Abstract
Rationale
There is a focus on developing D3 receptor antagonists as cocaine addiction treatments.
Objective
We investigated the effects of a novel selective D3 receptor antagonist, SR 21502, on cocaine reward, cocaine-seeking, food reward, spontaneous locomotor activity and cocaine-induced locomotor activity in rats.
Methods
In Experiment 1, rats were trained to self-administer cocaine under a progressive ratio (PR) schedule of reinforcement and tested with vehicle or one of three doses of SR 21502. In Experiment 2, animals were trained to self-administer cocaine under a fixed ratio schedule of reinforcement followed by extinction of the response. Then, animals were tested with vehicle or one of the SR 21502 doses on cue-induced reinstatement of responding. In Experiment 3, animals were trained to lever press for food under a PR schedule and tested with vehicle or one dose of the compound. In Experiments 4 and 5, in separate groups of animals, the vehicle and three doses of SR 21502 were tested on spontaneous or cocaine (10 mg/kg, IP)-induced locomotor activity, respectively.
Results
SR 21502 produced significant, dose-related (3.75, 7.5 and 15 mg/kg) reductions in breakpoint for cocaine self-administration, cue-induced reinstatement (3.75, 7.5 and 15 mg/kg) and cocaine-induced locomotor activity (3.75, 7.5 and 15 mg/kg) but failed to reduce food self-administration and spontaneous locomotor activity.
Conclusions
SR 21502 decreases cocaine reward, cocaine-seeking and locomotor activity at doses that have no effect on food reward or spontaneous locomotor activity. These data suggest SR 21502 may selectively inhibit cocaine’s rewarding, incentive motivational and stimulant effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Factors that contribute to cocaine addiction include cocaine reward and cue-induced relapse. The mesocorticolimbic dopamine (DA) system is critically involved in both reward and relapse (Pierce and Kumaresan 2006; Willuhn et al. 2010; Wise 1996, 2005). Given the critical role of DA in cocaine reward and seeking, it would seem logical that compounds that disrupt DA neurotransmission might be effective in treating these aspects of cocaine addiction. Traditional DA-selective compounds can reduce cocaine reward and cocaine-seeking, but they also cause extrapyramidal “side effects,” mainly through their antagonist actions at D2 receptors (for a review, see Platt et al. 2002). Thus, a major challenge in the search for effective cocaine addiction treatments is the development of a drug whose effects are relatively restricted to cocaine reward and cocaine-seeking with minimal side effects.
Several animal models have been used to investigate the effects of compounds on cocaine reward and cocaine-seeking. In the present set of studies, cocaine reward was investigated using the intravenous cocaine self-administration under progressive ratio (PR) schedule of reinforcement paradigm and cocaine-seeking using the extinction followed by cocaine-cue reinstatement of responding paradigm. We used these procedures to test the effects on cocaine reward and cocaine-seeking of a novel DA D3 subtype receptor antagonist.
Recently, there is growing interest in pharmacotherapies targeting DA D3 receptors. This approach seems promising because the distribution of D3 receptors appears to be mostly restricted to the mesolimbic system (Diaz et al. 1995, 2000; Murray et al. 1994; Sokoloff et al. 1990), a system strongly implicated in reward and motivation (Wise 1996, 2004).
DA D3 receptors were first implicated in cocaine reward with the demonstration that 7-OH-DPAT, a D3 receptor agonist, decreased cocaine self-administration rates under a fixed ratio (FR) schedule of reinforcement (Caine and Koob 1993), suggesting enhancement of cocaine reward. DA D3 receptor antagonists, such as SB-277011A, NGB-2904 and YQA14, have higher selectivities for D3 than D2 receptors, suggesting that they would be useful in reducing cocaine’s rewarding and incentive motivational effects with minimal side effects (for reviews, see Heidbreder 2008; Heidbreder and Newman 2010). In self-administration studies with low FR schedules of reinforcement, D3 receptor antagonists produce inconclusive effects; they either fail to inhibit cocaine self-administration (Gal and Gyertyan 2003; Xi et al. 2005) or significantly decrease it (Song et al. 2011). But when work demands are increased, as in higher FR and PR schedules of reinforcement, SB-277011A, NGB-2904 and YQA14 significantly reduce responding (Song et al. 2011; Xi and Gardner 2007; Xi et al. 2005). Under a PR schedule of reinforcement, the response requirement increases with each subsequent reward until responding ceases. The final ratio completed that results in reward—referred to as the breakpoint (BP)—indicates the strength of motivation to obtain the drug and, therefore, the reward value of the drug. And it appears that treatment with DA receptor antagonists reduces BPs for cocaine self-administration, suggesting a reduction in cocaine’s rewarding effect (Ranaldi and Wise 2001).
DA D3 receptors also are involved in cocaine-seeking. Both SB277011A and NGB-2904 block reinstatement of cocaine-seeking triggered by cocaine itself (Vorel et al. 2002; Xi and Gardner 2007; Xi et al. 2006), cocaine cues (Gilbert et al. 2005; Xi and Gardner 2007) or foot-shock stress (Xi et al. 2004). Thus, DA D3 receptor antagonists can reduce cocaine reward and cocaine-seeking.
In the search for DA D3 receptor selective ligands as potential cocaine addiction therapeutics, compounds possessing a basic acylaminobutylpiperazine pharmacophore have received considerable attention. Well-studied examples of this class of ligands include compounds such as BP 897 (Pilla et al. 1999), NGB 2904 (Xi et al. 2006), PG01037 (Grundt et al. 2007; Mason et al. 2010) and YQA14 (Song et al. 2011), all possessing a phenyl group on the piperazine nitrogen (Fig. 1). In the development of DA D3 antagonist ligands as drugs, identification of ligands with desired potency, selectivity and drug-like characteristics has been particularly challenging. For example, ligands such as NGB 2904 and PG01037 are highly lipophilic (CLogP >5), and high lipophilicity, in general, has been associated with poor developability (i.e., the potential of a compound to become a clinical candidate/therapeutic agent) of the compounds as drugs (Ritchie et al. 2013). In a recent effort, ligands incorporating nitrogen-containing heterocycles at both ends of the molecule were pursued as an approach to discover compounds possessing D3 receptor binding selectivity, optimal lipophilicity and developability characteristics. From this effort, SR 21502 (Fig. 1) possessing a pyrimidinyl group on the piperazine nitrogen and an imidazo[1,2-a]pyrimidine on the acyl carbon was identified as a ligand possessing a favorable profile compared to the standard ligands (Table 1). SR 21502 displayed good affinity at the DA D3 receptor with >120-fold binding selectivity over the DA D2 receptor. In the functional activity assay based on inhibition of forskolin-stimulated cAMP accumulation, SR 21502 displayed weak partial agonist activity at DA D3 receptor (27.7 ± 7.0 % inhibition normalized to inhibition by the full agonist quinpirole) and antagonist activity at DA D2 receptor (-1.9 ± 13.3 %). In the agonist-stimulated mitogenesis assay, this compound was characterized as an antagonist at D3 (IC50 = 157 ± 34 nM) and D2 (IC50 = 2300 ± 1100 nM) receptors (Ananthan et al. 2012). On the basis of these favorable characteristics, we chose SR 21502 as a compound of interest for evaluating in vivo activity against cocaine reward and cocaine-seeking. We hypothesized that SR 21502 would reduce BPs for cocaine self-administration under a PR schedule of reinforcement and reduce responding for cues that were previously associated with cocaine taking. We also examined the effects of this compound on food reward, spontaneous locomotor activity and cocaine-induced locomotor activity.
Methods
The protocols used in the present experiments were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Queens College Institutional Animal Care and Use Committee.
Subjects
Subjects were male Long–Evans rats bred in our facility from males and females obtained from Charles River Laboratories (Raleigh, NC). The animals were housed individually with free access to food (Lab Diet) and water and maintained on a reverse 12 h light:12 h dark cycle (lights were turned on at 6 p.m.) and tested during the animals’ active period (the dark cycle).
Surgery
Surgeries were conducted as described previously (Ranaldi et al. 2011). Briefly, rats were anesthetized and silastic catheters were inserted into the jugular vein. The catheters were passed subcutaneously to the top of the skull and mounted to the skull with dental acrylic. The catheter was flushed with heparin solution (200 U/ml) immediately after surgery and every day thereafter.
Apparatus
Cocaine self-administration chambers
Cocaine self-administration sessions were conducted in 16 operant conditioning chambers, eight measuring 30 × 22 × 27 cm (l × w × h) and the other eight measuring 26 × 26 × 30 cm (l × w × h). Each chamber was situated inside a sound-attenuating box and equipped with two retractable levers and a white light above each lever. Polyethylene tubing, covered by a metal tether, connected the animal, through a fluid swivel, to a syringe in a syringe pump (Razel, 3.33 rpm).
Food self-administration chambers
Training and testing took place in ventilated, sound-attenuated operant conditioning chambers measuring 30 × 21 × 18 cm. Each chamber contained two levers, one light above each lever and a food trough. Each lever was positioned 2.5 cm from the rod floor and the food trough (measuring 5 × 5 cm) was centered between the two levers.
Locomotor activity chambers
Locomotor activity chambers measuring 43 × 43 × 30 cm (l × w × h) were used for the locomotor activity experiments. Each chamber was equipped with 32 photo-emitters, 16 positioned along the length and 16 along the width of the chamber, 4 cm above the floor. Each photo-emitter was paired directly opposite a photocell. Locomotor activity counts were registered when adjacent beams were broken consecutively.
Drugs
Cocaine, a gift from the National Institute on Drug Abuse (Bethesda, MD), was dissolved in 0.9 % saline to achieve doses of 0.375, 0.75, 1.00 and 1.5 mg/kg/injection. SR 21502 was provided by Southern Research Institute (Birmingham, AL). It was dissolved in distilled water to achieve doses of 0, 1.875, 3.75, 7.5 and 15 mg/kg and administered in volumes of 1 ml/kg.
Procedure
Experiment 1. Intravenous cocaine self-administration under PR schedule of reinforcement
Animals were trained to self-administer cocaine (0.75 mg/kg/injection) initially under a FR 1 schedule of reinforcement during daily 3-h sessions. Each session began with a priming injection, and subsequent drug injections were earned by lever presses. Responding on the active lever activated the syringe pump for 4.5 s, causing the intravenous delivery of cocaine and the onset of a white light above the active lever for 20 s. Responses on the inactive lever were counted but had no consequences. The active and inactive levers (left/right) were counterbalanced across animals. After the animals demonstrated a steady rate of self-administration for three consecutive sessions, they were placed on a PR schedule of reinforcement starting with one of three randomly chosen doses of cocaine (0.375, 0.75 or 1.5 mg/kg/injection). All PR sessions—training and tests—were 7 h long. The PR schedule required an animal to emit progressively more responses (1, 2, 4, 6, 9, 12, 15, 20…) in order to obtain successive cocaine infusions during a session (see details in Richardson and Roberts 1996). Under this schedule, the requirement for lever pressing becomes so high that eventually the animals stop responding and reach a BP. The BP was operationally defined as the total number of infusions earned prior to a 1-h period during which no infusions were obtained. Animals were tested with a dose of the D3 antagonist when they demonstrated stable BPs. Stable BPs were operationally defined as three consecutive BPs that did not differ by more than two ratio steps and did not show descending or ascending trends. On the test day, the animals were injected with SR 21502 intraperitoneally 10 min prior to the test session. Each animal was tested with one dose of the D3 antagonist (doses were 0, 3.75, 7.5 and 15; N values = 16, 9, 11 and 14, respectively), randomly determined, at as many of the three cocaine doses as possible. After a particular test, the animal’s cocaine dose was changed to one of the others (randomly determined), and its baseline BP on the new dose was established.
Experiment 2. Cue-induced reinstatement
Separate groups of animals were trained to self-administer cocaine (1 mg/kg/injection), initially on a FR1 schedule of reinforcement during 3-h sessions. Responding on the active lever activated the injection pump for 4.5 s and turned on the light cue above the active lever for 20 s. During that 20-s period, the animals could not self-administer cocaine. Responding on the inactive lever was counted but had no programmed consequences. The animals remained on the FR1 schedule for the next five consecutive sessions or until they showed stable responding. Afterward, animals were placed on a FR3 schedule of reinforcement for 10 consecutive sessions followed by an extinction phase. Each extinction session was 3 h long, and responding on either lever produced no consequences. This phase continued until extinction criteria were met; extinction criteria were defined as seven or fewer lever presses per hour on each of the levers for three consecutive sessions. The following day, the animals were injected with one of the SR 21502 doses (0, 1.875, 3.75, 7.5 or 15 mg, N values = 7, 8, 8, 8 and 8, respectively), 20 min prior to the onset of the session, and tested in cue-induced reinstatement. Two presentations of the drug cues, which consisted of the light (20-s duration) and pump activation (4.5-s duration) were made 2 min apart at the beginning of the session. Each response on the active lever was reinforced with the drug cues (but no cocaine). Responding on the inactive lever produced no consequences.
Experiment 3. Food self-administration under PR schedule of reinforcement
A different group of animals (n = 8) was trained to press a lever reinforced by food under a FR1 schedule of reinforcement. Each lever press resulted in the delivery of two food pellets and presentation of the light stimulus for 3 s. When animals demonstrated learning of the lever press response, operationally defined as responding for five consecutive 10-min sessions where the total number of rewards per session was greater than 100, the animals were placed on a PR schedule. After BPs stabilized (stability defined similarly to cocaine self-administration described above), the animals were injected with 0 or 3.75 mg/kg of SR 21502 10 min prior to the test session. Afterward, animals remained on the PR schedule for at least three sessions and until stable BPs were demonstrated before being tested with the other dose of SR 21502.
Experiments 4 and 5. Locomotor activity
Before receiving any drug, the animals were placed in locomotor activity chambers for habituation, for 2 h/day on 3 consecutive days. Based on the ranked total locomotor activity counts observed during the third habituation session, animals were assigned to one of nine treatment groups. Four groups were used to determine whether SR 21502 alone altered locomotor activity and the remaining five groups to determine whether SR 21502 altered cocaine-induced locomotor activity. For the SR 21502 alone experiments, each of the four groups received one of four doses of SR 21502 (0, 3.75, 7.5 or 15 mg/kg, N values = 8 for each group) 10 min prior to placement in the activity chambers. For the SR 21502/cocaine experiments, each of four groups were injected with one of the four doses of the compound (0, 3.75, 7.5 or 15 mg/kg, N values = 8, 8, 9 and 8, respectively) and 10 min later received 10 mg/kg of cocaine as a second injection. The fifth group (N = 8) received the SR 21502 vehicle as the first injection and saline as the second. All animals were placed in the locomotor activity chambers immediately after the second injection.
Data analysis
Experiment 1. Cocaine self-administration under a PR schedule of reinforcement
BP during the test session was expressed as the percentage of the average BP obtained during the last three baseline sessions. These values and final ratio were analyzed using two separate two-way analyses of variance (ANOVAs) with SR 21502 and cocaine dose as between-groups factors. Because not all animals were tested at each level of the cocaine dose factor, this factor was treated as a between-groups rather than as a repeated-measures factor, resulting in a more conservative test. Significant effects were followed by Tukey’s post hoc tests.
Experiment 2. Cue-induced reinstatement
The numbers of lever presses on the active and inactive levers during the reinstatement test were analyzed using a two-way ANOVA with SR 21502 dose as between-groups and lever as within-group factors. Any significant interaction was followed by tests of simple effect of SR 21502 dose on each lever. Significant effects were followed by Tukey’s post hoc tests.
Experiment 3. Food self-administration under a PR schedule of reinforcement
Percentages of baseline BPs for PR responding for food were analyzed using a dependent measures t-test comparing the 0 and 3.75 mg doses of SR 21502.
Experiments 4 and 5. Locomotor activity
The locomotor counts in the SR 21502 alone and SR 21502/cocaine experiments were analyzed using two separate, mixed-design ANOVAs with SR 21502 dose (between groups) and 5-min bins (repeated measures) as factors. Significant interactions were followed by tests of simple effects of SR 21502 dose at each time interval.
Results
Experiment 1. Cocaine self-administration under a PR schedule of reinforcement
SR 21502 caused a dose-related reduction in the percentage of baseline BPs such that the two higher doses (7.5 and 15 mg/kg) reduced BPs to a greater degree than the 3.75 mg/kg dose (Fig. 2b). The patterns of BP reductions with the 7.5 and 15 mg/kg doses did not appear different from each other. A two-way ANOVA on BPs with SR 21502 dose and cocaine dose as factors revealed significant main effects of the D3 receptor antagonist (F 3,97 = 13.11, p < 0.05) and cocaine (F 2,97 = 5.52, p < 0.05). Tukey’s post hoc comparisons among SR 21502 doses revealed that the animals receiving 3.75, 7.5 or 15 mg of SR 21502 showed significant reductions in the percentage of baseline BPs (ps < 0.05) compared to vehicle. The 3.75 mg dose of SR 21502 differed significantly from the 15 mg dose (p < 0.05). We also investigated the effects of SR 21052 on final ratio. For each dose of SR 21502 responding under the influence of the antagonist increased as a function of cocaine dose (Fig. 2b). This was supported by a two-way ANOVA (SR 21502 dose × cocaine dose) on final ratio data revealing significant SR 21502 dose and cocaine dose effects (F 3,97 = 5.14 and F 2,97 = 7.44; ps < 0.05, respectively). Therefore, animals responded at higher rates under the same dose of the antagonist when cocaine reward was increased, demonstrating surmountable antagonism.
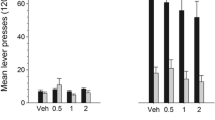
a Effects of various doses of SR 21502, administered intraperitoneally 10 min prior to the start of the session, on BPs of intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. * SR 21502 doses significantly different from vehicle, # dose of SR 21502 significantly different from 3.75 mg/kg, SR SR 21502. b Effects of various doses of SR 21502, administered intraperitoneally 10 min prior to the start of the session, on final ratios of intravenous cocaine self-administration under a PR schedule of reinforcement
Experiment 2. Cue-induced reinstatement
Cue-induced reinstatement of responding on the active lever was reduced with SR 21502 in a manner related to the dose, the higher the dose the greater the reduction in lever pressing (Fig. 3). In all groups, active lever pressing was greater than inactive lever pressing (Fig. 3). These observations were supported by statistical analyses. A two-way ANOVA showed a significant dose by lever interaction (F 4,35 = 5.49, p < 0.05). Tests of simple effect of dose at each lever revealed a significant effect on the active lever (F 4,35 = 15. 5, p < 0.05), but not on the inactive lever. Tukey’s post hoc tests showed that the groups treated with 3.75, 7.5 and 15 mg of SR 21502 differed significantly in responding on the active lever from the vehicle group (p values < 0.05), and the 1.875 mg dose differed significantly from the 7.5 and 15 mg (p values < 0.05) groups.
Effects of various doses of SR 21502, administered intraperitoneally 20 min prior to the start of the session, on cue-induced reinstatement of lever pressing; the cocaine-associated light and pump activation were presented at the start of the session and again 2 min later. Active lever presses were reinforced with the light-pump stimulus but not with cocaine. Inactive lever presses produced no consequences. * Active lever pressing significantly different from vehicle, + active lever presses significantly different from 1.875 mg/kg at p < 0.05
Experiment 3. Food self-administration under a PR schedule of reinforcement
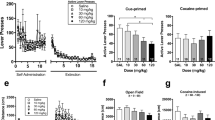
The 3.75 mg dose of SR 21502, the lowest dose that caused a significant reduction in cocaine BPs (Fig. 2b) and cue-induced reinstatement (Fig. 3), did not affect food self-administration under a PR schedule of reinforcement. A repeated-measures t-test failed to show a significant difference in the percentage of baseline BP between the 0 and 3.75 mg doses.
Experiments 4 and 5. Locomotor activity
Figure 4a shows the effects of SR 21502 administered alone on spontaneous locomotor activity. The 3.75 mg dose, which significantly reduced cocaine BPs and cue-induced reinstatement but had no effect on food BPs, had no effect on spontaneous locomotor activity. For the 7.5 and 15 mg doses, locomotor activity in the first 5 min of testing was lower than for the 3.75 and vehicle doses, but this was not significant. A two-way mixed-design ANOVA with SR 21502 dose (between groups) and time interval (repeated measures) as factors showed a significant time effect (F 23,621 = 64.12, p < 0.05) but no other effects or interactions.
a Effect of various doses of SR 21502, administered intraperitoneally 10 min prior to the test sessions, on spontaneous locomotor activity. b Effect of SR 21502, administered intraperitoneally 10 min prior to the test sessions, on cocaine-induced locomotor activity. Cocaine was administered intraperitoneally, immediately prior to the start of the sessions. * The vehicle–cocaine dose is significantly different from the vehicle–vehicle dose, # the vehicle–cocaine dose is significantly different from the 15 mg/kg dose, & the vehicle–cocaine dose is significantly different from the 7.5 mg/kg dose, ^ the vehicle–cocaine dose is significantly different from the 3.75 mg/kg dose, SR SR 21502
Figure 4b shows the effects of SR 21502 on cocaine-induced locomotor activity. Groups treated with any of the SR 21502 doses and cocaine showed less locomotor activity than the vehicle–cocaine group, but not less than the vehicle–vehicle group, during the first 20 min of the session. This reduction of cocaine-induced locomotor activity appeared to be dose-related; the higher SR 21502 doses produced greater reductions in cocaine-induced locomotion as compared to the lower doses. A two-way, mixed-design ANOVA with SR 21502 dose (between groups) and time interval (repeated measures) as factors showed a significant SR 21502 dose by time interaction (F 92,805 = 2.9, p < 0.05), a significant SR 21502 dose effect (F 4,35 = 5.91, p < 0.05) and a significant time effect (F 23,805 = 73.4, p < 0.05). Tests of simple effects of SR 21502 dose at each time interval showed significant dose effects at the 5-, 10-, 15-, 20-, 30- and 35-min intervals. Tukey’s post hoc tests revealed that the vehicle–cocaine group differed significantly from the vehicle–vehicle group at the 10-, 15-, 20-, 30- and 35-min intervals, from the SR 21502 15 mg dose at the 5-, 10-, 15- and 20-min intervals, from the SR 21502 7.5 mg dose at the 5- and 15-min intervals and from the SR 21502 3.75 mg dose at the 15-min interval (p values < 0.05).
Discussion
The purpose of the present study was to investigate whether or not the novel DA D3 receptor antagonist, SR 21502, reduces cocaine reward and cocaine-seeking in self-administration procedures. We found that SR 21502 caused a dose-related reduction in BPs for cocaine under a PR schedule of reinforcement. We also found that SR 21502 caused a dose-related reduction in cue-induced and cue-reinforced responding during the reinstatement test. Furthermore, the lowest dose of SR 21502 that caused a significant reduction in BPs for cocaine reward and reinstatement did not affect BPs for food reward. SR 21502 did not significantly affect spontaneous locomotor activity at any time during the test session. However, SR 21502 did significantly reduce cocaine-induced locomotor activity in a dose-related fashion.
The reduction in lever pressing for cocaine cannot be explained as resulting from incapacitation of the lever press response since rats treated with any one dose of SR 21502 demonstrated that they were able to produce higher rates of the lever press response under the same dose of SR 21502 if the cocaine dose was raised (see Fig. 2b). This suggests that antagonist-reduced responding was not due to incapacitation of the lever press response, since greater active lever pressing was demonstrated under the same doses of antagonist that reduced responding if motivation to respond was increased by increasing the cocaine reward magnitude (dose). Also, SR 21502 did not reduce inactive lever pressing in the reinstatement study, suggesting that its effects were selective rather than nonspecific. Likewise, the reduced active lever pressing (in the self-administration and reinstatement studies) cannot be explained by reductions in general motoric activity since none of the SR 21502 doses caused significant reductions in spontaneous locomotor activity. Thus, the best explanation for the observed reduction in BPs for cocaine is that SR 21502 reduces cocaine reward. Likewise, the best explanation for the reduced cue-induced reinstatement of responding is that it reduces cocaine-seeking. Although SR 21502 failed to significantly affect spontaneous locomotor activity, it did significantly reduce cocaine-induced locomotor activity. Finally, we found that a dose of SR 21502 that caused significant reductions in BPs for cocaine and cue-induced reinstatement failed to affect BPs for food reward. All in all, these findings suggest that this compound’s effects are relatively selective for the rewarding, incentive motivational and stimulant effects of cocaine.
The present findings suggest a role for DA D3 receptors in cocaine reward and are in accord with previous studies showing cocaine reward reductions by other D3 receptor antagonists. For instance, SB 277011A, NGB 2904, S33138 and YQA14 have been tested in cocaine self-administration under the PR schedule of reinforcement (Peng et al. 2009; Song et al. 2011; Xi and Gardner 2007; Xi et al. 2005) and have been shown to reduce BPs.
It is difficult to make direct comparisons of the behavioral effects of these compounds, because different methods and protocols and doses of cocaine were used for the different tests in different labs. Nevertheless, we could attempt comparisons in cases when experimental design features come close. We found that under the PR schedule, SR 21502 produced as much as 35 % reduction in BPs at the 0.75 mg/kg dose of cocaine, an effect that was surmountable by increasing the dose of cocaine. SB 277011A produced approximately 70 % reduction on BP when tested at all doses of cocaine (0.25, 0.5 and 1.0 mg/kg; Xi et al. 2005); the absence of surmountable antagonism makes it difficult to interpret whether these reductions were cocaine reward deficits or performance deficits. In reinstatement tests, we found that SR 21502 produced as much as 40 % reduction in cue-induced reinstatement. SB-77011A produced as much as 80 % and NGB 2904 as much as 60 % reduction in cue-induced responding (Gilbert et al. 2005). However, our training cocaine dose was higher; we used an FR3 schedule during training, and our extinction criteria were less stringent than in the other study making our baseline reinstatement effect perhaps more resistant to antagonism. Again, the lack of uniformity among protocols makes it difficult to make valid comparisons among these compounds.
The capacity of D3 receptor antagonists to reduce cocaine reward also has been assessed using the FR1 schedule of reinforcement; however, the results from such studies remain inconclusive. Typical reward-attenuating effects of DA antagonists on psychostimulant self-administration under FR schedules of reinforcement consist of compensatory increases in self-administration rate at low antagonist doses and cessation of responding at higher doses (Caine and Koob 1994; de Wit and Wise 1977; Ettenberg et al. 1982; Woolverton and Virus 1989). However, NGB-2904 and SB-277011A failed to reduce cocaine self-administration (Gal and Gyertyan 2003; Xi et al. 2005), while YQA14 produced only decreases in cocaine intake (Song et al. 2011), making it difficult to conclude that any antagonist effects were on cocaine reward and not on response capacity or other related behaviors.
However, a role for D3 receptors in cocaine reward has been shown in brain stimulation reward (BSR) studies. Acute administration of SB-277011A or NGB 2904 blocks the enhancement of BSR by cocaine in rats (Vorel et al. 2002; Xi et al. 2006). Interestingly, when these D3 receptor antagonists were administered alone, they failed to affect BSR thresholds, suggesting that D3 receptor stimulation itself is not involved in BSR per se but is involved in cocaine reward. While reductions in BSR thresholds measure the direct rewarding value of cocaine, cocaine-induced conditioned place preference measures cocaine reward indirectly, by providing information about the rewarding effects of contextual cues associated with cocaine. There is evidence that D3 receptor stimulation is involved in expression of cocaine conditioned place preference; when D3 receptor antagonists were administered after conditioning and just prior to the conditioned place preference test, they blocked the expression of the preference for the cocaine-paired place (Cervo et al. 2005; Macdonald et al. 2003; Micheli et al. 2007; Vorel et al. 2002). When the antagonists were administered prior to conditioning sessions, but not prior to the preference test, animals either failed to show the conditioned place preference (Vorel et al. 2002) or did show the conditioned place preference (Gyertyan and Gal 2003), suggesting a blockade or no effect on the acquisition, respectively, of cocaine conditioned place preference by D3 receptor antagonism. Thus, as in tests of the D3 receptor antagonists in FR1 cocaine self-administration, tests of the effects of D3 antagonists on cocaine reward, as determined by the acquisition of a cocaine place preference, remain inconclusive.
Growing evidence indicates that these D3 receptor antagonists reduce not only cocaine reward but also cocaine-seeking. In the reinstatement paradigm, both SB277011A and NGB-2904 reduce the reinstatement of cocaine-seeking triggered by cocaine itself (Vorel et al. 2002; Xi and Gardner 2007; Xi et al. 2006), cocaine cues (Gilbert et al. 2005; Xi and Gardner 2007) or foot-shock stress (Xi et al. 2004). Thus, D3 receptor antagonists, under most circumstances, reduce both cocaine reward and cocaine-seeking. Such findings are congruent with our results indicating that SR 21502 is effective at reducing incentive motivation to self-administer cocaine and seek it.
A large number of studies indicate that D3 receptors are involved in reactivity to rewards (i.e., drugs) and to cues that are associated with rewarding effects of drugs. In humans, such cues can elicit cravings and lead to relapse (Childress et al. 1988, 1993; Ehrman et al. 1992); in animals, they induce reinstatement of extinguished drug-seeking responses (Arroyo et al. 1998; de Wit and Stewart 1981; Shalev et al. 2002). DA D3 receptor ligands, therefore, by reducing the reinstatement of drug-seeking, may be effective in preventing cocaine-seeking in humans (for review, see Heidbreder 2008; Heidbreder and Newman 2010).
In conclusion, the present findings demonstrate that SR 21502, a novel selective DA D3 receptor antagonist decreases cocaine reward, cocaine-seeking and cocaine-induced locomotor activity at doses that have no effect on food reward or spontaneous locomotor activity. Such a behavioral pharmacological profile—selective reduction of cocaine reward, cocaine-seeking and stimulant effects—suggests that this compound has potential as a psychotherapeutic agent for cocaine addiction and certainly merits further investigation as such.
References
Ananthan S, Saini SK, Zhou G, Hobrath JV, McDowell S, Mishra Y, Griffin SA, Luedtke RR (2012) Structure–activity relationships in a novel series of DA D3 receptor selective ligands. Soc Neurosci Meet 2012. Abstract Number 780.20
Arroyo M, Markou A, Robbins TW, Everitt BJ (1998) Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology 140:331–344
Caine SB, Koob GF (1993) Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science 260:1814–1816
Caine SB, Koob GF (1994) Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270:209–218
Cervo L, Burbassi S, Colovic M, Caccia S (2005) Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol Biochem Behav 82:727–734
Childress AR, Ehrman RN, McLellan AT, O’Brien CP (1988) Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr 81:74–80
Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137:73–95
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143
de Wit H, Wise RA (1977) Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with noradrenergic blockers phentolamine and phenoxybenzamine. Can J Psychol 31:195–203
Diaz J, Levesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P (1995) Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 65:731–7345
Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P (2000) Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20:8677–8684
Ehrman RN, Robbins SJ, Childress AR, O'Brien CP (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107:523–529
Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982) Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 78:204–209
Gal K, Gyertyan I (2003) Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull 61:595–601
Gilbert JG, Newman AH, Gardner EL, Ashby CR Jr, Heidbreder CA, Pak AC, Peng XQ, Xi ZX (2005) Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse 57:17–28
Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH (2005) Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem 48(3):839–848
Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, Jenkins BG, Luedtke RR, Newman AH (2007) Heterocyclic analogues of N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem 50:4135–4146
Gyertyan I, Gal K (2003) Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. Neuroreport 14:93–98
Heidbreder C (2008) Selective antagonism at dopamine D3 receptors as a target for drug addiction pharmacotherapy: a review of preclinical evidence. CNS Neurol Disord Drug Targets 7:410–421
Heidbreder CA, Newman AH (2010) Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci 1187:4–34
Macdonald GJ, Branch CL, Hadley MS, Johnson CN, Nash DJ, Smith AB, Stemp G, Thewlis KM, Vong AK, Austin NE, Jeffrey P, Winborn KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Watson JM, Wood M, Parker SG, Ashby CR Jr (2003) Design and synthesis of trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxadiazolyl))- phenyl)carboxamido)cyclohexyl)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1 H-3-benzazepine (SB-414796): a potent and selective dopamine D3 receptor antagonist. J Med Chem 46:4952–4964
Mason CW, Hassan HE, Kim KP, Cao J, Eddington ND, Newman AH, Voulalas PJ (2010) Characterization of the transport, metabolism, and pharmacokinetics of the dopamine D3 receptor-selective fluorenyl- and 2-pyridylphenyl amides developed for treatment of psychostimulant abuse. J Pharmacol Exp Ther 333:854–864
Micheli F, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Curcuruto O, Damiani F, Fabio RD, Donati D, Gentile G, Gribble A, Hamprecht D, Tedesco G, Terreni S, Tarsi L, Lightfoot A, Stemp G, Macdonald G, Smith A, Pecoraro M, Petrone M, Perini O, Piner J, Rossi T, Worby A, Pilla M, Valerio E, Griffante C, Mugnaini M, Wood M, Scott C, Andreoli M, Lacroix L, Schwarz A, Gozzi A, Bifone A, Ashby CR Jr, Hagan JJ, Heidbreder C (2007) 1,2,4-triazol-3-yl-thiopropyl-tetrahydrobenzazepines: a series of potent and selective dopamine D(3) receptor antagonists. J Med Chem 50:5076–5089
Murray AM, Ryoo HL, Gurevich E, Joyce JN (1994) Localization of dopamine D 3 receptors to mesolimbic and D 2 receptors to mesostriatal regions of human forebrain. Proc Natl Acad Sci U S A 91:11271–11275
Peng XQ, Ashby CR Jr, Spiller K, Li X, Li J, Thomasson N, Millan MJ, Mocaer E, Munoz C, Gardner EL, Xi ZX (2009) The preferential dopamine D3 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking behavior in rats. Neuropharmacology 56:752–760
Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30:215–238
Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P (1999) Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 400:371–375
Platt DM, Rowlett JK, Spealman RD (2002) Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 163:265–282
Ranaldi R, Wise RA (2001) Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: possible role for dendritically released dopamine. J Neurosci 21:5841–5846
Ranaldi R, Kest K, Zellner M, Hachimine-Semprebom P (2011) Environmental enrichment, administered after establishment of cocaine self-administration, reduces lever pressing in extinction and during a cocaine context renewal test. Behav Pharmacol 22:347–353
Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Ritchie TJ, Macdonald SJF, Peace S, Pickett SD, Luscombe CN (2013) Increasing small molecule drug devolopability in sub-optimal chemical space. Med Chem Commun 4:673–680
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Scwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D 3) as a target for neuroleptics. Nature 347:146–150
Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ, Li X, Gaal J, Xi ZX, Gardner EL (2011) YQA14: a novel dopamine D(3) receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D(3) receptor-knockout mice. Addict Biol 17:14
Vorel SR, Ashby CR Jr, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22:9595–9603
Willuhn I, Wanat MJ, Clark JJ, Phillips PE (2010) Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci 3:29–71
Wise RA (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Wise RA (2005) Forebrain substrates of reward and motivation. J Comp Neurol 493:115–1121
Woolverton WL, Virus RM (1989) The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav 32:691–697
Xi ZX, Gardner EL (2007) Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev 13:240–259
Xi ZX, Gilbert J, Campos AC, Kline N, Ashby CR Jr, Hagan JJ, Heidbreder CA, Gardner EL (2004) Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology (Berl) 176:57–65
Xi ZX, Gilbert JG, Pak AC, Ashby CR Jr, Heidbreder CA, Gardner EL (2005) Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci 21:3427–3438
Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR Jr, Gitajn L, Gardner EL (2006) The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology 31:1393–1405
Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JW, Thurkauf A (1998) NGB 2904 and NGB 2849: two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett 8(19):2715–2718
Acknowledgments
We want to thank Adjoa Anor and Myriam Waheed for their technical assistance in conducting these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galaj, E., Ananthan, S., Saliba, M. et al. The effects of the novel DA D3 receptor antagonist SR 21502 on cocaine reward, cocaine seeking and cocaine-induced locomotor activity in rats. Psychopharmacology 231, 501–510 (2014). https://doi.org/10.1007/s00213-013-3254-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3254-y