatment × conditioning interaction: F 1,24 = 50.57, p < 0.0001; Fig. 3b). However, when not paired with morphine conditioning, white noise did not restore morphine CPP in Oprd1 −/− mice (gender effect: F 1,24 < 1; genotype effect: F 1,24 < 1; pairing effect: F 1,24 < 1; conditioning effect: F 1,24 = 56.10; p < 0.0001; genotype × pairing × conditioning effect: F 1,24 = 6.12, p < 0.05; post-hoc: unpaired in Oprd1 −/− mice different from unpaired and paired in WT animals, p < 0.05). These data indicate that providing internal drug or external auditory cues predicting morphine to Oprd1 −/− animals allows expression of a WT-like morphine-induced CPP under non-matching time conditions.
Drug or auditory cues predicting morphine reward restore morphine CPP in Oprd1 −/− mice under non-matching time conditions (AM–PM paradigm). a WT and Oprd1 −/− mice displayed similar place preference to morphine (10 mg/kg) when tested under the effects of the drug. b WT and mutant animals showed a similar strong preference for the morphine-paired compartment when a white noise previously paired with morphine (10 mg/kg) was emitted during test session. When not paired to morphine exposure, however, white noise stimulus was ineffective in restoring morphine CPP in Oprd1 −/− mice (control groups). Place preference data show mean (±SEM) time spent in the drug-paired compartment (expressed as a percentage of time spent in the two compartments) during the 20-min pre- and post-conditioning sessions (n = 3–5 per gender, genotype and dose). Solid star: comparison to vehicle group (one-way analysis of variance); asterisk: comparison between genotypes under the unpaired condition (one-way analysis of variance). One symbol: p < 0.05; three symbols: p < 0.001
Experiment 3: circadian or auditory cues predicting food restored morphine-induced CPP in Oprd1 −/− mice
We finally questioned whether morphine-induced CPP in Oprd1 −/− mice could be entrained by food. We again used the non-matching AM–PM condition to test the influence of food-predicting circadian or auditory cues on the expression of a morphine-induced CPP. When tested immediately before expected feeding time, WT and Oprd1 −/− animals displayed similar strong preference for the morphine-paired chamber (gender effect: F 1,24 < 1; genotype effect: F 1,24 < 1; treatment effect F 1,24 = 50.65, p < 0.0001; conditioning effect: F 1,24 = 63.53, p < 0.0001; treatment × conditioning interaction: F 1,24 = 53.57, p < 0.0001; Fig. 4a). When feeding was not time-scheduled, mice displayed less preference for morphine-paired compartment (gender effect: F 1,24 < 1; genotype effect: F 1,24 < 1; time schedule effect: F 1,24 = 6.21, p < 0.05; conditioning effect: F 1,24 = 64.68, p < 0.0001; conditioning × genotype effect: F 1,24 = 5.79, p < 0.05; conditioning × time-schedule effect: F 1,24 = 9.67, p < 0.01; Fig. 4a) with mutant mice failing to express significant CPP under the non time-scheduled feeding condition (gender effect: F 1,12 < 1; genotype effect: F 1,12 = 1.17, NS; conditioning effect: F 1,12 = 8.60, p < 0.05; conditioning × genotype effect: F 1,12 = 5.32, p < 0.01). In line with these results, both WT and mutant mice showed significant morphine-induced CPP when an auditory cue previously paired with food presentation was displayed during testing session, (gender effect: F 1,21 = 1.08, NS; genotype effect: F 1,21 < 1; treatment effect F 1,21 = 43.82, p < 0.0001; conditioning effect: F 1,21 = 45.58, p < 0.0001; treatment × conditioning interaction: F 1,21 = 44.72, p < 0.0001; Fig. 4b). Thus, Oprd1 −/− mice expressed normal morphine-induced CPP when provided with circadian or auditory cues predicting a food reward.
Circadian or auditory cues predicting food reward restore morphine CPP in Oprd1 −/− mice under non-matching time conditions (AM–PM paradigm). a Food-deprived WT and Oprd1 −/− mice displayed similar place preference to morphine (10 mg/kg) when tested immediately before expected feeding time. However, when circadian cues were not predictive of feeding (unscheduled feeding), Oprd1 −/− mice failed to express significant CPP. b Food-deprived animals from WT and Oprd1 −/− mouse lines exhibited preference for a morphine-paired compartment when exposed to a white noise previously paired to food exposure. Place preference data show mean (±SEM) time spent in the drug-paired compartment (expressed as a percentage of time spent in the two compartments) during the 20-min pre- and post-conditioning sessions (n = 3–5 per gender, genotype and dose). Solid star: comparison to vehicle group (one-way analysis of variance). Asterisk: comparison between genotypes under the unscheduled feeding condition (one-way analysis of variance). One symbol: p < 0.05; three symbols: p < 0.001
Discussion
In the present study, we were first able to identify experimental conditions under which Oprd1 −/− mice display a morphine-induced CPP in a drug-free state. Notably under those conditions, the expression of morphine CPP did not require a peripheral morphine injection as in our previous study (Le Merrer et al. 2011), indicating that morphine CPP in Oprd1 −/− mice can take place without activation of mu opioid receptors on the test day. Other cues, however, were necessary, since we observed that mutant mice showed a marked preference for the morphine-paired chamber only when circadian time of testing matched the time of morphine pairings. These animals thus expressed morphine CPP in a circadian time-state-dependent manner. In mutant animals therefore, circadian cue-drug association likely facilitates the expression of morphine CPP, which is otherwise hampered by altered drug-context association.
The failure of mutant mice in expressing morphine-induced CPP under non-matching circadian time conditions could however result from different sensitivity of these animals to partial morphine withdrawal. This was unlikely though, since Oprd1 −/− animals expressed marked CPP when the interval of time elapsed between the last morphine injection and the testing session was 24 h (matching conditions) but equally failed to express such CPP when this interval was 17 h (PM–AM paradigm) or 31 h (AM–PM paradigm). Moreover, naloxone-induced somatic signs of withdrawal were preserved in mutant mice (see Supplementary Methods and Supplementary Figure 1), which supports the notion that morphine dependence is not altered in the absence of delta opioid receptors.
Time-state-dependent place conditioning has been previously described for wheel running and foot-shocks in hamsters and for food reward in rats (Cain et al. 2004a, b; Ralph et al. 2002). This phenomenon can be interpreted as a state-dependent effect (Overton 1978), where different circadian times correspond to different internal states (Tzschentke 2007). Animals would use internal states associated to circadian cues as conditioned stimuli for place preference learning. Accordingly, in previous place preference studies (Chefer and Shippenberg 2009; Le Merrer et al. 2011), mice received morphine and saline injections on the morning of alternate days, whereas in present work, drug and saline exposures were scheduled at distinct predictable hours. Under the latter, but not the former conditions, circadian cues acquired a predictive value for reinforcement, and Oprd1 −/− mice then seemed able to use these cues to express CPP to morphine. Moreover, these mice were also able to use internal drug cues to retrieve morphine CPP. Indeed, morphine administered immediately before test session restored CPP in Oprd1 −/− mice under an alternate days-conditioning paradigm (Le Merrer et al. 2011) and, in the present study, under non-matching time conditions. These data demonstrate that Oprd1 −/− mice, when provided with interoceptive circadian or drug cues, express morphine-induced CPP in a similar way as their WT counterparts.
Circadian and drug cues, interestingly, are not only based on internal states but they also share a common non-spatial nature. We thus questioned whether another non-spatial, but external cue, namely a discrete auditory cue, paired with morphine conditioning, would be able to restore CPP in mutant animals. Discrete unimodal cues are often combined to spatial cues to facilitate the acquisition of place preference (Cunningham et al. 2011). We associated a discrete white noise with morphine pairings during conditioning phase. Remarkably, Oprd1 −/− mice displayed similar morphine-induced CPP as WT animals when tested in the presence of the morphine-paired white noise stimulus. When not paired to morphine exposure, however, such stimulus was ineffective in restoring morphine CPP in mutant animals. Mice lacking the delta opioid receptors can therefore use various morphine-predicting stimuli, either internal or discrete but not spatial, to retrieve a CPP to morphine. Such ability is in line with the view that conditioned associations in place conditioning not only encode the affective properties of the unconditioned stimulus (US) but also include information regarding the feature of the US (Bevins and Murray 2011; Corbit and Balleine 2005; Delamater and Holland 2008; Reichel et al. 2010).
The neuronal substrates underlying time-state dependency remain to be identified. Remarkably, lesions of the brain circadian clock suprachiasmatic nucleus (SCN) fail to affect time-dependent place conditioning in hamsters (Cain and Ralph 2009). Hence, this phenomenon seems independent from the SCN and could alternatively rely on a food-entrainable pacemaker (Mistlberger et al. 1996; Pezuk et al. 2010; Stephan 1983). Whether time-state dependent morphine-induced CPP in mice lacking the delta receptor involves a food-entrainable oscillator implies that CPP expression in these animals may be food-entrainable as well. We challenged this hypothesis by submitting Oprd1 −/− mice and their WT controls to a time-scheduled restricted feeding paradigm. Food-deprived animals were then tested for morphine CPP under the AM–PM condition immediately before the expected feeding time. In agreement with our hypothesis, expression of a morphine-induced CPP in Oprd1 −/− mice was food-entrainable: mutant and WT animals displayed similar place preference under these conditions. Importantly, food restriction was not sufficient to restore the expression of a place preference in Oprd1 −/− mice, as they failed to display morphine-induced CPP when timing cues were not predictive of feeding. Therefore, time-state dependent CPP in knockout mice is very likely to depend on a food-entrainable oscillator.
Interestingly, in previous food-entrainment experiment, circadian cues acquired a predictive value for food reward. Food-entrainment of CPP expression in mutant animals could thus rely strictly on circadian mechanisms or instead result from conditioning to food-predictive cues. To address this question, we assessed the ability of a discrete auditory cue predicting food-reward independently from circadian cues to restore CPP in Oprd1 −/− mice. Non-contingent presentation of a white noise previously paired with non time-scheduled exposure to food restored the expression of morphine CPP in these animals under the AM–PM condition. Therefore, circadian or discrete auditory cues predicting food were equally able to trigger CPP expression in mutant animals, suggesting that food-entrainment relied mainly on conditioning processes. Stimuli previously associated with rewarding outcomes were shown to influence instrumental performance by increasing motivational arousal, a phenomenon called general pavlovian-instrumental transfer PIT; (Corbit and Balleine 2005, 2011). Following a similar process, food expectancy in our experiments may have generated internal cues that generalized to the drug-associated cues, and permitted the retrieval of place conditioning in Oprd1 −/− mice.
Associative learning processes are primary components of place preference conditioning. These processes involve parallel and distributed neural systems in the brain (O'Keefe and Nadel 1978; Packard et al. 1989; White and McDonald 2002). Delta opioid receptors are expressed in several key brain regions for place conditioning, namely the hippocampus (HPC), nucleus accumbens (NAc), and amygdala (Le Merrer et al. 2009). A classical CPP paradigm, in which spatial cues are predominant, recruits primarily the HPC and the shell region of the NAc (Ferbinteanu and McDonald 2001; Ito and Hayen 2011; Ito et al. 2006, 2008; Meyers et al. 2003). Impaired expression of a CPP to morphine under conditions where only spatial cues are available (Chefer and Shippenberg 2009; Le Merrer et al. 2011) and present results) therefore argue for deficient function of the HPC and/or NAc shell in Oprd1 −/− mice. Further experiments will be needed to explore this hypothesis.
In conclusion, our study reveals how different variables such as circadian time, interoceptive cues, or discrete auditory cues can restore a morphine-induced place conditioning in Oprd1 −/− mice. From a methodological point of view, this work stresses the difficulty to draw robust conclusions regarding the hedonic value of a drug using a classical CPP paradigm in genetically modified animals (Cunningham et al. 2011; Stephens et al. 2010). As regards to delta opioid receptor function, our results suggest that delta receptors are not necessary in regulating non-contextual cues contributing to CPP, which are otherwise used by delta receptor-deficient mice as an alternative strategy to express place conditioning. On the contrary, delta receptors play a key role in the processing of contextual information and facilitate spatial learning. The latter activity may have important implications when HPC-dependent contextual information gains control over behavioral responses, as for example in situations of context-induced relapse for addicted individuals.
References
Bevins RA, Murray JE (2011) Internal stimuli generated by abused substances: role of Pavlovian conditioning and its implications for drug addiction. In: Schachtman TR, Reilly S (eds) Associative learning and conditioning: human and non-human applications. Oxford University Press, New York, pp 270–289
Cain SW, Ralph MR (2009) Circadian modulation of conditioned place avoidance in hamsters does not require the suprachiasmatic nucleus. Neurobiol Learn Mem 91:81–84
Cain SW, Chou T, Ralph MR (2004a) Circadian modulation of performance on an aversion-based place learning task in hamsters. Behav Brain Res 150:201–205
Cain SW, Ko CH, Chalmers JA, Ralph MR (2004b) Time of day modulation of conditioned place preference in rats depends on the strain of rat used. Neurobiol Learn Mem 81:217–220
Chefer VI, Shippenberg TS (2009) Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology 34:887–898
Corbit LH, Balleine BW (2005) Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer. J Neurosci 25:962–970
Corbit LH, Balleine BW (2011) The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J Neurosci 31:11786–11794
Cunningham CL, Groblewski PA, Voorhees CM (2011). Place conditioning. Neuromethods 53:167-189
David V, Matifas A, Gavello-Baudy S, Decorte L, Kieffer BL, Cazala P (2008) Brain regional Fos expression elicited by the activation of mu- but not delta-opioid receptors of the ventral tegmental area: evidence for an implication of the ventral thalamus in opiate reward. Neuropsychopharmacology 33:1746–1759
Delamater AR, Holland PC (2008) The influence of CS-US interval on several different indices of learning in appetitive conditioning. J Exp Psychol Anim Behav Process 34:202–222
Ferbinteanu J, McDonald RJ (2001) Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus 11:187–200
Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL (2000) Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25:195–200
Hutcheson DM, Matthes HW, Valjent E, Sanchez-Blazquez P, Rodriguez-Diaz M, Garzon J, Kieffer BL, Maldonado R (2001) Lack of dependence and rewarding effects of deltorphin II in mu-opioid receptor-deficient mice. Eur J Neurosci 13:153–161
Ito R, Hayen A (2011) Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J Neurosci 31:6001–6007
Ito R, Robbins TW, McNaughton BL, Everitt BJ (2006) Selective excitotoxic lesions of the hippocampus and basolateral amygdala have dissociable effects on appetitive cue and place conditioning based on path integration in a novel Y-maze procedure. Eur J Neurosci 23:3071–3080
Ito R, Robbins TW, Pennartz CM, Everitt BJ (2008) Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci 28:6950–6959
Kotlinska JH, Gibula-Bruzda E, Pachuta A, Kunce D, Witkowska E, Chung NN, Schiller PW, Izdebski J (2010) Influence of new deltorphin analogues on reinstatement of cocaine-induced conditioned place preference in rats. Behav Pharmacol 21:638–648
Le Merrer J, Becker JA, Befort K, Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412
Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL (2011) Deletion of the delta opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol Psychiatry 69:700–703
Longoni R, Cadoni C, Mulas A, Di Chiara G, Spina L (1998) Dopamine-dependent behavioural stimulation by non-peptide delta opioids BW373U86 and SNC 80: 2. Place-preference and brain microdialysis studies in rats. Behav Pharmacol 9:9–14
McBride WJ, Murphy JM, Ikemoto S (1999) Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101:129–152
Menkens K, Bilsky EJ, Wild KD, Portoghese PS, Reid LD, Porreca F (1992) Cocaine place preference is blocked by the delta-opioid receptor antagonist, naltrindole. Eur J Pharmacol 219:345–346
Meyers RA, Zavala AR, Neisewander JL (2003) Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport 14:2127–2131
Mistlberger RE, de Groot MH, Bossert JM, Marchant EG (1996) Discrimination of circadian phase in intact and suprachiasmatic nuclei-ablated rats. Brain Res 739:12–18
Morales L, Perez-Garcia C, Alguacil LF (2001) Effects of yohimbine on the antinociceptive and place conditioning effects of opioid agonists in rodents. Br J Pharmacol 133:172–178
Moron JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA (2010) Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology 35:955–966
O'Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Oxford University Press, Oxford
Overton DA (1978) Basic mechanisms of state-dependent learning. Psychopharmacol Bull 14:67–68
Packard MG, Hirsh R, White NM (1989) Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 9:1465–1472
Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M (2010) Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms 25:432–441
Ralph MR, Ko CH, Antoniadis EA, Seco P, Irani F, Presta C, McDonald RJ (2002) The significance of circadian phase for performance on a reward-based learning task in hamsters. Behav Brain Res 136:179–184
Reichel CM, Wilkinson JL, Bevins RA (2010) Reference place conditioning procedure with cocaine: increased sensitivity for measuring associatively motivated choice behavior in rats. Behav Pharmacol 21:323–331
Sanchis-Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11:2–38
Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL (2004) The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci 19:2239–2248
Shippenberg TS, Bals-Kubik R, Herz A (1987) Motivational properties of opioids: evidence that an activation of delta-receptors mediates reinforcement processes. Brain Res 436:234–239
Stephan FK (1983) Circadian rhythm dissociation induced by periodic feeding in rats with suprachiasmatic lesions. Behav Brain Res 7:81–98
Stephens DN, Duka T, Crombag HS, Cunningham CL, Heilig M, Crabbe JC (2010) Reward sensitivity: issues of measurement, and achieving consilience between human and animal phenotypes. Addict Biol 15:145–168
Suzuki T, Yoshiike M, Mizoguchi H, Kamei J, Misawa M, Nagase H (1994) Blockade of delta-opioid receptors prevents morphine-induced place preference in mice. Jpn J Pharmacol 66:131–137
Suzuki T, Tsuji M, Mori T, Ikeda H, Misawa M, Nagase H (1997) Involvement of dopamine-dependent and -independent mechanisms in the rewarding effects mediated by delta opioid receptor subtypes in mice. Brain Res 744:327–334
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
White NM, McDonald RJ (2002) Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem 77:125–184
Acknowledgements
We thank Dr. J.A.J. Becker for help in performing experiments, Drs. D.N. Stephens, and D. Massotte for their helpful comments on a draft of the manuscript, and G. Duval and D. Memetov for animal care.
Fundings and grants
This work was supported by the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale and Université de Strasbourg. We thank the European Union (GENADDICT/FP6 005166) and the National Institutes of Health (NIAAA AA-16658 and NIDA DA-16768/DA-005010) for financial support.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 88 kb)
Rights and permissions
About this article
Cite this article
Le Merrer, J., Faget, L., Matifas, A. et al. Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology 223, 99–106 (2012). https://doi.org/10.1007/s00213-012-2693-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2693-1