Abstract
Rationale
Varenicline is the most effective drug for smoking cessation, but its use decreased because of reports of depressogenic side effects. However, because smoking and smoking cessation on their own are associated with depression, it remains unclear whether reported depressogenic effects are attributable to varenicline, or to smoking, and/or smoking cessation themselves.
Objectives
Previously, we observed no depressogenic effects of varenicline on a psychological level. In the present study, we aimed at investigating potential depressogenic effects of the partial nicotinergic acetylcholine receptor agonist varenicline on a biological level. A possible pathway would be an effect of varenicline on the hypothalamic–pituitary–adrenal (HPA) axis, considering the relation between the HPA axis and (1) the cholinergic system and (2) depression.
Methods
In a randomized, double-blind design, we administered varenicline or placebo for 7 days (0.5 mg/day first 3 days, then 1 mg/day) to healthy never-smoking subjects, thereby eliminating bias by (previous) smoking status. We used repeated measures (before and after treatment) of the salivary free cortisol awakening response to measure HPA axis activity and flexibility.
Results
Salivary cortisol data of 34 subjects were included in the analysis. Results showed no effect of varenicline on height (F 1,32 = 0.405; P = 0.529) or shape (F 2,31 = 0.110; P = 0.164) of the cortisol awakening response.
Conclusions
Results do not suggest depressogenic effects of varenicline on the HPA axis. Although this does not preclude other biological depressogenic effects of varenicline, it seems that concerns about effects of varenicline on the HPA axis should not limit its potential to treat nicotine and related addictions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco use, resulting from nicotine addiction, is the leading cause of preventable disability and death. Smoking cessation can potentially reverse this disease burden; however, chances of success for smoking cessation attempts without therapy are low (Cahill et al. 2012).
Varenicline is the most effective smoking cessation treatment available. As a partial competitive agonist of α4β2 nicotinic acetylcholine (nACh) receptors, varenicline prevents nicotine-induced reward and withdrawal symptoms (Brandon et al. 2011; Cahill et al. 2012; Perkins et al. 2010). In addition, varenicline has been proposed in the treatment of alcohol abuse (Mitchell et al. 2012; Sotomayor-Zárate et al. 2013). Despite its effectiveness, varenicline use decreased because of reported associations with depression and suicidal ideation, leading to an FDA-boxed warning (Moore et al. 2011).
However, because smoking and smoking cessation on their own can cause depression, it remains unclear whether reported adverse effects can be explained by varenicline use, or by smoking and/or smoking cessation themselves. Moreover, in contrast to reported depressogenic effects, varenicline has been proposed as potential antidepressant (Philip et al. 2010). Meta-analysis of randomized controlled clinical trials of varenicline reported increased sleep disorder incidence, but found no association with psychiatric adverse events (Tonstad et al. 2010). However, these trials were underpowered to detect rare—but serious—adverse events.
We previously investigated whether risk for psychiatric adverse events should limit varenicline's potential to decrease the nicotine addiction-associated burden of disease (Mocking et al. 2013). In a randomized, placebo-controlled trial design, we administered varenicline to never-smoking subjects, thereby eliminating possible biases by smoking status. Findings suggest that short-term varenicline does not lead to early predictive depressogenic biases in a sensitive neuropsychological test battery.
However, besides this psychological pathway, varenicline may exert potential depressogenic effects on a biological level. A possible candidate may be the hypothalamic–pituitary–adrenal (HPA) axis because (1) altered HPA axis activity and flexibility is associated with depression, and interestingly, (2) the cholinergic system and the HPA axis are intimately linked.
Literature implicates altered HPA axis activity and flexibility in depression (Stetler and Miller 2011). Furthermore, HPA axis alterations, e.g., Cushing's disease or corticosteroid treatment, may induce depression (Langenecker et al. 2012). In addition, HPA axis alterations predict depression development or recurrence (Appelhof et al. 2006; Goodyer et al. 2000). Therefore, the HPA axis has been used as a measure of risk of depression development (Browning et al. 2012).
With regard to the link between the cholinergic system and the HPA axis, stimulation of nACh receptors raises concentrations of HPA axis hormones corticotropin releasing hormone (CRH), adrenocorticotropic hormone, and cortisol, possibly through nACh receptors on CRH-releasing neurons (Philip et al. 2010). Furthermore, mecamylamine, a nACh receptor antagonist, prevents hypothalamic CRH release (Philip et al. 2010). Short-term nACh receptor stimulation activates the HPA axis (Kirschbaum and Hellhammer 1994); chronic stimulation (e.g., habitual smoking) seems to induce HPA axis hyperactivity and reduced flexibility (Philip et al. 2010; Vreeburg et al. 2009). Effects of nACh receptor modulation on the HPA axis can already occur within a short time frame, i.e., minutes to hours (Fuxe et al. 1989; Philip et al. 2010; Raber et al. 1995; Rhodes et al. 2001).
So even though varenicline does not seem to exert depressogenic biases in emotional processing, varenicline might induce depressogenic alterations in HPA axis activity and flexibility. In addition, through the association between HPA axis alterations and sleep (Vreeburg et al. 2009), an HPA axis effect of varenicline could explain varenicline-associated sleep disturbances.
To investigate whether altered HPA axis activity and flexibility may explain reported depressogenic effects of varenicline, independent of smoking status, we aimed at assessing the effects of short-term varenicline use on HPA axis activity and flexibility in never-smoking subjects. We hypothesized that short-term varenicline use would induce HPA axis hyperactivity and decreased flexibility.
Methods
Participants
Methods of this randomized, placebo-controlled trial have been published previously (Mocking et al. 2012). We recruited participants aged 18–35, physically fit (assessed by physicians) with a body mass index of 18.5–30 kg/m2. To exclude possible biases by (previous) nicotine use, we excluded subjects currently or previously using any form of tobacco. We used the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al. 1996) to exclude subjects with personal and/or family histories of drug and/or alcohol dependency, psychiatric illnesses, or suicidal ideation or acts, to minimize risk of adverse events for ethical reasons. We excluded subjects who were taking psychotropic medication, had taken part in studies involving medication, or used recreational drugs within the last 3 months. All participants gave written informed consent. The study was reviewed by the Berkshire Research Ethics Committee (10/H05050/26). Participants received £125 reimbursement for participation.
Procedure
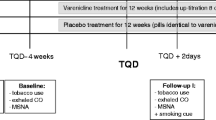
We randomized participants to receive either ten capsules of 0.5 mg varenicline tartrate (intervention; manufactured by Pfizer pharmaceuticals) or placebo (control; lactose pills), identically packed to guarantee blinding. We administered capsules for 7 days, using titration as recommended by the manufacturer. We instructed participants to take one capsule at 0800 hours on days 1–3 and day 7, and an additional capsule on days 4–6 at 2000 hours.
Measures
We measured HPA axis activity and flexibility both before (day 1, before the first capsule) and after (day 7, before the last capsule) the intervention, to increase power by modeling intra-subject variability. We used salivary free cortisol awakening responses as a sensitive, reliable, and non-invasive measurement of HPA axis activity and flexibility (Kirschbaum and Hellhammer 1994). To measure cortisol-awakening responses, we provided participants with two different saliva sampling sets, each consisting of three saliva tubes (Salivette™, Sarstedt). Of each set, we asked participants to use the first sample tube immediately after waking up in the morning while still lying in bed, the second tube 15 min later, and the third tube 30 min later.
We instructed patients not to eat, drink, brush their teeth, or engage in strenuous activity during saliva sampling, and to refrain from alcohol and recreational drugs during the study. In addition, we asked participants to store samples in their home freezer until taking them with them when they came for the testing. We centrifuged saliva samples and stored them at −20 °C, until cortisol was measured using a commercially available double-antibody radio-immunoassay and expressed in nanomoles per liter. Both intra- and inter-assay variability were <5 %.
Statistical analyses
We did not have to exclude any outliers and observed no deviations from normality after logarithmic transformation of cortisol data. We analyzed data with SPSS IBM 19 using a full-factorial repeated-measures analysis of variance (RMANOVA) with time (baseline/follow-up) and moment (awakening/+15 min/+30 min) as within-subject factors, treatment (varenicline/placebo) as between-subject factor, and cortisol as outcome variable. Whenever the assumption of sphericity was violated, we used Greenhouse–Geisser corrected results.
We performed a power calculation with G*Power 3.1.3. With 34 participants, divided over two groups, using two repeated measures, we had adequate power (0.80) to detect within–between interaction effects on the upper border of small-sized effects (f = .248).
Results
Of 41 included participants, 21 were randomized and allocated to receive varenicline and 20 placebo. Due to side effects [nausea (N = 1, on day 2) and flu-like symptoms (N = 1, on day 3)], two participants (<10 %) withdrew from the varenicline group. Because one more participant in the intervention group had to be excluded due to missing data, 38 participants successfully delivered salivary cortisol samples (varenicline group, N = 18; placebo group, N = 20).
Due to a laboratory error, saliva samples of four participants had to be excluded. This resulted in an equal distribution of 17 participants in the varenicline group and 17 in the placebo group at both time points for HPA axis analyses. Age and sex did not significantly differ between both groups (Table 1).
The RMANOVA showed no significant between-subject treatment effect (F 1 = 0.074, P = 0.788), nor significant time (F 1,32 = 0.376, P = 0.544), or time × moment effects (F 2,31 = 1.59, P = 0.219). This means that the varenicline and placebo groups showed no cortisol differences present both before and after treatment, and the overall cortisol response to awakening does not differ between the beginning and the end of the study. The factor “moment” was highly significant (F 2,31 = 21.6, P < 0.001), which reflects the expected rise in cortisol after awakening.
With regard to the effect of treatment (differences before and after varenicline vs. placebo), the RMANOVA showed no significant time × treatment effect (F 1,32 = 0.405, P = 0.529), nor a significant time × moment × treatment effect (F 2,31 = 1.92, P = 0.164). This implies that, compared to placebo, varenicline treatment induced no significant changes in cortisol awakening response overall height or shape (Fig. 1).
Cortisol morning awakening responses at day 7 of randomized treatment with either placebo (N = 17) or varenicline (N = 17). Results of the repeated-measures analysis of variance including baseline (before treatment) and day 7 (after treatment) cortisol concentrations; time: F 1,32 = 0.376, P = 0.544; moment: F 2,31 = 21.6, P < 0.001; treatment: F 1 = 0.074, P = 0.788; time × moment: F 2,31 = 1.59, P = 0.219; time × treatment: F 1,32 = 0.405, P = 0.529; moment × treatment: F 2,31 = 0.820, P = 0.450; time × moment × treatment: F 2,31 = 1.92, P = 0.164. T0 immediately after waking up, T1 15 min after T0, T2 30 min after T0
Discussion
To investigate biological explanations for suggested depressogenic effects of varenicline, the present study aimed at assessing varenicline's influence on HPA axis activity and flexibility independent from smoking cessation. In a double-blind, randomized study design, never-smoking participants were asked to provide saliva samples for repeated cortisol measurements, before and after treatment with either varenicline or placebo for 7 days. Results show no significant influence of varenicline on cortisol, thereby not suggestive of depressogenic effects on the HPA axis.
The observed absence of effects of varenicline on overall height and/or shape of the cortisol awakening response implies that the hypotheses of varenicline-induced HPA axis hyperactivity and reduced flexibility had to be rejected. Because of the link between the HPA axis and both (1) the cholinergic system (Philip et al. 2010) and (2) depression and sleep (Appelhof et al. 2006; Browning et al. 2012; Goodyer et al. 2000; Langenecker et al. 2012; Stetler and Miller 2011; Vreeburg et al. 2009), alterations in HPA axis activity and flexibility would provide a possible biological explanation for varenicline's sleep disturbing and suggested depressogenic effects. However, our results show no effect of varenicline on the HPA axis, which may have several implications.
First, it might be that varenicline does not exert any biological depressogenic effects. This would be in accordance with the finding that varenicline also shows no depressogenic effects in non-smokers on a psychological level (Mocking et al. 2012). In addition, a pooled analysis of ten placebo-controlled, double-blind controlled trials showed no significant increase in overall psychiatric disorders in varenicline-treated, smoking subjects (Tonstad et al. 2010). However, these trials were underpowered to detect rare—but serious—adverse events.
Alternatively, it may be that varenicline exerts its suggested depressogenic effects through other less well-known biological pathways. This would fit with associations of varenicline with depression and suicide reported in post-marketing surveillance studies (Moore et al. 2011). However, these studies could have been subject to several forms of biases (Moore et al. 2011). Furthermore, it may be that varenicline only has a depressogenic effect on the HPA axis in people with a high baseline cholinergic stimulation [e.g., current smokers, subjects with a (family) history of depression], which were excluded in the current study. Future randomized controlled trials in diverse populations measuring both clinical and biological outcome factors could provide further insight in the suggested depressogenic effects of varenicline.
Besides clinical implications, our results also provide insight in the physiological relationship between the cholinergic system and the HPA axis. While we hypothesized that varenicline would induce hyperactivation and reduced flexibility of the HPA axis, we observed no effects. This may be because we had to base our hypotheses on the HPA axis effects of the full nAch receptor agonist nicotine (Philip et al. 2010; Vreeburg et al. 2009) because to our knowledge, this is the first study investigating the effect of a partial nACh receptor agonist on the HPA axis.
Some limitations should be addressed. First, as explained previously (Mocking et al. 2012), a lower dosage than recommended by the manufacturer (0.5 mg BID vs. 1 mg BID) was used because (1) manufacturer's recommendations are intended for smokers, and may cause tolerability issues in our never-smoking population; (2) used dose results in still 60 % of steady-state kinetics using manufacturer's recommendations (Faessel et al. 2010); and (3) used varenicline dose is still therapeutically effective, with only slightly lower abstinence rates compared to manufacturer's dose (Oncken et al. 2006). In addition, the two dropouts from the varenicline group due to, e.g., nausea, and the significantly higher nausea scores in the varenicline group (Mocking et al. 2013), suggest that the used dose was at the upper tolerability limit in this sample. Second, the used methodology allowed investigation of 7 days varenicline treatment. It may be that potential depressogenic effects of varenicline on the HPA axis occur at later stages of varenicline treatment. Nevertheless, preclinical literature and data on cigarette smoking suggest rapid effects of nACh receptor modulation on the HPA axis, i.e., minutes to hours (Fuxe et al. 1989; Philip et al. 2010; Raber et al. 1995; Rhodes et al. 2001). Future research applying more chronic and/or higher dosages of varenicline treatment should investigate the effects of varenicline on the HPA axis in the long term and in higher concentrations.
Third, no drug screening was used to affirm nicotine abstinence. However, absence of cortisol outliers suggests no participants used nicotine. Fourth, no measure of HPA axis feedback was used (e.g., dexamethasone suppression test). However, the cholinergic system is not suggested to affect HPA axis feedback. Fifth, it is not possible to completely rule out non-compliance to the saliva sampling procedure. We verbally ascertained that no severe deviations occurred, which is corroborated by the significant moment factor in our model. Nevertheless, minor irregularities may have distorted results. Sixth, we did not extensively measure sleep characteristics. This would have been interesting considering the association of varenicline with sleep changes, and sleep changes can influence morning cortisol. However, we previously showed that both groups did not differ on reported rates of insomnia or odd dreams (Mocking et al. 2013). In addition, if changes in sleep due to varenicline would have resulted in different morning cortisol concentrations, this would be shown in the applied model. Finally, due to a laboratory error, saliva samples of four participants had to be excluded. Since (1) this missingness was completely at random, and (2) power analysis shows that adequate power remained to detect effects at the upper level of small-sized effects, this likely has not changed results.
The present study also had particular strengths. To our knowledge, this is the first study investigating the effects of a partial nACh receptor agonist on HPA axis activity and flexibility. In addition, by studying never-smoking subjects, influence of varenicline remained unconfounded from any bias by current and/or previous smoking status.
In conclusion, 7 days varenicline treatment in healthy non-smoking subjects had no influence on the cortisol awakening response, thereby not suggestive of depressogenic effects on the HPA axis. This fits with earlier findings suggesting no neuropsychological depressogenic effects, but does not preclude other biological depressogenic effects of varenicline. Further trials in other populations using biological and clinical outcome measures—combined with results from post-marketing surveys—are needed to determine whether risk for serious adverse neuropsychiatric events should limit varenicline's potential to treat nicotine and related addictions, and thereby reduce the tobacco use-associated health impact.
References
Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, Hoogendijk WJ, Tijssen JG, Wiersinga WM, Schene AH (2006) Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression). Biol Psychiatry 59:696–701
Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC, Karver SB, Small BJ (2011) Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacol 218:391–403
Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ (2012) Using attentional bias modification as a cognitive vaccine against depression. Biol Psychiatry 72:572–579
Cahill K, Stead LF, Lancaster T (2012) Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 4, CD006103
Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH (2010) A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet 49:799–816
First MB, Spitzer RL, Gibbon M, Williams JB (1996) User's guide for the Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Association, Washington
Fuxe K, Andersson K, Eneroth P, Härfstrand A, Agnati LF (1989) Neuroendocrine actions of nicotine and of exposure to cigarette smoke: medical implications. Psychoneuroendocrinology 14:19–41
Goodyer IM, Herbert J, Tamplin A, Altham PM (2000) Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. Br J Psychiatry 177:499–504
Kirschbaum C, Hellhammer DH (1994) Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 19:313–333
Langenecker SA, Weisenbach SL, Giordani B, Briceño EM, Guidotti Breting LM, Schallmo MP, Leon HM, Noll DC, Zubieta JK, Schteingart DE, Starkman MN (2012) Impact of chronic hypercortisolemia on affective processing. Neuropharmacology 62:217–225
Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL (2012) Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacol 223:299–306
Mocking RJ, Patrick PC, Pringle A, Parsons E, McTavish SF, Cowen PJ, Harmer CJ (2013) Effects of short-term varenicline administration on emotional and cognitive processing in healthy, non-smoking adults: a randomized, double-blind, study. Neuropsychopharmacology 38:476–484
Moore TJ, Furberg CD, Glenmullen J, Maltsberger JT, Singh S (2011) Suicidal behavior and depression in smoking cessation treatments. PLoS One 6:e27016
Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K (2006) Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med 166:1571–1577
Perkins KA, Mercincavage M, Fonte CA, Lerman C (2010) Varenicline's effects on acute smoking behavior and reward and their association with subsequent abstinence. Psychopharmacol 210:45–51
Philip NS, Carpenter LL, Tyrka AR, Price LH (2010) Nicotinic acetylcholine receptors and depression: a review of the preclinical and clinical literature. Psychopharmacol 212:1–12
Raber J, Koob GF, Bloom FE (1995) Interleukin-2 (IL-2) induces corticotropin-releasing factor (CRF) release from the amygdala and involves a nitric oxide-mediated signaling; comparison with the hypothalamic response. J Pharmacol Exp Ther 272:815–824
Rhodes ME, O'Toole SM, Wright SL, Czambel RK, Rubin RT (2001) Sexual diergism in rat hypothalamic-pituitary-adrenal axis responses to cholinergic stimulation and antagonism. Brain Res Bull 54:101–113
Sotomayor-Zárate R, Gysling K, Busto UE, Cassels BK, Tampier L, Quintanilla ME (2013) Varenicline and cytisine: two nicotinic acetylcholine receptor ligands reduce ethanol intake in University of Chile bibulous rats. Psychopharmacology (Berl) 227:287–298
Stetler C, Miller GE (2011) Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73:114–126
Tonstad S, Davies S, Flammer M, Russ C, Hughes J (2010) Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf 33:289–301
Vreeburg SA, Kruijtzer BP, van Pelt J, van Dyck R, DeRijk RH, Hoogendijk WJ, Smit JH, Zitman FG, Penninx BW (2009) Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology 34:1109–1120
Acknowledgments
We are most grateful to the participants of our study.
Role of funding source
Funding support for this study was provided by the Medical Research Council G0801432/1. Study's funding source had no role in study design; collection, analysis, and interpretation of data; writing of the report; and decision to submit the paper for publication. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.
Conflicts of interest
AB received speakers' fees from Astra Zeneca. CJH serves on the advisory board of P1vital, and receives consultancy fees from and has shares in the company, and is also a director of Oxford Psychologists. PJC has been a paid member of advisory boards of Eli Lilly, Lundbeck, and Servier, and has received remuneration for scientific advice given to legal representatives of GlaxoSmithKline. The remaining authors declare no possible conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
AP, PJC, and CJH designed the experiment. RJTM, CPP, AP, EP, and SFM collected the data. RJTM, SAW, CPP, and EP analyzed the data. RJTM and SAW drafted the manuscript. All authors participated in interpretation of the final results and editing of the report. All authors saw and approved the final version of the report.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Participant flow. Participant flow chart and dropout (based on a template from Consort 2010) (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Mocking, R.J.T., Wever, S.A., Pflanz, C.P. et al. Effects of short-term varenicline administration on cortisol in healthy, non-smoking adults: a randomized, double-blind, study. Psychopharmacology 231, 143–148 (2014). https://doi.org/10.1007/s00213-013-3213-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3213-7