Abstract
Rationale
Prior exposure to drugs of abuse may increase or decrease the reinforcing effects of the drug in later consumptions. Based on the initial locomotor activity (LA) response to an acute drug administration or to novelty in an open-field arena, animals can be classified as low or high LA responders (LR or HR). Few studies have used this classification with nicotine, and the results are controversial. Some authors suggested that nicotine can induce conditioned-place preference (CPP) following prior nicotine exposure, whereas others suggested that previous nicotine exposure extinguishes nicotine-CPP.
Objective
To explore if the administration of nicotine in a novel environment without explicit behavioral consequences to classify animals in low and high nicotine responders (LNR and HNR) could affect the establishment of nicotine CPP in male Sprague–Dawley rats.
Results
Prior exposure to a single dose of nicotine (0.4 mg/kg, subcutaneously) induced CPP in LNR rats after 14 days of conditioning (seven-trial) but not after two or eight conditioning days. In contrast, HNR rats did not show CPP under any condition. In addition, our results indicated that previous exposure to nicotine decreased its rewarding effects in eight conditioning days CPP (four-trial), which can be regularly established without prior exposure to nicotine.
Conclusion
The results suggested that response to a single exposure to nicotine predicts the acquisition of nicotine preference in a 14-day conditioning protocol only for LNR rats. Thus, our findings demonstrated the relevance of using LNR and HNR classification when the individual susceptibility to nicotine preference is studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many people experiment with potentially addictive drugs at least once in their lifetime, but only a small percentage become addicted (Wagner and Anthony 2002; World Health Organization 2010). One of the major challenges in the drug of abuse field is to identify the mechanisms involved in the susceptibility of some individuals to make the transition from casual to compulsive drug use. Adolescents are more susceptible to the initiation of drug consumption (Vastola et al. 2002; Adriani et al. 2003; Belluzi et al. 2004; Mathews et al. 2011). In animal models of preference to drugs of abuse, if conditioning is initiated during adolescence, a higher and more stable nicotine- conditioned-place preference (CPP) is established (Vastola et al. 2002; Adriani et al. 2003; Pastor et al. 2011; Natarajan et al. 2011; also see “Materials and Methods”). Previously, we demonstrated that adolescent rats can re-establish nicotine-preference after 12 days of extinction using a single nicotine injection (Pascual et al. 2009). These results suggested that, if nicotine is administrated during adolescence, a preference could be maintained for long time; however, this was not the case when adult rats were used (Vastola et al. 2002; Belluzzi et al. 2004; Shram et al. 2006; Shearman et al. 2008).
Furthermore, recent studies suggested that first experiences with drugs of abuse may have long-term impact on the development of drug-seeking behavior (Miller et al. 2001; Brielmaier et al. 2007; Goriounova and Mansvelder 2012). On the other hand, it has been demonstrated that individual sensitivity or vulnerability to the reinforcing effects of drugs of abuse is one of the most important factors underlying the development of drug consumption, where genetic, age, and other individual neurobiological differences could contribute to nicotine reward (Sabeti et al. 2002; Allen et al. 2007; Mandt et al. 2008). Individual variability in the initial responsiveness to psychostimulants may predict subsequent risk for stimulant abuse (Cohen et al. 2005; Allen et al. 2007). Individual animal response to drugs of abuse is usually determined by changes in their locomotor activity (LA) after drug exposure (Jonah 1997; Mandt et al. 2008; Blanchard et al. 2009). Generally, LA is expressed as the amount of animal activity measured in an inescapable novel environment. Under these circumstances, animals may be classified as high (HR) and low responders (LR) by using the median split procedure (Piazza et al. 1989; Gulley et al. 2003; Allen et al. 2007; Mandt et al. 2008). HR and LR classification was initially used by considering the locomotor response to novelty (Piazza et al. 1989; Pierre and Vezina 1997; Suto et al. 2001; Nadal et al. 2005; Bhatti et al. 2007; Aydin et al. 2011), but recently it has been used also to characterize locomotor response to psychostimulants (Gulley et al. 2003; Cohen et al. 2005; Allen et al. 2007; Mandt et al. 2008). For instance, response to novelty did not predict the magnitude of cocaine-CPP (Gong et al. 1996). In contrast, locomotor response to cocaine was reported to predict cocaine-induced CPP but only in low responders to cocaine (Allen et al. 2007).
It has been suggested that individual differences in the sensitivity to nicotine depends on LA in response to a novel environment (Rosecrans 1995; Redolat et al. 2009). In fact, nicotine self-administration and nicotine CPP were increased by previous exposure to a novel environment (Brielmaier et al. 2012; Suto et al. 2001; Cain et al. 2006). Other studies demonstrated that only animals previously exposed to nicotine showed later a strong motivation for this drug of abuse (Shoaib et al. 1994). In contrast, recently, it was demonstrated that animals which consumed more nicotine in drinking water in their home cage (free choice task) for 6 weeks showed lower nicotine CPP score, regardless whether they were HR or LR (Nesil et al. 2011). Taken together, previous studies indicated that prior exposure to a novel environment or a drug of abuse may have a predictive value for drug of abuse consumption. However, the studies that examined nicotine effects are ambiguous. Thus, we evaluated in this work whether previous classification of rats in LR and HR to a single dose of nicotine had some incidence in the establishment of nicotine CPP.
Materials and methods
Animals
Adolescent male Sprague–Dawley rats, weighing 80–100 g (PN 25–26) (School of Pharmacy and Biochemistry, University of Buenos Aires) were housed in groups of four on a 12-h light/dark cycle with ad libitum access to food and water. Adolescent rats were used considering previous studies which demonstrated that high nicotine CPP scores can be obtained only if conditioning started between PN29 and PN35 (Vastola et al. 2002; Pascual et al. 2009; Pastor et al. 2011). Animals were handled during 5 min twice a day for 4 days prior to behavioral measurements. Animals were carefully cared for according to regulations specified in the Guide for the Care and Use of Laboratory Animals with the approval of the University of Buenos Aires.
Drugs
A dose of 0.4 mg/kg nicotine [hydrogen] tartrate (Sigma, St. Louis, MO) was dissolved in phosphate-buffered saline (PBS) and administered subcutaneously (SC) in a volume of 1 ml/kg body weight. An equal volume of PBS was injected for the control condition. Indicated doses are based on the molecular weight of the freebase.
Behavioral studies
Locomotor activity apparatus
Locomotor activity was quantified as previously described (Fuentealba et al. 2007). Horizontal locomotor activity was measured in Plexiglas cages (34 × 22 × 15 cm), fitted with a pair of photobeams, by using a device programmed to count only when beams at both ends of the cage were interrupted consecutively.
CPP apparatus
CPP took place in a three-compartment box. Boxes exhibited two similar compartments (28 × 21 × 21 cm), one black and the other white, separated by a small gray compartment (12 × 21 × 21 cm) with sliding doors. The two large compartments had different visual and tactile cues: The black compartment had a bar-grid floor, whereas the white one had a wire mesh floor. Each of the large compartments had pair of photobeams, and the software measured the interval between the first and second interruptions of the beam closest to the door as time spent in that compartment. Figure 1 shows a schematic diagram of the behavioral protocol. The conditioning box used in this study is considered biased because animals showed a significant preference for one compartment over the other prior to conditioning. In unbiased box, animals have not a preference for one compartment. Because previous data showed that two thirds of the studies in which nicotine-induced CPP were performed using biased procedure, we decided to use this type of conditioning box in our study (Calcagnetti and Schecter 1994; Le Foll and Goldberg 2005; Brielmaier et al. 2008).
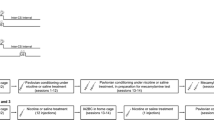
Schematic diagram showing the experimental procedure. First was performed the classification of rats in LNR and HNR, depending on their locomotor activity in response to a single nicotine injection (assay), after novelty and habituation period in the LA box. These two groups of animals underwent nicotine CPP using three different protocols, four-, one-, and seven-trial (see “Materials and Methods” for details). Arrows indicate nicotine (N), control saline (S) injections, and (S/N) indicate that saline or nicotine was administrated this day depending of the experimental group
Experiment 1, four-trial nicotine CPP
LA procedure
On day 1 after handling, animals were placed individually in LA cages during 60 min (the first 30 min novelty-induced response and the second 30 min habituation to the box were determined). Then, rats were removed from the box, injected SC with 0.4 mg/kg nicotine or 1 ml/kg saline (SAL, control animals), and immediately returned to the box, and the LA was measured for 30 min (LA assay session). LA in the assay session was defined as total consecutive beam interruptions. Nicotine-treated rats with activity scores that fell below the median distribution were defined as low nicotine-responders (LNR), while those with scores above the median were defined as high nicotine-responders (HNR) (according to Sabeti et al. 2002; Gulley et al. 2003; Allen et al. 2007; Mandt et al. 2008).
Conditioning-place preference in LNR and HNR rats
Following LA determination and after resting for 1 day in their home cage, animals classified as LNR or HNR and SAL underwent four-trial nicotine-CPP protocol.
Pre-conditioning phase (CPP-pretest)
The first day of CPP procedure, animals were placed in the CPP box with doors opened, which allowed them to roam freely the three compartments for 10 min. Time spent in each compartment was used to determine each animal’s compartment preference. Rats clearly preferred the black compartment. Only two animals from the total preferred the white side but with a preference around 60 %. These two animals were included in the CPP score.
Conditioning phase
The first conditioning day, rats were injected with PBS and immediately exposed to the preferred black compartment (door closed) for 30 min. The second conditioning day, rats were injected with either nicotine (NIC) or PBS (SAL) and immediately exposed to the non-preferred white compartment (door closed). This entire procedure was repeated four times (four-trial or 8-day protocol, Fig. 1). Locomotor horizontal activity in the white compartment (drug-paired compartment) was analyzed during the whole conditioning session every day.
Post-conditioning phase (CPP-test)
The day following the last conditioning session, animals were tested in a drug-free environment. Rats were allowed to explore the three compartments for 10 min with doors opened. Time spent in the white compartment was recorded for each animal.
Experiment 2 consisted of one trial and experiment 3 of seven-trial nicotine CPP
In both experiments, the LA was assessed as in experiment 1 to classify animals in LNR or HNR. For nicotine CPP, the general procedure was identical to experiment 1, except that in experiment 2 animals were trained only with one-trial (two conditioning days) and in experiment 3 with seven trials (14 conditioning days). The respective control groups included: absolute control (SAL): (LA: saline; CPP: saline); LNR-SAL: (LA: nicotine (LNR); CPP: saline) and HNR-SAL: (LA: nicotine (HNR); CPP: saline).
Data analysis
Because LA levels slightly varied between experiments over time, LNR/HNR classification was made within each distinct group tested in independent experiments and by treating all animals as a single population of different cohorts. Rats that were classified differently by both of these methods (i.e., within and between experiments) were excluded from final data analysis (Allen et al. 2007). Four or six rats were excluded by using these criteria.
Time spent in each compartment was converted into a preference score [Score (s) = time spent in the white compartment during test − time spent in the white compartment during pretest]. Preference scores data were analyzed using Student t test or one-way ANOVA followed by Scheffé post hoc test. LA data were analyzed by one- or two-way ANOVA followed by Scheffé. Results are expressed as means ± SEM. Significance was set at p ≤ 0.05.
Results
Experiment 1: four-trial nicotine CPP in HNR and LNR rats
Locomotor activity
Figure 2a shows the time course of LA measured every 5 min in the LA box. Two-way ANOVA revealed a significant group difference (F 2,39 = 11.085, p < 0.001). Scheffé post hoc comparisons showed significant differences in the LA at different times after nicotine administration but not before nicotine injection. The highest value was found at 10 min post-nicotine administration (SAL versus HNR, p < 0.001; LNR versus HNR, p < 0.001). Significant differences at 5 and 15 min were also observed (Fig. 2a). To verify if LNR and HNR classification correlated with novelty, animals classified as LNR and HNR were also classified as LR and HR by using novelty-induced individual values. Figure 2b dot graph shows the individual values of LR and HR, in which one can observe that LNR and HNR populations resulted mixed when they were reclassified considering novelty. To further compare novelty with nicotine effect on LA, the correlation between novelty (first 30 min) and LA assay sessions (30 min post-nicotine injection in LA box) was determined. No correlation between both parameters was found by using a linear regression analysis. Average LA was compared between saline (SAL), LNR, and HNR (Fig. 2b, bar graph), and significant group differences were found (F 2,39 = 53.861, p < 0.001). Post hoc significant differences were observed between SAL and HNR (p < 0.001) and LNR and HNR (p < 0.0001) groups.
Four-trial nicotine CPP without prior exposure to nicotine and following LNR and HNR classification. a Data are presented as total beam interruptions every 5 min over 60 min before (novelty-habituation) and 30 min after nicotine or control (SAL) injection. The arrow at 60 min indicates the time of injection. b Animals treated with a single injection of nicotine (0.4 mg/kg) were grouped according to their LA values and classified as HNR or LNR. In dot graphs individual LA in response to novelty (NOV) and following nicotine administration (ASSAY) are presented for LNR and HNR classification according to the data obtained during assay. Graph bars show total locomotor activity in response to saline (SAL) or nicotine (LNR/HNR) during the 30 min-assay. c Bars indicate CPP score in four-trial nicotine CPP without exposure to LA box (first two bars from the left) and in rats previously classified as LNR or HNR (four bars from the right). d LA is expressed as total consecutive beam interruptions during each conditioning sessions with nicotine (LNR and HNR) or saline (SAL) in the white compartment. Results are expressed as means ± SEM. Data were analyzed by Student t test, or one- or two-way ANOVA followed by Scheffé post hoc test. n = 7–16 per group. *p < 0.05, ***p < 0.001, and +++ p < 0.001 versus SAL; # p < 0.05, ## p < 0.01, and ### p < 0.001 versus LNR. SAL: control rats; NIC: nicotine-treated rats; LNR: low nicotine-responders; HNR: high nicotine-responders; LA: locomotor activity
Conditioning-place preference
Figure 2c shows four-trial CPP score in rats and four-trials CPP in LNR and HNR. Four-trial CPP without prior exposure to nicotine showed a significantly positive score (p < 0.05, first two bar graphs), in agreement with our previous studies (Pascual et al. 2009; Pastor et al. 2011). When CPP was performed after prior exposure to nicotine, with LNR and HNR classification, both groups of rats failed to show CPP. Figure 2d shows LA in the white compartment (drug-paired compartment). Two-way ANOVA indicated significant differences in group (F 2,39 = 73.351, p < 0.001), pairing (F 3,117 = 5.462, p < 0.01), and pairing × group (F 3,117 = 5.722, p < 0.001). Sheffé post hoc comparisons showed significant differences between SAL and LNR (p < 0.001 for pairings 1–4) and between SAL and HNR (p < 0.001 for pairings 1–4). It is important to remark that HNR were consistently more active than LNR during nicotine pairing 1 (p < 0.001), but not during pairings 2, 3, and 4.
Experiment 2: one-trial CPP in HNR and LNR rats
Data from experiment 1 suggested that a single prior exposure to nicotine decreased preference for the drug in a four-trial protocol. Because HNR and LNR showed LA differences during the first conditioning day, we examined both groups by using one-trial nicotine-CPP.
Locomotor activity
Different groups of rats were first classified in LNR and HNR, and one trial CPP was performed. Time course of LA during novelty-habituation and nicotine assay periods was analyzed by using two-way ANOVA, which showed a significant group difference (F 2,37 = 32.790, p < 0.001) (Fig. 3a). Scheffé post hoc comparisons showed that the highest value of LA was exhibited at 5 min post-nicotine administration (SAL-HNR, p < 0.001; SAL-LNR, p < 0.05, and LNR-HNR, p < 0.001), but significant differences were also observed at 10 and 15 min for HNR and 10 min for LNR (Fig. 3a). No significant differences in LA were observed during the novelty-habituation period among groups. Figure 4b displays individual values of LNR or HNR (dot graph). The novelty group was built by using the individual novelty-induced LA values in animals that were later classified as LNR (gray) or HNR (black) in the assay session. As described above, we aimed to evaluate a possible correlation between novelty- and nicotine-evoked LA in LNR or HNR. The LNR and HNR populations became mixed when individual novelty values were used as data. Further analyses demonstrated no significant correlation between novelty and nicotine effects on LA levels. The LA average values from LNR and HNR were compared with each other and control rats (Fig. 3b bars) showing significant differences among groups (F 2,37 = 74.660, p < 0.001). Moreover, SAL versus HNR (p < 0.001), SAL versus LNR (p = 0.001), and LNR versus HNR (p < 0.001) were significantly different.
One-trial CPP after LNR and HNR classification. a Data are expressed as total beam interruptions every 5 min. The arrow at 60 min indicates the time of injection. b Dot graphs show individual LA in response to novelty (NOV) and after nicotine administration (ASSAY) presented for LNR or HNR rats classification according to the data obtained after assay. Bars represent total beam interruptions in response to saline (SAL) or nicotine injection. c Bars indicate CPP score using one-trial nicotine CPP in rats which were previously classified as LNR or HNR and SAL. d Graph indicates the LA in response to saline or nicotine injection during the 30-min conditioning session in the white compartment. Results are expressed as means ± SEM. Data were analyzed by one- or two-way ANOVA followed by Scheffé post hoc test. n = 8–16 per group. *p < 0.05, **p < 0.01, ***p < 0.001, and +++p < 0.001 versus SAL; # p < 0.05, ## p < 0.01, and ### p < 0.001 versus LNR. SAL: control rats; LNR: low nicotine responders; HNR: high nicotine responders
Seven-trial CPP after LNR and HNR classification. a Data are expressed as total beam interruptions every 5 min. The arrow at 60 min indicates the time of injection. b In the dot graphs, individual locomotor activity in response to novelty (NOV) and following nicotine administration (ASSAY) are presented for rats classified as LNR or HNR. Bars show total LA in response to SAL or LNR and HNR. c Bars indicate CPP score following seven-trial nicotine-CPP in rats which were previously classified as LNR, HNR, and the saline groups (SAL, LNR-SAL, and HNR-SAL). d LA was measured during each conditioning sessions with nicotine (or saline) in the white compartment. Data were analyzed by one- or two-way ANOVA. Scheffé post hoc tests were performed when required. Significance was set at p ≤ 0.05. n = 5–16 per group. In graphs a and b, one- or two-way ANOVA followed by Scheffé test was applied, *p < 0.05, **p < 0.01, and ***p < 0.001 versus SAL; # p < 0.05, ## p < 0.01, and ### p < 0.0001 versus LNR. In graph c, data were analyzed by one-way ANOVA followed by Scheffé, *p < 0.05. In graph d, data were analyzed by two-way ANOVA followed by Scheffé, + p < 0.05, +++ p < 0.001 LNR versus SAL; X p < 0.05, XX p < 0.01, XXX p < 0.001 LNR versus LNR-SAL; # p < 0.05 LNR versus HNR; ***p < 0.001 HNR versus SAL and HNR versus HNR-SAL. SAL: control rats; NIC: nicotine-treated rats; LNR-SAL, and SAL-HNR by HNR-SAL: LNR-saline during CPP; SAL-HNR: HNR saline during CPP; LNR: low nicotine-responders; HNR: high nicotine-responders
Conditioning place preference
One-trial CPP score in LNR and HNR rats showed not significant differences (Fig. 3c). Figure 3d depicts LA expressed as total consecutive beam interruptions during the conditioning protocol with nicotine or saline in the white compartment (drug-paired compartment). One-way ANOVA (F 2,37 = 31.926, p < 0.001) and post hoc test revealed significant differences between SAL and LNR (p < 0.001), SAL and HNR (p < 0.001), and LNR and HNR (p < 0.001).
Experiment 3: seven-trial nicotine CPP in HNR and LNR rats
Considering that preference for nicotine was not established using four- or one-trial CPP in LNR and HNR, we evaluated whether more conditioning days could help to establish nicotine CPP in LNR and HNR.
Locomotor activity
In Fig. 4a, we analyzed the time course of LA by using two-way ANOVA (F 2,27 = 15.913, p < 0.001), followed by Scheffé post hoc comparisons which revealed significant differences at different times after nicotine administration among SAL, LNR, and HNR. The highest value was found at 5 min post-nicotine administration (SAL-HNR, p < 0.001; SAL-LNR, p < 0.05, and LNR-HNR, p < 0.05). Significant differences were also observed at 10 and 15 min. As described for the previous experiments, there was no correlation between novelty- and nicotine-induced LA assay sessions (dot graph in Fig. 4b). LA during nicotine assay revealed significant group differences (F 2,27 = 99.524, p < 0.001). Post hoc comparisons showed significant differences between SAL and HNR (p < 0.001), SAL and LNR (p < 0.01), and LNR and HNR (p < 0.001).
Conditioning-place preference
In seven-trial CPP, the score revealed significant differences between absolute saline, LNR + HNR saline, and LNR + HNR nicotine groups (first three bars from the left in Fig. 4c) (F 2,49 = 6.873, p < 0.01). Post hoc Scheffe showed significant differences in LNR + HNR nicotine compared with absolute saline (p < 0.05) and LNR + HNR saline (p < 0.05). Considering LNR and HNR separately, one-way ANOVA revealed significant differences between groups (F 4,47 = 4.971, p < 0.01). Post hoc analysis showed that only LNR developed CPP compared with absolute control (p < 0.05) and LNR saline animals (p < 0.05). No significant difference was observed between LNR- and HNR-CPP and HNR-SAL and HNR-CPP animals (Fig. 4c).
To evaluate whether nicotine had differential behavioral effects on LNR or HNR during the seven-trial CPP, we analyzed the LA at every conditioning day. Figure 4d shows significant differences in group (F 2,27 = 25.021, p < 0.001) and pairing × group (F 6,162 = 2.395, p < 0.01). Significant differences were found between SAL and LNR (p < 0.05 for pairing 1; p < 0.001 for pairings 2–7), SAL and HNR (p < 0.001 for pairings 1–7). HNR were consistently more active than LNR only during the first pairing (p < 0.05).
Discussion
The present study explored whether individual differences in initial locomotor responsiveness to nicotine in rats can predict the rewarding properties of nicotine using a CPP task.
We decided to use the median split of a Gaussian distribution method, according to extensive previous evidences (Piazza et al. 1989; Allen et al. 2007; Mandt et al. 2008), to divide animals in LNR and HNR categories because our intention was to include almost the whole population in this classification. We considered that other methods discard more animals for further analysis, particularly those close to the median value. Therefore, the median split takes into account the majority of animals allowing a more representative interpretation of data, which might be biologically more relevant. It is important to remark that LA parameters measured previously to classification in LNR and HNR, which involved novelty and habituation to the new environment showed similar values in the three experiments performed in this work. Moreover, to classify the animals in LNR and HNR during the 15 min following nicotine administration in the LA box, the number of beam interruptions, as a measure of LA, was similar in the three experiments, indicating the robustness of this methodology. Previous studies have suggested that novelty and psychostimulants increase locomotor activity through different mechanisms (Bevins and Besheer 2001; Gulley et al. 2003; Coolon and Cain 2009). Furthermore, a correlation between a novel environment and psychostimulant administration effects on LA has been previously reported (Piazza et al. 1989, 2000; Deroche et al. 1993). However, our results demonstrated that groups classified according to novelty (LR/HR) or in response to acute nicotine (LNR/HNR) were not coincident, indicating that these two methods to classify low and high LA responders probably involve different mechanisms, as it was suggested for cocaine (Gulley et al. 2003).
On the other hand, the age of exposure to a particular drug affects developmental outcomes. Prenatal exposure influences drug-taking propensity of the off-spring, and adolescents are particularly vulnerable to drug seeking and consumption (Kandel et al. 2006; Substance Abuse and Mental Health Services Administration 2010). Adolescence represents a crucial phase in development, characterized by specific neurobiological and behavioral features such as impulsivity and risk-taking (Adriani and Laviola 2004). Nicotine provokes differential effects in adolescent and adult rodents by influencing LA and CPP. Upregulation of nicotinic receptors and modulation of excitatory signaling in midbrain DA neurons are triggered by nicotine exposure, and these changes were age-dependent. In particular, adolescent rats showed greater responses to nicotine (Placzek et al. 2009). Considering these previous findings, we decided to use adolescent animals in the present work.
Once the animals were classified in LNR and HNR, they were submitted to CPP to evaluate nicotine reward. To our surprise, LNR and HNR rats were unable to establish four-trial CPP, a protocol used regularly in our studies (Pascual et al. 2009; Pastor et al. 2011). The psychostimulant effect of nicotine was demonstrated because during each conditioning day LNR and HNR rats showed significant increases in LA compared with controls, as it has been previously demonstrated (Stolerman et al. 1973; Clarke and Kumar 1983; Benwell and Balfour 1992; Nisell et al. 1996; Miller et al. 2001; Vezina et al. 2007). It has been recently reported that rats exposed to free choice nicotine (oral consumption for several weeks) did not develop CPP in a four-trial schedule, suggesting a tolerance effect (Nesil et al. 2011). In contrast, Lister rats treated with nicotine for 7 days prior to four conditioning trials established significant nicotine CPP with 0.6 mg/kg of nicotine (Shoaib et al. 1994). Reasons for the failure of nicotine to induce four-trial CPP in animals previously exposed to the drug are unknown; however, this exposure appears to induce key changes in the generation of CPP. One possibility to consider is that the exposure to the LA box and the CPP box (during pretest) could generate latent inhibition (Lubow 1973). Latent inhibition may reduce the association between environment and nicotine, thus decreasing four-trial CPP score.
The fact that animals previously classified as LNR and HNR showed similar LA response during the first conditioning day indicated that this behavioral classification is conserved after few days. This difference in LA could suggest sensitization in LNR or tolerance in HNR animals. However, both groups reached the same level of LA from the second trial, indicating that no further sensitization was induced. Since a constant level of LA was observed during the following days, tolerance cannot be discarded during conditioning, as it was suggested previously (Nesil et al. 2011; Shoaib et al. 1994). Considering the above results and the fact that nicotine CPP could be established with only one nicotine pairing (Spina et al. 2006; Brielmaier et al. 2007), we decided to test if the difference in LA activity observed during the first conditioning day could participate in the induction of one-trial CPP. LNR or HNR were unable to establish a preference by using one-trial nicotine-CPP, in agreement with previous studies indicating that only one nicotine pairing is not enough to develop a preference (Laviollette and van der Kooy 2004; Le Foll and Goldberg 2005; Markou 2008; Barik and Wonnacott 2009). Nevertheless, other groups found single-trial CPP to nicotine in early adolescent but not in adult rats, by using either biased or unbiased designs (Belluzzi et al. 2004; Brielmaier et al. 2008). Likewise, we have previously found that nicotine induced a significant one-trial CPP in early adolescent rats; however, those experiments were carried out without prior exposure to nicotine (unpublished data).
It is known that more conditioning sessions can increase the probability of establishing nicotine-CPP (Laviollette and van der Kooy 2004; Le Foll and Goldberg 2005); therefore, we conducted a seven-trial protocol. Our results showed that, after seven trials, LNR exhibited a positive CPP score. Analyzing combined LNR and HNR data, the CPP score was also positive, but only LNR were responsible for this significant effect. These results are in agreement with previous observations in which cocaine low responders but not high responders developed cocaine-CPP (Allen et al. 2007). These results evidenced that nicotine and cocaine provoke a similar effect on CPP when animals are previously exposed to these drugs. Recently, it was demonstrated by using CPP that nicotine primed the response to cocaine, but cocaine did not prime the response to nicotine, showing that previous administration of nicotine could facilitate cocaine preference (Levine et al. 2011). The potential effect of prior exposure to nicotine on other drugs of abuse in CPP raises an interesting matter, particularly in view of the observation that among adults aged 18 to 34 years who had used drugs of abuse at least once, 90 % had smoked cigarettes before they began to use psychostimulants (Kandel et al. 2006; Substance Abuse and Mental Health Services Administration 2010).
Noteworthy, rats classified as LNR and HNR during prior exposure to nicotine coincided with the animals that later showed low or high LA in response to nicotine during the first conditioning day, which may indicate an individual intrinsic response of each animal to nicotine exposure. Therefore, the first exposure to nicotine in the CPP box could be used for LNR and HNR classification without the need of measuring the LA in the LA box. Using this method of classifying LNR and HNR animals that considers particular individual responsiveness to nicotine may be an effective strategy for identifying genetic, epigenetic, and cellular mechanisms that can contribute to nicotine vulnerability. Difference between LNR and HNR might be due in part to the differential expression of nicotinic receptors in those animals, even though this hypothesis needs further corroboration. Moreover, considering previous studies which demonstrated that transcription and epigenetic factors are involved in nicotine CPP (Pascual et al. 2009; Pastor et al. 2011), it is possible that these factors influence behavioral differences among animals.
Our data suggest that LA response classification to a previous nicotine administration predicts the establishment of nicotine CPP in a subgroup of animals at least after seven trials, but abolishes the preference for nicotine when a four-trial schedule is used. The classification methodology in HNR and LNR modifies the CPP outcome and a stronger association between environmental cues and nicotine is necessary to generate a significant CPP score.
In summary, our data suggested that rats with low initial locomotor responsiveness to nicotine in an environment without explicit behavioral consequences exhibit a phenotype of increased susceptibility to develop nicotine CPP. Our findings also highlighted the fact that using outbred rats as a homogeneous population could lead to ambiguous conclusions and misinterpretation of data. We believe that LNR/HNR classification is a more realistic strategy to evaluate nicotine preference-related behaviors, in spite of increasing conditioning trials. Finding out neurochemical or molecular differences between LNR and HNR might clarify the mechanisms involved in the differential response to nicotine and might help to determine the individual vulnerability to nicotine consumption.
References
Adriani W, Laviola G (2004) Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol 15:341–352
Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV (2003) Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci 23:4712–4716
Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR (2007) Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague–Dawley rats. Pharmacol Biochem Behav 86:37–44
Aydin C, Oztan O, Isgor C (2011) Vulnerability to nicotine abstinence-related social anxiety-like behavior: molecular correlates in neuropeptide Y, Y2 receptor and corticotropin releasing factor. Neurosci Lett 490:220–225
Barik J, Wonnacott S (2009) Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol 192:173–207
Belluzzi JD, Lee AG, Oliff HS, Leslie FM (2004) Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacol (Berl) 174:389–395
Benwell ME, Balfour DJ (1992) The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. Br J Pharmacol 105:849–856
Bevins RA, Besheer J (2001) Individual differences in rat locomotor activity are diminished by nicotine through stimulation of central nicotinic acetylcholine receptors. Physiol Behav 72:237–244
Bhatti AS, Hall P, Ma Z, Tao R, Isgor C (2007) Hippocampus modulates the behaviorally-sensitizing effects of nicotine in a rat model of novelty-seeking: potential role for mossy fibers. Hippocampus 17:922–933
Blanchard MM, Mendelsohn D, Stamp JA (2009) The HR/LR model: further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev 33:1145–1154
Brielmaier JM, McDonald CG, Smith RF (2007) Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol 29:74–80
Brielmaier JM, McDonald CG, Smith RF (2008) Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav 89:94–100
Brielmaier J, McDonald CG, Smith RF (2012) Effects of acute stress on acquisition of nicotine conditioned place preference in adolescent rats: a role for corticotropin-releasing factor 1 receptors. Psychopharmacol (Berl) 219:73–82
Cain ME, Dotson WF, Bardo MT (2006) Individual differences in the effect of novel environmental stimuli prior to amphetamine self-administration in rats (Rattus norvegicus). Exp Clin Psychopharmacol 14:389–401
Calcagnetti DJ, Schechter MD (1994) Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry 18:925–933
Clarke PB, Kumar R (1983) Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol 80:587–594
Cohen C, Perrault G, Griebel G, Soubrié P (2005) Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30:145–155
Coolon RA, Cain ME (2009) Individual differences in response to novelty and the conditioned locomotor effects of nicotine. Behav Pharmacol 20:322–329
Deroche V, Piazza PV, Le Moal M, Simon H (1993) Individual differences in the psychomotor effects of morphine are predicted by reactivity to novelty and influenced by corticosterone secretion. Brain Res 623:341–344
Fuentealba JA, Gysling K, Andrés ME (2007) Increased locomotor response to amphetamine induced by the repeated administration of the selective kappa-opioid receptor agonist U-69593. Synapse 61:771–777
Gong W, Neill DB, Justice JB Jr (1996) Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav 53:191–196
Goriounova NA, Mansvelder HD (2012) Nicotine exposure during adolescence leads to short- and long-term changes in spike timing-dependent plasticity in rat prefrontal cortex. J Neurosci 32:10484–10493
Gulley JM, Hoover BR, Larson GA, Zahniser NR (2003) Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology 28:2089–2101
Jonah BA (1997) Sensation seeking and risky driving: a review and synthesis of the literature. Accid Anal Prev 29:651–665
Kandel DB, Yamaguchi K, Klein LC (2006) Testing the gateway hypothesis. Addiction 101:470–472
Laviolette SR, van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55–65
Le Foll B, Goldberg SR (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacol (Berl) 178:481–492
Levine A, Huang Y, Drisaldi B, Griffin EA Jr, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER (2011) Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med 3:107ra109
Lubow RE (1973) Latent inhibition. Psychol Bull 79:398–407
Mandt BH, Schenk S, Zahniser NR, Allen RM (2008) Individual differences in cocaine-induced locomotor activity in male Sprague–Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology 201:195–202
Markou A (2008) Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci 363:3159–3168
Mathews IZ, Kelly H, McCormick CM (2011) Low doses of amphetamine lead to immediate and lasting locomotor sensitization in adolescent, not adult, male rats. Pharmacol Biochem Behav 97:640–646
Miller DK, Wilkins LH, Bardo MT, Crooks PA, Dwoskin LP (2001) Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacol (Berl) 156:469–476
Nadal R, Rotllant D, Márquez C, Armario A (2005) Perseverance of exploration in novel environments predicts morphine place conditioning in rats. Behav Brain Res 165:72–79
Natarajan R, Wright JW, Harding JW (2011) Nicotine-induced conditioned place preference in adolescent rats. Pharmacol Biochem Behav 99:519–523
Nesil T, Yararbas G, Mola G, Kanit L, Pogun S (2011) Previous chronic exposure eliminates the conditioning effect of nicotine in rats. Brain Res Bull 85:339–345
Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH (1996) Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22:369–381
Pascual MM, Pastor V, Bernabeu RO (2009) Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacology 207:57–71
Pastor V, Host L, Zwiller J, Bernabeu R (2011) Histone deacetylase inhibition decreases preference without affecting aversion for nicotine. J Neurochem 116:636–645
Piazza PV, Deminière JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245:1511–1513
Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M (2000) Vertical shifts in self-administration dose–response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci 20:4226–4232
Pierre PJ, Vezina P (1997) Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology 129:277–284
Placzek AN, Zhang TA, Dani JA (2009) Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochem Pharmacol 78:686–692
Redolat R, Pérez-Martínez A, Carrasco MC, Mesa P (2009) Individual differences in novelty-seeking and behavioral responses to nicotine: a review of animal studies. Curr Drug Abuse Rev 2:230–242
Rosecrans JA (1995) The psychopharmacological basis of nicotine's differential effects on behavior: individual subject variability in the rat. Behav Genet 25:187–196
Sabeti J, Gerhardt GA, Zahniser NR (2002) Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J Pharmacol Exp Ther 302:1201–1211
Shearman E, Fallon S, Sershen H, Lajtha A (2008) Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res Bull 76:626–639
Shoaib M, Stolerman IP, Kumar RC (1994) Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology 113:445–452
Shram MJ, Funk D, Li Z, Lê AD (2006) Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology 186:201–208
Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G (2006) Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacol (Berl) 184:447–455
Stolerman IP, Fink R, Jarvik ME (1973) Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia 30:329–342
Substance Abuse and Mental Health Services Administration (2010) Office of Applied Studies, National Survey on Drug Use and Health, 2009 [Computer file], ICPSR29621-v1, Ann Arbor, MI : Inter-university Consortium for Political and Social Research [distributor], 11-16
Suto N, Austin JD, Vezina P (2001) Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology 158:175–180
Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP (2002) Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77:107–114
Vezina P, McGehee DS, Green WN (2007) Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry 31:1625–1638
Wagner FA, Anthony JC (2002) From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 26:479–488
World Health Organization (2010) Atlas 2010 of drug of abuse around the world
Acknowledgments
This work was supported by FONCyT, and CONICET grants (R.B.), Argentina, and MCI P10/063F, Chile. We thank M. P. Faillace for helpful comments and discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pastor, V., Andrés, M.E. & Bernabeu, R.O. The effect of previous exposure to nicotine on nicotine place preference. Psychopharmacology 226, 551–560 (2013). https://doi.org/10.1007/s00213-012-2928-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2928-1