Abstract
Rationale
Neuronal nicotinic acetylcholine receptors (nAChRs) are pharmacological targets that have recently been implicated in the reinforcing effects of many drugs of abuse, including ethanol. Varenicline and cytisine are nAChR partial agonists in clinical use as smoking cessation aids. However, their efficacies to reduce alcohol consumption have not been fully studied.
Objectives
This study aims to compare the effects of varenicline and cytisine on ethanol consumption by rats bred for many generations as high ethanol drinkers (UChB).
Results
Repeated dosing (0.5 or 1.0 mg/kg/day i.p.) of varenicline or cytisine, for three consecutive days, to male UChB rats pre-exposed to 10 % (v/v) ethanol and water 24 h/day for 4 weeks, significantly reduced alcohol intake and preference of ethanol over water during 1- and 24-h ethanol access periods. This effect was specific for ethanol intake and was not observed for 0.2 % saccharin or water consumption. Varenicline appears to be more effective than cytisine, probably due to its more favorable pharmacokinetic and pharmacodynamic properties. Long-term use of both nAChRs ligands for more than 8–10 days induced tolerance to their effects on ethanol consumption.
Conclusions
This preclinical study in UChB rats demonstrated that both varenicline and cytisine reduce alcohol intake, with varenicline producing a greater and longer-lasting reduction than cytisine. However, dose adjustment will have to be considered as a possible way to counter tolerance arising after continued use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol addiction is a worldwide public health problem. The World Health Organization Global Status Report on Alcohol and Health-2011 showed that the average per capita consumption in liters of pure alcohol between 2003 and 2005 was 8.7 for the Americas, 12.2 for Europe, 6.3 for the Western Pacific, and 2.2 for South/East Asia. In this report, some countries in these areas of the world have maintained a stable per capita consumption while others have increased it. In this regard, increased alcohol consumption associated with specific genetic and environmental conditions can lead to uncontrolled consumption of alcohol and more serious pathological conditions such as alcoholism (WHO 2011).
Alcohol and nicotine are commonly co-abused (Madden and Heath 2002; Talcott et al. 1998). Approximately 60–80 % of heavy drinkers smoke tobacco (Moss et al. 2007), and it has been suggested that common genes are involved in the susceptibility to both alcohol and nicotine dependence (Bergstrom et al. 2003; Collins et al. 1993; de Fiebre et al. 1991, 2002; Kendler et al. 2007; Madden and Heath 2002). Additionally, it has been shown that alcohol can directly or indirectly interact with neuronal nicotinic acetylcholine receptors (nAChRs) (Blomqvist et al. 1992; Davis and de Fiebre 2006; Le et al. 2000).
The α4β2 nAChR is the predominant nAChR subtype in the brain, and it is now well established that it plays an essential role in mediating nicotine's rewarding properties (Picciotto et al. 1998; Tapper et al. 2004). Consistent with an important role of α4β2 nAChR in nicotine addiction, two α4β2 nAChR partial agonists, cytisine, a plant alkaloid (Papke and Heinemann 1994; Rollema et al. 2010), and varenicline, which was developed from cytisine, (Coe et al. 2005; Rollema et al. 2007a) have been shown to be efficacious as smoking cessation aids (Cahill et al. 2009; Etter 2006; Reus et al. 2007; Tutka and Zatoński 2006). Varenicline and cytisine are high-affinity α4β2 nicotinic acetylcholine receptor partial agonists. However, both drugs can bind to other neuronal nAChRs with lower affinity. For example, K i values for varenicline are 0.4, 86, 125, and 8,200 nM for α4β2, α3β4, α7, and α1βγδ receptors, respectively, while the corresponding values for cytisine are 2.0, 480, 5,890, and 492 nM, respectively, for the same receptors (Rollema et al. 2010, 2007a, b, Coe et al. 2005). Further, Rollema et al. (2010) reported that varenicline has higher efficacy (22 ± 2.5 %) than cytisine (6.5 ± 0.2 %) relative to the effect of 100 μM acetylcholine. Comparisons between both drugs indicated that varenicline resulted in higher rates of abstinence from smoking than cytisine (Cahill et al. 2009; Etter 2006). The greater effectiveness of varenicline than cytisine was suggested not only to be associated with their respective pharmacodynamic properties (Rollema et al. 2010) but also to their different pharmacokinetics. Varenicline has a longer half-life than cytisine (4 and 1.5 h, respectively) and a greater in vitro binding affinity for the α4β2 nAChR (K i = 0.4 and 2.0 nM, respectively) (Obach et al. 2006; Rollema et al. 2010, Coe et al. 2005).
It has been hypothesized that the clinical efficacy of varenicline and cytisine involves not only their high affinity for α4β2 nAChR allowing them to compete with nicotine and preventing the binding of the latter to these receptors, but also their partial agonist character, that in the absence of nicotine might enhance the activity of α4β2 nAChR sufficiently to blunt craving and withdrawal (Coe et al. 2005; Cohen et al. 2003; Hogg and Bertrand 2007; Rollema et al. 2007b).
Preclinical studies have provided evidence that either varenicline (Kamens et al. 2010; Steensland et al. 2007) or cytisine (Sajja and Rahman 2011) decreases ethanol intake in rodents. These previous studies evaluated the effects of varenicline and cytisine only after a single or short-term administration. Humans attempting to quit alcohol consumption would have to receive medication daily and for long periods of time to treat their drug addiction. Although the existing literature indicates that short-term treatments with varenicline or cytisine reduce ethanol consumption in rodents, the effect of longer varenicline or cytisine treatment times on ethanol consumption in an animal model of alcoholism have not been investigated thus far. Therefore, the aim of the present study was to compare the effects of short- and long-term repeated administration of varenicline and cytisine on the voluntary ethanol consumption displayed by high-alcohol-drinking University of Chile bibulous (UChB) rats (for reviews, see Mardones and Segovia-Riquelme 1983; Quintanilla et al. 2006; Tampier and Quintanilla 2010) using the continuous and limited access two-bottle choice paradigms.
Materials and methods
Animals
The studies were performed in male Wistar-UChB rats bred for over 86 generations to ingest 10 % ethanol solution in preference to water (Mardones and Segovia-Riquelme 1983; Quintanilla et al. 2006). Rats of the UChB line satisfy the essential criteria proposed for an animal model of alcoholism and have been used previously as a tool for screening alcoholism medications (Quintanilla et al. 2008). Animal experimental procedures were approved by the Institutional Animal Experimentation Ethics Board and followed international guidelines (NIH Guide for the Care and Use of Laboratory Animals).
Drugs and drinking solutions
Varenicline and cytisine, purchased from Sigma-Aldrich, Inc. (St. Louis, Missouri, USA) or isolated from Calia (Sophora) secundiflora seeds, were dissolved in 5 mL/kg physiological saline to yield desired concentrations calculated based on the body weight of the animal. Ethanol solution (10 % v/v) was prepared by mixing absolute ethanol (Merck Darmstadt, Germany) with distilled water. Saccharin was purchased from Sigma-Aldrich (Bellefonte, PA) and dissolved in distilled water to constitute 0.2 % (w/v). Both drinking solutions, 10 % (v/v) ethanol and 0.2 % (w/v) saccharin, were chosen based on prior studies in UChB rats. Thus, it has been shown that UChB rats drink greater amounts of a 10 % (v/v) ethanol solution (Mardones 1951) and also a greater amount of a 0.2 % (w/v) saccharin solution (Tampier and Quintanilla 2005) than the amounts of relatively lower or higher concentrations of ethanol or saccharin.
Ethanol intake studies
Assessment of voluntary ethanol consumption
All rats used for ethanol intake studies (250–300 g) were previously tested for their voluntary ethanol consumption in the following way: Two-month-old rats were housed in individual cages in temperature- and humidity-controlled rooms under a 12-h light/12-h dark cycle. Ethanol was provided under the standard, homecage two-bottle free-choice regimen between ethanol (10 % in distilled water, v/v) and distilled water, with unlimited access (24 h/day). All fluids were presented in 50-mL graduated glass cylinders with glass drinking spouts, which had been previously tested to ensure that they did not spill fluid. The glass cylinders were inserted daily through two grommets in front of the cage until a baseline of ethanol consumption was established (4 weeks). The placement of the ethanol bottle was alternated daily to control for side preferences. Food was provided ad libitum and water and ethanol intake were read daily, immediately before lights off. After ethanol consumption, ethanol intake of the last fifteen drinking days was averaged to obtain the mean voluntary ethanol consumption for each rat and expressed as grams per kilogram body weight per day.
Experiment 1: effect of repeated administration of varenicline or cytisine on ethanol intake using the limited access paradigm
The short plasma half-life (t½) of cytisine (1.5 h) implies a short-lasting effect. Therefore, to determine the dose–response relationships for varenicline and cytisine, we tested whether a dose of 0.5 or 1.0 mg/kg of each drug administered for three consecutive days was able to reduce ethanol intake under limited alcohol access conditions (1 h a day). The doses of varenicline and cytisine and the treatment regimen used were based on previous work (Steensland et al. 2007; Bell et al. 2009). In the present experiment, 30 rats were used (n = 5 rats per group), that had been given continuous 24/h access to 10 % ethanol versus water for 4 weeks. At the end of this period, the rats were offered limited access to 10 % ethanol for 1 h everyday (from 14:00 to 15:00 o'clock) for ten consecutive days, but food and water were freely available. The baseline value was calculated as the average intake by each individual animal obtained on the last 4 days before injecting the drug. Water intake was not measured at the 1-h time point because of the low baseline consumption of water (less than 0.2 mL/1 h) but was recorded daily. Ethanol consumed was recorded at the end of the daily ethanol access period.
Drug administration began after the rats had maintained stable baseline drinking levels of the 10 % (v/v) ethanol solution (after 10 days). On the first drug treatment day, rats were divided into six groups (n = 5 rats per group), matched for ethanol intake over 5 days. To evaluate the effect of repeated intraperitoneal (i.p.) administration of each nicotinic acetylcholine receptor ligand, varenicline (0.5 mg/kg) or saline, and varenicline (1.0 mg/kg) or saline, were administered daily, 30 min before the onset of the 1-h ethanol access period (at 14:00 o'clock), during three consecutive days. In separate groups of rats, cytisine (0.5 mg/kg) or saline, and cytisine (1.0 mg/kg) or saline (i.p.) were administered daily during three consecutive days.
Experiment 2: effect of repeated administration of varenicline or cytisine on ethanol intake using the 24-h continuous access paradigm
To evaluate whether the effect of the higher dose of varenicline or cytisine evaluated in experiment 1 (1.0 mg/kg) is long-lasting, we tested both nAChR ligands in the continuous (24 h/day) 10 % ethanol versus water paradigm. In this experiment, a new group of rats (not used before) was first given 4 weeks of continuous (24 h/day) access to 10 % ethanol and water. At the end of this period, the rats were divided into five groups, matched for ethanol intake over 15 days before the start of the drug treatment. To evaluate the effect of repeated administration of the α4β2 nAChR partial agonists, rats (n = 5 per group) were given, 30 min before the lights were turned off, an i.p. injection of varenicline (1.0 mg/kg) or saline. In separate groups of rats, cytisine (1.0 or 1.5 mg/kg; n = 5 rats per group) was administered for three consecutive days. Ethanol and water intake were recorded daily.
Experiment 3: effect of extended repeated administration of varenicline or cytisine on ethanol intake using the 24-h continuous access paradigm
To evaluate the effect of extended repeated administration of varenicline and cytisine on voluntary continuous ethanol intake, a new group of 20 rats (not used before) was first given 4 weeks of continuous (24 h/day) access to 10 % ethanol and water. When these rats had reached stable baseline levels of ethanol consumption after 4 weeks, they were divided into 4 groups (n = 5 per group). Two groups were given, 30 min before the lights were turned off, an i.p. injection of varenicline (1.0 mg/kg) or saline for eleven consecutive days. In separate groups of rats, cytisine (1.5 mg/kg) or saline were administered daily for 13 consecutive days.
To evaluate the effect of repeated administration of varenicline or cytisine on voluntary continuous saccharin intake, a different group of rats (5 rats per group) was given continuous access (24 h/day) to 0.2 % (w/v) saccharin and distilled water for three consecutive weeks. Food was provided ad libitum and water and saccharin intake recorded daily, immediately before lights off. After the 3 weeks, saccharin intake of the last ten drinking days was averaged to obtain the mean voluntary saccharin consumption for each rat and expressed as milliliter per kilogram body weight per day. On the first drug treatment day, the rats were divided into two groups matched for saccharin intake over 10 days, and varenicline (1.0 mg/kg) or saline (i.p.) were administered daily for three consecutive days. In separate groups of rats, cytisine (1.5 mg/kg) or saline were administered daily for three consecutive days.
Statistics
Statistical analysis was performed using Graph Pad Prism (Graph Pad, San Diego, CA). Ethanol and saccharin intake data from chronic treatments in the two-bottle-choice paradigms were analyzed using two-way ANOVA followed by the Newman–Keuls post hoc test when a significant overall main effect was found (p < 0.005).
Results
Experiment 1: effect of repeated administration of varenicline or cytisine on ethanol intake using the limited access paradigm
To determine the dose–response relationships for varenicline and cytisine, we tested whether a dose of 0.5 or 1.0 mg/kg of each drug administered for three consecutive days was able to reduce ethanol intake under limited alcohol access conditions (1 h a day). Scheduling alcohol availability to 1 h resulted in a stable ethanol intake of approximately 1.0 g/kg body weight and less than 0.2 mL of water during the daily 60-min free-choice period.
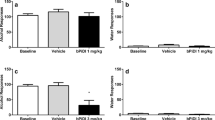
The effects of varenicline compared to saline on ethanol intake under limited access, in doses of 0.5 and 1.0 mg/kg, administered for three consecutive days, are illustrated in Fig. 1. Two-way ANOVA (treatment × day) of saline, 0.5 mg/kg and 1.0 mg/kg varenicline group data shown in Fig. 1 indicated that varenicline treatment (0.5 and 1.0 mg/kg) caused a significant reduction of ethanol intake [F (2,96) = 48.55, P < 0.001] compared to the saline group, the magnitude of which was a function of day [F (6,96) = 9.44, P < 0.001]. This was supported by a significant treatment × day interaction [F (6,96) = 2.30, P < 0.01]. When both varenicline doses were compared among them in a separate ANOVA, it was found that the effects of both doses were significantly different [F (1,48) = 14.20, P < 0.001]. The magnitude of the effect was a function of day [F (6,48) = 6.44, P < 0.001], but the 0.5 mg/kg dose did not have the same effect as the 1.0 mg/kg dose throughout the 3-day period [dose × day interaction: F (6,48) = 1.8, N.S.]. Post hoc analysis revealed that 0.5 mg/kg varenicline (Fig. 1; gray columns) significantly decreased ethanol intake for the 1-h test period in the last two treatment days, while the 1.0 mg/kg dose (Fig. 1; black columns) decreased ethanol intake all three treatment days compared with the saline group (P < 0.005, Newman–Keuls test). The magnitude of reduction compared with the saline-treated rats and calculated over the entire treatment period, averaged approximately 40 % in the 0.5 mg/kg and 60 % in the 1.0 mg/kg varenicline rats. Further, the effect of treatment with 1.0 mg/kg varenicline was long-lasting, as the ethanol intake remained significantly reduced with respect to the intake of the saline group (P < 0.001) for 2 days after the treatment ended (Fig. 1; post-treatment days 4 and 5; black columns). On the other hand, comparison of the effect of each varenicline dose versus ethanol intake displayed by the same group of rats during baseline days (data not shown), also indicated that 0.5 mg/kg varenicline decreased ethanol intake significantly the last 2 days of treatment, while the 1.0 mg/kg dose decreased ethanol intake from the first day (the three treatment days) and also the first two post-treatment days.
Short treatment (3-day) with varenicline (0.5 or 1.0 mg/kg/day) significantly decreases ethanol intake in rats under limited access. Rats were exposed to 10 % ethanol for only 1 h each day, with water and food freely available (24 h/day). These animals were previously submitted to continuous (24 h/day) free choice between 10 % ethanol and water for four consecutive weeks and further allowed access to 10 % ethanol for 1 h every day during ten consecutive days (last four drinking days was averaged to obtain baseline ethanol intake). On day 11, rats were assigned into three groups (n = 5 rats per group) each group received 0.5, 1.0 mg/kg varenicline or saline intraperitoneally 30 min before the start of the 1 h drinking session. Data are expressed as mean ± SEM (two-way ANOVA within each treatment group followed by Newman–Keuls post hoc test) *p < 0.05 versus saline controls
The effects of cytisine on the ethanol intake under limited access in doses of 0.5 and 1.0 mg/kg or saline, administered for three consecutive days, are illustrated in Fig. 2. Two-way ANOVA (treatment × day) of all ethanol intake data shown in Fig. 2 indicated that 0.5 and 1.0 mg/kg cytisine caused a significant effect on ethanol intake [F (2,96) = 6.78, P < 0.001] compared to the saline group, the magnitude of which was not a function of day [F (6,96) = 1.13,N.S.] although both doses showed effects throughout the days [treatment × day interaction F (6,96) = 2.07, P < 0.03]. When the 0.5 and 1.0 mg/kg doses were compared among them in a separate ANOVA, it was found that the effects of both doses were not significantly different [F (1,48) = 1.70, N.S.], the magnitude of the effect was not a function of day [F (6,48) = 2.2, N.S.], and the 0.5 mg/kg dose did not have the same effect as the 1.0 mg/kg dose throughout the days [dose × day interaction, F (6,48) = 0.88, N.S.]. Post hoc comparisons indicated that cytisine caused a smaller reduction of ethanol consumption than varenicline. Indeed, both 0.5 mg/kg (Fig. 2; gray columns) and 1.0 mg/kg (Fig. 2; black columns) of cytisine significantly decreased ethanol consumption for the 1-h test period only the first 2 days of treatment (P < 0.01). The magnitude of this reduction compared with the saline-treated rats and calculated over the entire treatment period, averaged approximately 40 % in the 0.5 mg/kg and the 1.0 mg/kg doses (P < 0.01). On the other hand, comparison of ethanol consumption during the cytisine treatment days with respect to intake during the baseline days, before beginning the drug treatment (data not shown), indicated that 0.5 mg/kg cytisine decreased ethanol intake significantly on the first day of treatment, while the 1.0 mg/kg dose only decreased ethanol intake the last 2 days of treatment. No change in body weight was observed during the 3 days of varenicline or cytisine treatment.
Short treatment (3-day) with cytisine (0.5 or 1.0 mg/kg/day) significantly decreases ethanol intake in rats under limited access. Rats were exposed to 10 % ethanol for only 1 h each day, with water and food freely available (24 h/day). These animals were previously submitted to continuous (24 h/day) free choice between 10 % ethanol and water for four consecutive weeks and further allowed access to 10 % ethanol for 1 h every day during ten consecutive days (last four drinking days was averaged to obtain baseline ethanol intake). On day 11, rats were assigned into three groups (n = 5 rats per group) each group received 0.5, 1.0 mg/kg cytisine or saline intraperitoneally 30 min before the start of the 1 h drinking session. Data are expressed as mean ± SEM (two-way ANOVA within each treatment group followed by Newman–Keuls post hoc test) *p < 0.05 versus saline controls
Experiment 2: effect of repeated administration of varenicline or cytisine on ethanol intake using the 24-h continuous access paradigm
The continuous access paradigm was used to evaluate whether varenicline (1.0 mg/kg) or cytisine (1.0 or 1.5 mg/kg) might have a long-lasting action and if these drugs are able to reduce the consumption of ethanol displayed during a 24-h drinking session, following drug treatment. Rats given continuous (24 h/day) access to 10 % ethanol and water consumed an average of 7.0 ± 0.5 g ethanol/kg/day and 29 ± 5 mL water/kg/day at the end of the 4-week free-choice period. The effect of varenicline in doses of 1.0 mg/kg or saline, administered for three consecutive days, on ethanol intake under continuous access is illustrated in Fig. 3a. Two-way ANOVA of the saline and varenicline data revealed a significant effect of varenicline treatment [F (1,109) = 75.76, P < 0.001] compared to saline, the magnitude of which was a function of day [F (9,109) = 3.19, P < 0.01]. This was supported by a significant treatment × day interaction [F (9,109) = 2.70, P < 0.005]. Post hoc comparisons indicated that treatment with 1.0 mg/kg varenicline produced a statistically significant reduction of 24 h ethanol intake on all treatment days compared with the saline group (P < 0.01). The magnitude of reduction calculated over the entire treatment period averaged approximately 42 % (P < 0.001). As seen in the limited access experiment, the effect of 1.0 mg/kg varenicline on continuous drinking was also long-lasting, as the ethanol intake remained significantly reduced for 2 days after treatment ended (P < 0.001). On the other hand, comparison of ethanol consumption during all days of varenicline administration with respect to the intake during the four baseline days, before beginning treatment, indicated that 1.0 mg/kg varenicline significantly (P < 0.01) reduced ethanol intake (Fig. 3a) and ethanol preference (Table 1) on the last 2 days of treatment and the first two post-treatment days. In contrast, water consumption was not significantly affected by varenicline on any of the treatment days (data not shown).
Effect of short treatment (3-day) with varenicline or cytisine significantly decreases ethanol intake in rats under continuous ethanol access. Rats were previously submitted to continuous (24 h/day) free choice between 10 % ethanol and water for four consecutive weeks. Thereafter, varenicline 1.0 mg/kg or saline (a) was administered intraperitoneally 30 min before the lights were turned off to two different group of rats on each of three consecutive days. Cytisine 1.0, 1.5 mg/kg or saline (b) was administered to two different group of rats on each of three consecutive days. Data are expressed as mean ± SEM (two-way ANOVA within each treatment group followed by Newman–Keuls post hoc test), *p < 0.05 versus saline controls
The effects of cytisine at 1.0 mg/kg and saline on ethanol consumption during a 24-h drinking session are illustrated in Fig. 3b (black diamonds). Furthermore, to determine if increasing the dose from 1.0 to 1.5 mg/kg, cytisine could reduce ethanol intake to a similar extent as seen with 1.0 mg/kg varenicline, a different group of rats was injected with 1.5 mg/kg cytisine for three consecutive days (Fig. 3b; black squares). Two-way ANOVA (treatment × day) of saline, 1.0 mg/kg and 1.5 mg/kg cytisine group data, shown in Fig. 3b, indicated that 1.0 and 1.5 mg/kg doses caused a significant effect on ethanol intake [F (2,96) = 6.3, P < 0.0025] compared to the saline group, the magnitude of which was a function of day [F (6,96) = 4.48, P < 0.001]. This was supported by a significant treatment × day interaction [treatment × day interaction: F (6,96) = 2.10, P < 0.01]. When both cytisine doses were compared in a separate ANOVA, it was found that the effects of both doses were significantly different [F (1,48) = 4.00, P < 0.05], the magnitude of the effect was a function of day [F (6,48) = 7.21, P < 0.001], but the 1.0 mg/kg dose did not have the same effect as the 1.5 mg/kg dose throughout the days [dose × day interaction: F (6,48) = 0.72, N.S.]. Post hoc comparisons indicated that cytisine at a dose of 1.0 mg/kg (Fig. 3b; black diamonds), administered for three consecutive days, reduced ethanol consumption and also ethanol preference (Table 1). However, unlike varenicline, the reduction elicited by cytisine reached statistical significance on the third day of treatment, and the reduction was approximately 34 % (P < 0.01). Post hoc analysis indicated that cytisine at a dose of 1.5 mg/kg (Fig. 3b; black squares) significantly reduced ethanol intake and ethanol preference (Table 1) for the 24-h test period on all 3 days of treatment compared with the saline group, and the magnitude of reduction was approximately 48 % (P < 0.01), which is similar to the reduction obtained with 1.0 mg/kg varenicline (Fig. 3a). In addition, comparison of ethanol consumption during all days of cytisine administration with respect to the intake during the four baseline days, before initiating treatment (Fig. 3b; black squares), indicated that 1.5 mg/kg cytisine decreased the ethanol intake significantly on all 3 days of treatment. In contrast, water consumption (data not shown) was not significantly affected by cytisine on any of the treatment days. Taken together, the results obtained in limited- and continuous-access experiments showed that varenicline exhibits a stronger and longer-lasting effect than cytisine. No change in body weight was observed during the 3 days of varenicline or cytisine treatment.
To evaluate the specificity of both nAChR ligands on ethanol intake, varenicline in a dose of 1.0 mg/kg, or cytisine in a dose of 1.5 mg/kg, or saline, was administered for three consecutive days to rats under the two-bottle free-choice regimen between saccharin 0.2 % (w/v) and water with unlimited access (24 h/day). Two-way ANOVA of saccharin intake data displayed by the rats of the varenicline- and saline-treated groups (Table 2) revealed no significant effect of treatment [F (1,62) = 0.60, P > 0.05], day [F (6,62) = 0.67, P > 0.05] or treatment × day interaction [F (6,62) = 0.07, P > 0.05]. Similarly, two-way ANOVA of saccharin intake data displayed by rats of the cytisine and saline groups (Table 2) revealed no significant effect of treatment [F (1,62) = 0.61, P > 0.05], day [F (6,62) = 0.24, P > 0.05] or treatment × day interaction [F (6,62) = 0.19, P > 0.05].
Experiment 3: effect of extended repeated administration of varenicline or cytisine on ethanol intake using the 24-h continuous access paradigm
The effects of chronic administration of both nAChR partial agonists on ethanol intake during the 24-h drinking sessions were evaluated with repeated dosing of 1.0 mg/kg varenicline, which was administered for 11 consecutive days at 24 h intervals or 1.5 mg/kg cytisine administered for 13 consecutive days at 24 h intervals. In this experiment, rats were divided into four groups. One group of rats received varenicline and another saline. The other two groups received cytisine or saline. However, because both saline groups were not statistically different, ethanol intake data of both saline groups were pooled.
Figure 4a illustrates ethanol consumption in varenicline and saline groups during baseline, treatment and post-treatment days. Two-way ANOVA of the first 14 ethanol intake data shown in Fig. 4a revealed a significant effect of varenicline treatment [F (1,111) = 64.32, P < 0.001], the magnitude of which is a function of day [F (15,111) = 2.78, P < 0.01]. This was supported by a significant treatment × day interaction [F (15,111) = 1.77, P < 0.05]. Post hoc analysis revealed that varenicline reduces ethanol consumption significantly compared with the saline group on days 1 to 7 of drug treatment (P < 0.001). The ethanol intake reduction calculated over the first 7 days of varenicline treatment averaged approximately 51 %. This effect practically disappeared on days 8–10 of chronic treatment even though varenicline was still administered. Comparison of ethanol consumption during all days of varenicline administration with respect to intake during the five baseline days, before initiating treatment (Fig. 4a), indicated that 1.0 mg/kg varenicline decreased the ethanol intake significantly only on the first 7 days, but not on the last 4 days of chronic administration. Varenicline treatment during 11 consecutive days did not reduce body weight (means ± SEM, n = 5; 280 ± 3 g, the first treatment day, versus 290 ± 6 g the last day of treatment).
Extended repeated administration of varenicline or cytisine reduced ethanol intake until the seventh or eleventh day of treatment, respectively, when tolerance developed. a Effect of long-term (11-day) varenicline (1.0 mg/kg/day i.p.) treatment on voluntary ethanol intake in UChB rats previously submitted to continuous (24 h/day) free choice between 10 % ethanol and water for four consecutive weeks. b Effect of long-term (13-day) cytisine (1.5 mg/kg/day i.p.) treatment on voluntary ethanol intake in adult high alcohol-drinking UChB rats previously submitted to continuous (24 h/day) free choice between 10 % ethanol and water for four consecutive weeks. In this case, rats also received a single dose of cytisine (3.0 mg/kg i.p.) or saline on day 17. Varenicline, cytosine, or saline was administered intraperitoneally 30 min before the lights were turned off. Data are expressed as mean ± SEM (two-way ANOVA within each treatment group followed by Newman–Keuls post hoc test) *p < 0.05 versus saline controls, ¤p < 0.05 versus baseline drinking levels
Figure 4b illustrates the effect of chronic cytisine administration on ethanol intake. These animals first received a dose of 1.5 mg/kg of cytisine for 13 consecutive days (day 1 to 13), and after 3 days without any treatment (day 14 to 16), a single dose of 3.0 mg/kg of cytisine was injected on day 17. Two-way ANOVA of the first 16 ethanol intake data shown in Fig. 4b revealed a significant effect of 1.5 mg/kg cytisine treatment [F (1,118) = 487.8, P < 0.001], the magnitude of which is a function of the day [F (16,118) = 12.33, P < 0.001]. This was supported by a significant treatment × day interaction [F (16,118) = 7.88, P < 0.001]. Post hoc analysis revealed that 1.5 mg/kg cytisine reduces ethanol consumption significantly compared with the saline group on treatment days 1 to 10 (P < 0.001). The magnitude of this reduction compared with saline-treated rats and calculated over the first 10 days of cytisine treatment averaged approximately 43 %. The effect practically disappeared on days 11–13 even though cytisine was still administered. Comparison of ethanol consumption during all days of cytisine administration with respect to the intake during the five baseline days, before starting treatment (Fig. 4b), indicated that 1.5 mg/kg cytisine decreased ethanol intake significantly only the first 10 days of administration, but not the last 3 days of chronic administration. Once the acquired tolerance remained stable from day 14 to 16, these animals were injected with a single higher dose of cytisine (3.0 mg/kg) or saline on day 16 when the light was turned off (at 7 o'clock PM). The 3.0 mg/kg cytisine dose was able to reduce ethanol intake significantly on day 17 compared with the ethanol intake data of the respective saline-treated rats. Thus, with multiple repeated administrations, tolerance to the effect induced by both drugs appears to develop at different time points, on day 8 of chronic varenicline—and on day 11 of chronic cytisine treatment. Cytisine treatment during 13 consecutive days did not reduce body weight (means ± SEM, n = 5; 295 ± 3 g, the first treatment day, versus 299 ± 6 g the last day of treatment)
Discussion
Our results show that varenicline or cytisine elicited a dose-dependent reduction in voluntary ethanol consumption displayed by the high-alcohol-drinking UChB rats. These rats have been bred for many generations as high ethanol drinkers representing a unique animal model to study alcohol addiction (for reviews see Mardones and Segovia-Riquelme 1983; Quintanilla et al. 2006; Tampier and Quintanilla 2010). The reduction in alcohol intake was evident in a 1-h limited access paradigm (Figs. 1 and 2) and in a 24-h continuous access paradigm (Figs. 3 and 4). Varenicline and cytisine treatment under a short course of repeated dosing (0.5 or 1.0 mg/kg), for three consecutive days, significantly decreased voluntary ethanol intake in the high ethanol consumer (UChB) rats. These results are consistent with previous findings in rodents examining the effect of varenicline (Bito-Onon et al. 2011; Kamens et al. 2010; Steensland et al. 2007; Wouda et al. 2011) and cytisine (Bell et al. 2009; Hendrickson et al. 2009; Sajja and Rahman 2011) on ethanol intake and further support a role for nAChR in ethanol consumption. Comparison of the effects of both nAChRs ligands showed that varenicline elicited a greater reduction of ethanol intake than cytisine. In fact, 0.5 and 1.0 mg/kg doses of varenicline, administered for three consecutive days to rats under limited access, reduced the intake of ethanol displayed during a 1-h drinking session by 40 % and 60 %, respectively, during two (the 0.5 mg/kg dose) and three (the 1.0 mg/kg dose) days of treatment (Fig. 1), while the magnitude of reduction induced by cytisine compared with the saline-treated rats and calculated over the entire treatment period, reached an average of approximately 40 % in the 0.5 mg/kg and the 1.0 mg/kg doses (Fig. 2). These results can be rationalized, at least in part, by the finding that after oral administration, varenicline is more potent than cytisine in stimulating dopamine turnover in the rat nucleus accumbens (Rollema et al. 2010). Also, the present results indicate that the inhibitory effect of varenicline on ethanol consumption is longer-lasting compared to that of cytisine (Figs. 1 and 2). The differences in their durations of action may be associated with their respective pharmacokinetic and/or pharmacodynamic properties (Coe et al. 2005; Obach et al. 2006; Rollema et al. 2010). Short varenicline or cytisine treatments (for three consecutive days) were also able to reduce the voluntary consumption of ethanol displayed during a 24-h drinking session paradigm without reducing water intake (Table 1) or another highly desired drinking solution for UChB rats (Tampier and Quintanilla 2005) such as 0.2 % (w/v) saccharin (Table 2).
In the present study, it is difficult to determine the precise role of the individual subunits of the nAChR playing a role in ethanol consumption in UChB rats due to the fact that varenicline and cytisine have diverse pharmacological profiles. Both ligands are high-affinity partial agonists at α4β2 nAChRs and low-affinity partial agonists at α3β4, α3β2, α4β4, and α6 nAChRs, and also low-affinity agonists at α7 nAChRs (Coe et al. 2005). On the other hand, although it is well established that the α4β2 nAChR has an essential role in mediating nicotine's rewarding properties (Picciotto et al. 1998; Tapper et al. 2004), the subunit composition involved in ethanol's rewarding properties remains controversial, since the role of other nAChR subtypes has been demonstrated. It has been shown that systemic ethanol increases dopamine release in the mesolimbic dopamine system, an effect that appears to require the stimulation of nAChRs (Blomqvist et al. 1997; Ericson et al. 2009; Tizabi et al. 2007). The ability of mecamylamine, a nonselective nAChRs antagonist, to inhibit alcohol intake (Hendrickson et al. 2009) and to antagonize the mesolimbic dopamine-activating properties of ethanol (Blomqvist et al. 1993) support this hypothesis. A number of studies have indicated that ethanol interacts directly with α4β2 nAChRs where it acts as an allosteric modulator (Aistrup et al. 1999; Cardoso et al. 1999; Covernton and Connolly 1997). However, the finding that blocking α4β2 nAChR with a selective antagonist, dihydro-β-erythroidine (DHβE), failed to reduce ethanol consumption in rodents (Larsson et al. 2002; Le et al. 2000) did not support this notion. In addition, Chatterjee et al. (2011) hypothesized that partial agonists at α3β4 nAChRs decrease ethanol self-administration and consumption by indirectly modulating the mesolimbic dopaminergic system. They demonstrated that high-affinity partial agonists at α3β4 nAChRs, CP-601932 and PF-4575180, reduce ethanol consumption and seeking in rats. Interestingly, these authors also suggested that varenicline at doses of 1 and 2 mg/kg, that reduce ethanol intake, has a functional interaction with α3β4 nAChRs. Likewise, cytisine also has affinity for α3β4 nAChRs. Taken together, these results suggest that following 0.5 or 1.0 mg/kg doses, varenicline and cytisine might reach brain concentrations high enough to interact functionally with α3β4 nAChRs in UChB rats. Furthermore, Kuzmin et al. (2009) have previously demonstrated that α-conotoxin MII, a selective antagonist of α3β2, β3, and α6 subunits decreases ethanol self-administration in rats, suggesting that these subunits might be involved in mediating ethanol intake. Because the diverse pharmacological profiles of varenicline and cytisine also involve the α6 subunit (Rollema et al. 2010), it is possible that both ligands could modulate the α6 nAChR subunit in the VTA to mediate the reduction of ethanol consumption.
Consistent with an important role of α4β2 nAChRs in alcohol drinking behavior, Rezvani et al. (2010) have shown that the selective desensitizer of α4β2 nAChRs, sazetidine-A (Xiao et al. 2008), reduced alcohol consumption in alcohol-preferring (P) rats. Because sazetidine-A has both agonistic and desensitizing effects on α4β2 nAChRs, but its desensitizing effect is much more profound (Xiao et al. 2008), the authors suggest that the reduction of ethanol intake by sazetidine-A is mediated via desensitization of the α4β2 nicotinic receptor (Rezvani et al. 2010). Because it has been shown that both varenicline and cytisine desensitize nAChRs (Billen et al. 2012; Rollema et al. 2010; Mihalak et al. 2006; Lu et al. 1999), it is possible that a functional antagonism of nAChRs through desensitization may reduce ethanol intake in UChB animals by decreasing the firing rate of VTA neurons.
The present study also shows that long-term administration of varenicline (1.0 mg/kg/day) or cytisine (1.5 mg/kg/day) for eleven or thirteen consecutive days, respectively, induced a decrease in ethanol intake during the first days of treatment followed by development of tolerance by day 8 of varenicline and by day 11 of cytisine administration. Our finding that the loss of effect in reducing ethanol intake after 10 days of daily administration of 1.5 mg/kg of cytisine was overcome by the administration of a higher dose of 3.0 mg/kg cytisine, supports the notion that the loss of effect was due to tolerance development. There are no preclinical studies showing development of tolerance to the reduction of alcohol intake induced by varenicline or cytisine, because varenicline or cytisine have not been administered for more than 6 days in rodents before the present study. However, our finding of tolerance development to the effect of varenicline and cytisine did not agree with results of a recent study showing that continuous sazetidine-A infusions (6 mg/kg/day) over a period of 4 weeks reduced nicotine self-administration without attenuation in its effectiveness over 4 weeks (Johnson et al. 2012). This discrepancy may be due to differences in the up-regulation of nAChR which is a powerful influence in the efficacy of drugs. Thus, Hussmann et al. (2012) comparing the ability of chronic administration of nicotine (18 mg/kg/day), varenicline (1.8 mg/kg/day), and sazetidine-A (4.7 – 9.4 mg/kg/day) over a period of 2 weeks, found that nicotine and varenicline up-regulate brain nAChRs in rats and mice, while sazetidine-A did not. These authors postulate that the increased receptors convey excess levels of neural activity, which triggers anxiety pathways that lead to craving when the replacement therapies either with nicotine or varenicline, are stopped (Hussmann et al. 2012). According to this notion, previous studies showed that cytisine injected twice daily at a dose of 1.0 mg/kg for 10 days (Schwartz and Kellar 1985) and varenicline injected at a dose of 1.8 mg/kg/day for 14 days also induced up-regulation of nicotinic receptors (Turner et al. 2011) in rat brain. If we assume that nicotinic receptor desensitization is the mechanism by which varenicline and cytisine reduce alcohol consumption in UChB rats, it is possible that the up-regulation of nicotinic receptors might account for the development of tolerance because the same varenicline and cytisine dose is not enough to desensitize the increased nAChRs to reduce ethanol consumption. Clinically, these findings would suggest that long-term treatment of high-alcohol-drinking individuals with varenicline or cytisine should consider dose adjustment to counter tolerance arising after continued use.
References
Aistrup GL, Marszalec W, Narahashi T (1999) Ethanol modulation of nicotinic acetylcholine receptor currents in cultured cortical neurons. Mol Pharmacol 55:39–49
Bell RL, Eiler BJ 2nd, Cook JB, Rahman S (2009) Nicotinic receptor ligands reduce ethanol intake by high alcohol-drinking HAD-2 rats. Alcohol 43:581–592
Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ (2003) Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res 27:1535–1547
Billen B, Spurny R, Brams M, Van Elk R, Valera-Kummer S, Yakel JL, Voets T, Bertrand D, Smit AB, Ulens C (2012) Molecular actions of smoking cessation drugs at α4β2 nicotinic receptors defined in crystal structures of a homologous binding protein. PNAS 109:9173–9178
Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE (2011) Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20 % ethanol operant self-administration in Sprague–Dawley rats. Addict Biol 16:440–449
Blomqvist O, Soderpalm B, Engel JA (1992) Ethanol-induced locomotor activity: involvement of central nicotinic acetylcholine receptors? Brain Res Bull 29:173–178
Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B (1993) The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol 249:207–213
Blomqvist O, Ericson M, Engel JA, Soderpalm B (1997) Accumbal dopamine overflow after ethanol: localization of the antagonizing effect of mecamylamine. Eur J Pharmacol 334:149–156
Cahill K, Stead L, Lancaster T (2009) A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Saf 32:119–135
Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA (1999) Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther 289:774–780
Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rollema H, Bartlett SE (2011) Partial agonists of the alpha3beta4* neuronal nicotinic acetylcholine receptor reduce ethanol consumption and seeking in rats. Neuropsychopharmacology 36:603–615
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD 3rd, O'Neill BT (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474–3477
Cohen C, Bergis OE, Galli F, Lochead AW, Jegham S, Biton B, Leonardon J, Avenet P, Sgard F, Besnard F, Graham D, Coste A, Oblin A, Curet O, Voltz C, Gardes A, Caille D, Perrault G, George P, Soubrie P, Scatton B (2003) SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther 306:407–420
Collins AC, Romm E, Selvaag S, Turner S, Marks MJ (1993) A comparison of the effects of chronic nicotine infusion on tolerance to nicotine and cross-tolerance to ethanol in long- and short-sleep mice. J Pharmacol Exp Ther 266:1390–1397
Covernton PJ, Connolly JG (1997) Differential modulation of rat neuronal nicotinic receptor subtypes by acute application of ethanol. Br J Pharmacol 122:1661–1668
Davis TJ, de Fiebre CM (2006) Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health 29:179–185
de Fiebre CM, Romm E, Collins JT, Draski LJ, Deitrich RA, Collins AC (1991) Responses to cholinergic agonists of rats selectively bred for differential sensitivity to ethanol. Alcohol Clin Exp Res 15:270–276
de Fiebre NC, Dawson R Jr, de Fiebre CM (2002) The selectively bred high alcohol sensitivity (HAS) and low alcohol sensitivity (LAS) rats differ in sensitivity to nicotine. Alcohol Clin Exp Res 26:765–772
Ericson M, Lof E, Stomberg R, Soderpalm B (2009) The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther 329:225–230
Etter JF (2006) Cytisine for smoking cessation: a literature review and a meta-analysis. Arch Intern Med 166:1553–1559
Hendrickson LM, Zhao-Shea R, Tapper AR (2009) Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 204:563–572
Hogg RC, Bertrand D (2007) Partial agonists as therapeutic agents at neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 73:459–468
Hussmann GP, Turner JR, Lomazzo E, Venkatesh R, Cousins V, Xiao Y, Yasuda RP, Wolfe BB, Perry DC, Rezvani AH, Levin ED, Blendy JA, Kellar KJ (2012) Chronic sazetidine-A at behaviorally active doses does not increase nicotinic cholinergic receptors in rodent brain. J Pharcol Exp Therap 343:441–450
Johnson JE, Slade S, Wells C, Petro A, Sexton H, Rezvani AH, Brown ML, Paige MA, McDowell BE, Xiao Y, Kellar KJ, Levin ED (2012) Assessing the effects of chronic sazetidine-A delivery on nicotine self-administration in both male and female rats. Psychopharmacology (Berl) 222(2):269–276
Kamens HM, Andersen J, Picciotto MR (2010) Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 208(4):613–626
Kendler KS, Myers J, Prescott CA (2007) Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry 64:1313–1320
Kuzmin A, Jerlhag E, Liljequist S, Engel J (2009) Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology (Berl) 203:99–108
Larsson A, Svensson L, Soderpalm B, Engel JA (2002) Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol 28:157–167
Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK (2000) Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res 24:155–163
Lu Y, Marks MJ, Collins AC (1999) Desensitization of nicotinic agonist-induced [3H]γ-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists. J Pharmacol Exp Ther 291:1127–1134
Madden PA, Heath AC (2002) Shared genetic vulnerability in alcohol and cigarette use and dependence. Alcohol Clin Exp Res 26:1919–1921
Mardones J (1951) On the relationship between deficiency of B vitamins and alcohol intake in rats. Q J Stud Alcohol 12:563–575
Mardones J, Segovia-Riquelme N (1983) Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains. Neurobehav Toxicol Teratol 5:171–178
Mihalak KB, Caroll FI, Luetje CW (2006) Varenicline is a partial agonist at alpha4-neta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70:801–805
Moss HB, Chen CM, Yi HY (2007) Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend 91:149–158
Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O'Connell TN, Zandi KS, Miller S, Coe JW (2006) Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos 34:121–130
Papke RL, Heinemann SF (1994) Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol Pharmacol 45:142–149
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177
Quintanilla ME, Israel Y, Sapag A, Tampier L (2006) The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol 11:310–323
Quintanilla ME, Perez E, Tampier L (2008) Baclofen reduces ethanol intake in high-alcohol-drinking University of Chile bibulous rats. Addict Biol 13:326–336
Reus VI, Obach RS, Coe JW, Faessel H, Rollema H, Watsky E, Reeves K (2007) Varenicline: new treatment with efficacy in smoking cessation. Drugs Today (Barc) 43:65–75
Rezvani AH, Slade S, Wells C, Petro A, Lumeng L, Li TK, Xiao Y, Brown ML, Paige MA, McDowell BE, Rose JE, Kellar KJ, Levin ED (2010) Effects of sazetidine-A, a selective alpha4beta2 nicotinic acetylcholine receptor desensitizing agent on alcohol and nicotine self-administration in selectively bred alcohol-preferring (P) rats. Psychopharmacology (Berl) 211:161–174
Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE (2007a) Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci 28:316–325
Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD 3rd, Williams KE (2007b) Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985–994
Rollema H, Shrikhande A, Ward KM, Tingley FD 3rd, Coe JW, O’Neill BT, Tseng E, Wang EQ, Mather RJ, Hurst RS, Williams KE, de Vries M, Cremers T, Bertrand S, Bertrand D (2010) Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol 160:334–345
Sajja RK, Rahman S (2011) Lobeline and cytisine reduce voluntary ethanol drinking behavior in male C57BL/6J mice. Prog Neuropsychopharmacol Biol Psychiatry 35:257–264
Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A 104:12518–12523
Schwartz RD, Kellar KJ (1985) In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem 45(2):427–433
Talcott GW, Poston WS 2nd, Haddock CK (1998) Co-occurrent use of cigarettes, alcohol, and caffeine in a retired military population. Mil Med 163:133–138
Tampier L, Quintanilla ME (2005) Saccharin consumption and the effect of a long-term exposure to a sweetened alcoholic solution in high- (UChB) and low- (UChA) alcohol-drinking rats. Alcohol 37:47–52
Tampier L, Quintanilla ME (2010) Ratas UChA y UChB: Un modelo animal para el estudio del alcoholismo. Rev Farmacol Chile 3:5–11
Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032
Tizabi Y, Bai L, Copeland RL Jr, Taylor RE (2007) Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol 42:413–416
Turner JR, Castellano LM, Blendy JA (2011) Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res 13:41–46
Tutka P, Zatoński W (2006) Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol Rep 58:777–798
WHO (2011) Global Status Report on Alcohol and Health 2011. World Health Organization,Switzerland http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ (2011) Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 216(2):267–277
Xiao Y, Yasuda RP, Sahibzada N, Horton L, DiPietro JR, Iwueze AF,Paige MA, McDowell BE, Brown ML,Wolfe BB, Kellar KJ (2008) Pharmacology properties of sazetidine-A, a selective ligand of α4β2 nicotinic acetylcholine receptors. Neuroscience Meeting Planner. Society for Neuroscience, Washington, DC
Acknowledgments
This work was funded by MSI Grants Nº P10/063-F and P05/001-F, and FONDECYT Grant N° 111-0392. The authors thank Dr. Patricio Iturriaga-Vásquez for a generous gift of cytisine.
Disclosure/Conflict of interest
The authors of this paper declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sotomayor-Zárate, R., Gysling, K., Busto, U.E. et al. Varenicline and cytisine: two nicotinic acetylcholine receptor ligands reduce ethanol intake in University of Chile bibulous rats. Psychopharmacology 227, 287–298 (2013). https://doi.org/10.1007/s00213-013-2974-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-2974-3