Abstract
Rationale
Initiation of tobacco use typically begins during adolescence, and the nature of these first experiences with nicotine may affect the probability of continued use. In rodents, a number of studies suggest that periadolescents are more responsive to the rewarding effects of nicotine compared to adults.
Objectives
This study was designed to determine if there are age differences in the rewarding and aversive effects of nicotine by using the conditioned place preference (CPP) and conditioned taste avoidance (CTA) paradigms, respectively. We also examined age differences in locomotor responses to nicotine.
Methods
In the CPP paradigm, male periadolescent and adult Wistar rats received nicotine (0.2, 0.4, or 0.8 mg/kg, s.c.) or vehicle prior to place conditioning trials. In the CTA paradigm, in separate groups of rats, periadolescents and adults were exposed to a 0.1% saccharin solution, followed by the administration of nicotine (0.2, 0.4, or 0.8 mg/kg, s.c.) or vehicle. Four saccharin–nicotine pairings were followed by a preference test and three extinction sessions.
Results
In the CPP paradigm, nicotine produced a dose-dependent place preference in periadolescent, but not in adult, rats. In the CTA paradigm, adult rats expressed a dose-dependent avoidance of saccharin after pairings with nicotine, whereas periadolescents were resistant to CTA formation. With regard to locomotor activity, adults and periadolescents showed comparable locomotor responses to nicotine.
Conclusions
These results suggest that periadolescent rats find nicotine more rewarding and less aversive, compared to adult rats. This shift in the balance between the rewarding and aversive effects of nicotine may make adolescents more susceptible to continued nicotine use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The high prevalence of initiation of tobacco use during adolescence has important implications. For example, subjects who began smoking as adolescents show a reduced probability of quitting compared to those who began as adults (Breslau and Peterson 1996; Chen and Millar 1998). Additionally, the early onset of tobacco use is a strong predictor of future alcohol and drug use, whereas the onset of use at later ages is not associated with such an increased risk (Grant 1998; Hanna and Grant 1999). Adolescence, therefore, appears to be a vulnerable developmental stage for exposure to tobacco.
The nature of the first experiences with a particular drug can have a long-term impact on the patterns of subsequent use in both animals (Kelley and Middaugh 1999; Brandon et al. 2001; Andersen et al. 2002; Rodd-Henricks et al. 2002a,b; but see Smith et al. 2002) and humans (Clark et al. 1998; Chen and Millar 1998; Eissenberg and Balster 2000). Therefore, studying how the motivational effects of drugs differ between adolescents and adults may shed light on both the greater susceptibility to abuse during the adolescent stage and the mechanisms underlying continued drug use in adults. In rodents, periadolescence is the developmental period comprising the 7–10 days before puberty and the several days after the onset of puberty, which occurs at approximately 40 days of age and is precipitated by a prepubertal surge of gonadal hormones (Compechot et al. 1981). This ontogenetic period, spanning postnatal days 28 to 42 (P28–42), is characterized by increased novelty seeking, playful behavior, and impulsivity, as well as a differential sensitivity to pharmacological manipulations, compared to adults (Spear and Brake 1983; for extensive review, see Spear 2000).
Place conditioning is a procedure commonly used to measure the motivational effects of rewarding (e.g., sex, food, social interaction, drugs of abuse) and aversive (e.g., stress, drug withdrawal) stimuli in laboratory rodents (Mucha et al. 1982; Carr and White 1983; Mucha and Iversen 1984; Higgins et al. 1992; Paredes and Alonso 1997; McBride et al. 1999; Douglas et al. 2004; see Tzschentke 1998 for greater detail). In this paradigm, which is based on principles of Pavlovian conditioning, the unconditioned stimulus (US), such as a drug, is repeatedly paired with a neutral environment containing distinctive cues (visual, tactile, olfactory). This environment eventually acquires secondary motivational properties and becomes a conditioned stimulus that, in the absence of the US, will come to elicit approach or avoidance, the conditioned response, depending on the nature of the US.
Most of these studies, done primarily in adults, show that preferences for environments previously associated with injections of opiate and psychostimulant drugs are reliably demonstrated (Mucha et al. 1982; Carr and White 1983; Mucha and Iversen 1984; McBride et al. 1999). Results using conditioned place preference (CPP) to examine the rewarding effects of nicotine in adult rats have, however, been inconsistent, with some studies demonstrating a preference (Fudala et al. 1985; Calcagnetti and Schechter 1994; Le Foll and Goldberg 2005), an aversion (Clarke and Fibiger 1987; Laviolette and van der Kooy 2004), or no preference (Vastola et al. 2002; Torrella et al. 2004; Belluzzi et al. 2004) to nicotine-associated environmental stimuli. One possible reason that may contribute to this discrepancy is the use of different methodologies, e.g., biased vs unbiased designs. To date, there are only a few studies that have examined age differences in place conditioning to nicotine (Vastola et al. 2002; Belluzzi et al. 2004; Torrella et al. 2004), and two of these (those by Vastola et al. and Torella et al.) used a biased design, which is vulnerable to false positive results (Carr and White 1986; Tzschentke 1998). Results from these studies suggest that periadolescent rats are more sensitive to the rewarding effects of nicotine than adults, but interpretation is difficult due to the design used and the limited range of nicotine doses employed.
Another technique used to assess the motivational effects of a drug is the conditioned taste avoidance (CTA) paradigm. In a CTA study, repeated pairings of an appetitive novel tastant with a drug may result in reduced tastant consumption, which is thought to reflect the aversive properties of that drug (Wise et al. 1976; Carr and White 1986; Mayer and Parker 1993). CTA has been demonstrated across all major classes of addictive substances in adult rats (Parker 1995). Interestingly, drugs that induce a place preference will also elicit a significant CTA (van der Kooy et al. 1983; Mayer and Parker 1993; Chester and Cunningham 1999). In adult rats and mice, CTA induced by nicotine is a reliable phenomenon and is dose-dependent (Kumar et al. 1983; Pratt and Stolerman 1984; Etscorn et al. 1987; Shoaib and Stolerman 1995; Shoaib et al. 2002; Sellings et al. 2005).
Few studies have examined the effects of age on a nicotine-induced CTA. The effect of age on the development of an aversion to a flavored fluid containing nicotine has, however, recently been examined. Wilmouth and Spear (2004) showed that periadolescent rats may be more resistant to the development of an aversion to nicotine-containing solutions. Typical CTA experiments are designed such that drug administration immediately follows exposure to a sweet solution and a discrete association is made between the solution and drug effect. The procedure used in the Wilmouth and Spear (2004) study differed, in that the tastant contained nicotine and animals were exposed to it for long periods. As such, the possibility of latent inhibition makes it difficult to interpret the results, and comparisons with more commonly used CTA methodologies employing brief exposures to a novel tastant followed by drug injections may be limited.
Taking these results from CPP and CTA studies together, it appears that, at least in adults, nicotine has both rewarding and aversive properties. It may therefore be that the relative balance between the rewarding and aversive effects influences the likelihood that nicotine would be consumed on a continuous basis. It may be speculated that periadolescent rats may differ from adults in one or both of these effects of nicotine, and therefore the parallel use of these two procedures may be helpful in determining the mechanisms underlying the relative susceptibility of periadolescents to the development of nicotine use or abuse.
In the current study, to explore how this relationship may be influenced by age, differences between periadolescent and adult rats in the rewarding effects of nicotine were examined using an unbiased CPP procedure and the aversive effects of nicotine were determined using a CTA procedure. We also examined age differences in the locomotor-activating effects of nicotine following acute and repeated nicotine administration as another correlate of the rewarding effects of nicotine.
Materials and methods
Experiment 1
Animals
Periadolescent (P21, n=48) and adult (P53–56, n=48) male Wistar rats (Charles River, Quebec, Canada) arrived at the animal facility and were group-housed in Plexiglas cages measuring 51×41×20 cm. Periadolescents were housed by litter (n=6 per litter) and adults were housed four per cage. The rats were maintained on a 12/12 h light/dark cycle (lights on at 0700 hours) in a temperature- and humidity-controlled vivarium. Water and Purina rat chow were available ad libitum throughout the experiment.
Apparatus
The CPP boxes (30×60×40 cm) were made of Plexiglas and aluminum and composed of two compartments identical in size, separated by an elevated platform. One compartment was white and had a textured floor, while the other was black and had a floor with 1-cm holes set 1 cm apart. The floors used in the black compartments were the same for both periadolescents and adults, while those for the white compartments differed slightly in texture between the two age groups; this was necessary to equate preference for the two sides. A removable partition was placed between the two compartments during conditioning, and a white-noise generator masked environmental noise. The time spent in each compartment and locomotor activity were recorded using infrared photocell beams positioned equidistantly throughout the boxes during the conditioning and the test sessions.
Procedure
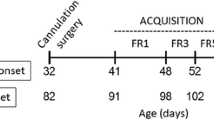
Prior to conditioning, animals (periadolescents, P28, and adults, P60–63) were allowed to habituate to the unpartitioned compartments for 15-min sessions on two consecutive days. During the conditioning sessions, animals received vehicle (0.9% saline) or one of three doses of nicotine tartrate (0.2, 0.4, or 0.8 mg/kg, s.c., expressed as base; Sigma-Aldrich, Oakville, ON, Canada) immediately prior to exposure to the CPP box. Nicotine administration was paired with one compartment type and vehicle with the other on alternate days during once-daily, 20-min conditioning sessions. There were a total of four nicotine pairings and four vehicle pairings. Injection order, i.e., nicotine or vehicle first, was counterbalanced across condition and compartment type. No more than two periadolescents from each litter were assigned to the same drug condition. Periadolescents were 30–37 days old and adults were 62–72 days old during conditioning.
On the test day for place preference, 24 h following the last conditioning session, the partition was removed and each rat was placed on the central platform and allowed to freely explore the unpartitioned compartments for 15 min; no injections were given during the test. Periadolescents were 38 days old and adults were 70–73 days old at time of testing.
Experiment 2
Animals
Animal ages and housing conditions were the same as in experiment 1. Animals had access to water for 10 h/day following conditioning trials to minimize the impact of water deprivation on the health of the developing periadolescents. This procedure differs from that typically used in CTA studies, in which animals are water-deprived for considerably longer periods.
Procedure
Periadolescent (P30) and adult (P62–65) animals were initially water-deprived for 24 h and subsequently familiarized with a 0.1% saccharin solution presented in a Richter tube for 1 h in novel cages. Saccharin–nicotine pairings began the following day, which was P31 for periadolescents and P63–66 for adults. All animals had daily access to saccharin for 30 min; water was unavailable. Vehicle or nicotine (0.2, 0.4, or 0.8 mg/kg, s.c.) was administered 20 min following saccharin exposure. No more than two periadolescents from each litter were assigned to the same drug condition. Four pairings occurred over a period of 4 days. On the test day for CTA, which occurred the day after the fourth pairing day, animals were given access to both saccharin and water for 30 min. This occurred on P35 and P67–70 for periadolescents and adults, respectively. The following 3 days comprised extinction trials, during which animals were treated in the same way as on the test day. On the test day and during the extinction phase, rats had a choice between saccharin and water, and no injections were administered.
Statistics and data presentation
For experiment 1, place conditioning to nicotine, the results of the test for place preference were calculated as difference scores (time on side paired with nicotine on test day − time on respective side during habituation test). The first 5 min of the test was analyzed, as we have found the most robust effects in this interval. These values were analyzed by two-way ANOVA, with the between-subjects factors of Age and Nicotine Dose. The number of photobeam breaks was used as an index of locomotor activity and was analyzed by repeated measures ANOVA, with the between-subjects factors of Age and Nicotine Dose and the repeated factor of Conditioning Trial. Baseline locomotor activity from the first vehicle-paired conditioning trial was used as a covariate in the analysis of nicotine’s effect on locomotor activity because adults were observed to be significantly more active under baseline conditions compared to periadolescents.
For experiment 2, saccharin consumption (in milliliters) from the test day was analyzed by two-way ANOVA with the between-subjects factors of Age and Nicotine Dose. To examine age differences in the extinction of the nicotine-induced CTA, data from all four extinction days were analyzed by repeated measures ANOVA, with the between-subjects factors of Age and Nicotine Dose and the within-subjects factor of Extinction Trial. Baseline consumption of saccharin on the first conditioning day was used as a covariate in the analysis of CTA because adults consumed significantly more saccharin compared to periadolescents.
For post hoc tests, Tukey’s Honestly Significant Difference was used where appropriate. Statistical significance for all tests was set at α=0.05, and was adjusted according to the number of levels in each factor for simple effect analysis. Statistical analyses were performed using Statistical Package for the Social Sciences version 12.0.
Results
Experiment 1: place conditioning with nicotine
There was a significant Age × Nicotine Dose interaction for time spent in the nicotine-paired compartment [F(3,49)=3.19, p<0.05]. As shown in Fig. 1a, nicotine produced a dose-dependent place preference in periadolescent rats [F(3,25)=5.34, p<0.01]. Post hoc tests revealed that periadolescent animals that received 0.8 mg/kg nicotine during place conditioning had a significant preference for the nicotine-paired side, compared with those administered vehicle or 0.2 mg/kg nicotine (p<0.05). In contrast, adults did not exhibit a significant place preference or aversion to the environment paired with any of the doses of nicotine (Fig. 1b).
Place conditioning to nicotine in a periadolescent (P38) and b adult (P70–73) male Wistar rats. Bars represent mean (±SEM) difference in time (seconds) spent on the nicotine-paired side prior to and following conditioning with 0, 0.2, 0.4, or 0.8 mg/kg nicotine (s.c.). Data were calculated as time spent in the nicotine-paired side during the first 5 min of the preference test minus the first 5 min of the second habituation session. n=6–8 per dose at each age. The asterisk represents p<0.05 compared to vehicle-treated animals
Locomotor activity
Figure 2a,b show periadolescent and adult locomotor activity, respectively, following nicotine administration during conditioning. Overall analysis of locomotion during conditioning revealed a significant Trial × Nicotine Dose interaction [F(9,144)=8.59, p<0.001]. This occurred due to the fact that locomotor activity tended to decline (adults), or remained the same (periadolescents) in the control groups over conditioning trials [F(3,33)=3.34, p<0.05], whereas locomotor activity increased with repeated nicotine administration (all doses, p<0.001, compared to control groups). Adults were consistently more active during conditioning, as revealed by a significant effect of Age [F(1,48)=5.99, p<0.05]. The factor of Age did not interact with Trial or Nicotine Dose.
Experiment 2: nicotine-induced conditioned taste avoidance
Analysis of the test day for CTA, the first day during which animals had a choice between saccharin and water, revealed a significant Age × Nicotine Dose interaction [F(3,50)=3.25, p<0.05]. Prior pairings with nicotine at any dose did not attenuate saccharin consumption in periadolescent rats (Fig. 3). In contrast, prior nicotine pairings reduced saccharin consumption in adult animals [F(3,23)=3.31, p<0.05]. The CTA was significant for adult animals previously administered 0.4 mg/kg nicotine, and a trend toward statistical significance was observed in those previously receiving 0.8 mg/kg nicotine.
CTA of a nicotine-paired solution in periadolescent (P35) and adult (P67–70) male Wistar rats. Bars represent mean (±SEM) saccharin solution consumed (milliliters) during a preference test (saccharin and water available) after the conditioning trials, during which animals were administered nicotine (0, 0.2, 0.4, or 0.8 mg/kg, s.c.) after exposure to a saccharin solution. n=6–8 per dose at each age. The asterisk represents p<0.05 vs vehicle
Analysis of the test day and three subsequent extinction trials showed a significant Age × Nicotine Dose interaction [F(3,50)=2.95, p<0.05]. Simple effects analysis split by Age demonstrated that animals from both age groups increased their saccharin consumption over these four trials. Over these four trials, prior pairings of nicotine with saccharin did not alter periadolescent saccharin consumption at any dose (Fig. 4a), whereas adult rats that had received pairings of 0.4 or 0.8 mg/kg nicotine consumed less saccharin compared to control adult rats (main effect of Nicotine Dose, F(3,24)=3.95, p<0.05, post hoc p=0.018 and p=0.059 for the rats that received 0.4 and 0.8 mg/kg nicotine, respectively, vs. control rats; Fig. 4b).
Extinction of a CTA to a nicotine-paired saccharin solution in a periadolescent and b adult male Wistar rats. Data points represent mean (±SEM) saccharin solution consumed (milliliters) over four extinction trials. Animals received a free choice between saccharin and water during this phase. No nicotine was administered during the extinction phase. n=6–8 per dose at each age. The asterisks represent p<0.05 vs vehicle
Analysis of water consumption during the CTA test and three extinction trials yielded no significant effect of nicotine dose nor an interaction between age and nicotine dose (data not shown).
Discussion
This experiment examined the influence of age on the motivational effects of nicotine using two different paradigms. In the CPP experiment, periadolescents spent more time in an environment previously paired with a moderate dose of nicotine, while adults showed no preference at any dose. Both age groups showed a progressive increase in locomotor activity with repeated, intermittent administrations of nicotine.
Findings from the present place conditioning experiment are consistent with previous reports describing the age-dependent effects of nicotine on CPP (Vastola et al. 2002; Belluzzi et al. 2004; Torrella et al. 2004). Using an unbiased place conditioning procedure, Belluzzi et al. (2004) also observed that periadolescent, but not adult, rats express a significant preference for environmental stimuli associated with nicotine. This previous experiment showed a significant place preference in periadolescent rats following a single conditioning trial; we employed a more commonly used, four-trial conditioning procedure. Belluzzi and colleagues also used a wide range of doses and observed that the highest nicotine dose (0.5 mg/kg) produced a place preference, whereas in our study, only the 0.8-mg/kg nicotine dose induced a significant place preference in the periadolescent rats. Our study also extends these previous findings in demonstrating age-dependent effects of nicotine on place preference in a different strain of rat (Wistar).
We did not observe a significant effect of age on the acute locomotor response to nicotine, an observation in agreement with a number of recent reports (Levin et al. 2003; Belluzzi et al. 2004; Rezvani and Levin 2004). Other laboratories have, however, demonstrated an acute locomotor-enhancing effect of nicotine in periadolescent, compared to older, rats (Adriani et al. 2002; Schochet et al. 2004; Elliott et al. 2005). In agreement with previous work (Vastola et al. 2002; Belluzzi et al. 2004; Elliott et al. 2005), we observed that periadolescents and adults showed comparable increases in locomotor activity with repeated, intermittent administration of the two higher doses of nicotine tested. In contrast, others have reported sensitization to the locomotor effects of nicotine in adults, but not in periadolescents (Schochet et al. 2004; Cruz et al., 2005). The reasons why age effects on locomotor responses to nicotine are inconsistently observed are not known. The variability may be related to the doses used, the method or duration of locomotor measurement, and the rat strain.
Although our design did not include a formal test for sensitization (i.e., low-dose nicotine challenge), it may be argued that the progressive increase in locomotor activity we observed can be taken as evidence for sensitization, as others have demonstrated sensitization to nicotine in designs employing similar dosing regimens (e.g., Ksir et al. 1987; Kosowski and Liljequist 2005). If the increases in locomotor activity indicate a sensitized response, the present findings would allow the speculation that the processes mediating the sensitization of nicotine-induced locomotor activity and place preference are dissociable in adult rats, as they appear to be with cocaine and amphetamine (Tirelli et al. 2003a,b).
The results from the CTA experiment suggest that periadolescent rats are less sensitive to the aversive effects of nicotine compared to adults, as measured by the amount of saccharin consumed on the test day. Expression of the nicotine-induced CTA was age-dependent, a finding that supports the previous findings by Wilmouth and Spear (2004), who, using a different methodology, found that after several days of exposure to a choice between a flavored nicotine solution and water, adults reduced their intake significantly more than periadolescents.
CTA induced by drugs of abuse has been suggested to reflect their aversive properties (Wise et al. 1976; Carr and White 1986; Mayer and Parker 1993). On the other hand, Hunt and Amit (1987) suggest that a drug-induced CTA is functionally related to the rewarding effects of a drug, with potent reinforcers inducing both CPP and CTA. The reward contrast hypothesis, which was proposed by Grigson (1997), postulates that the palatable tastant presented prior to the administration of a drug comes to predict the availability of a more potent reinforcer. The reduction in tastant consumption thus occurs as a result of the anticipation of this greater reward. The current findings do not support these hypotheses. If periadolescents were more sensitive to the rewarding effects of nicotine, periadolescents would be expected to demonstrate a CTA, which they did not. In accordance with our observations that periadolescent rats are less sensitive to the aversive effects of nicotine as measured using CTA, periadolescent rats are also insensitive to the stereotyped syndrome associated with d-amphetamine administration, compared to adults (Adriani and Laviola 2000).
The parallel use of CPP and CTA may allow us to examine the relative contribution of the rewarding and aversive properties of a drug to its overall effect. As shown by the CTA results, the aversive effects of nicotine may be smaller in periadolescents, an effect that may also have contributed to the development of CPP in these animals. On the other hand, in adults, the aversive effects of nicotine, as indexed by the strong nicotine-induced CTA, may block the development of a CPP.
Pharmacokinetic differences in metabolism may play a role in the age differences in the effects of nicotine observed in the present study. In a recent study, Slotkin (2002) reported that adults had four times the blood levels of nicotine as periadolescents following chronic administration using osmotic minipumps over a 17.5-day period. This may suggest that periadolescents metabolize nicotine more quickly, although the rapid growth of the periadolescents would have also altered the dose of nicotine being delivered, which was based on body weight. While this disparity in metabolism may help to explain the absence of a nicotine-induced CTA in the periadolescents, it is inconsistent with our observation of a robust CPP to nicotine in periadolescents. The results of another study suggest that periadolescents and adults respond differently to drugs, and this is not related to pharmacokinetic differences. Campbell et al. (1988) reported differences in cataleptic behavior between periadolescent and adult rats following intracerebroventricular administration of neuroleptics, a route of administration that would be largely free of such pharmacokinetic influences.
It is also possible that the conditioning session duration in experiment 1 (CPP) was optimal for periadolescents, but not for adults, due to the potential age difference in nicotine metabolism. Varying the duration of the conditioning sessions may therefore alter the development of CPP in both periadolescent and adult rats. Arguing against this hypothesis, however, are the results of numerous nicotine CPP studies conducted in adult rats using a range of conditioning session durations, which failed to report significant CPP (see Le Foll and Goldberg 2005 for a summary). It is unlikely, therefore, that session duration played a significant role in the results of the current study.
The present findings support previous research suggesting that periadolescents are more sensitive to the rewarding effects of nicotine compared to adults (Adriani et al. 2002; Levin et al. 2003; Belluzzi et al. 2004; Leslie et al. 2004; Belluzzi et al. 2005), or at least, periadolescents do not find it as aversive as adults do. However, the mechanism underlying these differences remains to be elucidated. Two possible substrates for these age differences may be the mesocorticolimbic dopamine and the central cholinergic systems.
The mesocorticolimbic dopamine system, which is strongly implicated in reward, is still developing during the periadolescent period (Spear 2000). The D3 subtype of dopamine receptor is only at 40% of adult levels in the nucleus accumbens during the periadolescent period (Stanwood et al. 1997). This, taken together with the recent observation that D3 receptor antagonists block nicotine place preference in adults (Le Foll and Goldberg 2005), suggests that differences in these receptors may underlie the effects of age we and others observe on the rewarding effects of nicotine.
The central cholinergic system also continues to develop during adolescence, and nicotine infusions have been shown to upregulate nicotinic receptors in periadolescents, compared to adults (Slotkin 2002). Exposure to nicotine during this developmental stage, furthermore, can have long-term consequences on later drug-taking behavior, as indexed by the elevated nicotine self-administration of rats pre-exposed to nicotine during periadolescence, compared to rats pre-exposed to nicotine during adulthood (Adriani et al. 2003).
Taking these results together, it is tempting to speculate that the reward–aversion balance is shifted during adolescence such that nicotine’s aversive properties are weaker. This may help to explain the development of CPP and absence of a CTA to nicotine in this age group. Further studies are needed to pinpoint the neurotransmitters and mechanisms involved in these effects.
The results from the current study support previous reports suggesting that adolescence is a unique developmental stage during which nicotine exerts differential motivational effects compared to those observed in adulthood, and complement the finding that early tobacco smoking has long-term effects on future drug-taking behavior. If adolescents are indeed more sensitive to the rewarding effects, or less sensitive to the aversive effects, of nicotine, they may be more likely to maintain smoking behavior and become dependent despite the associated negative health consequences.
References
Adriani W, Laviola G (2000) A unique hormonal and behavioral hyporesponsivity to both forced novelty and d-amphetamine in periadolescent mice. Neuropharmacology 39:334–346
Adriani W, Macri S, Pacifici R, Laviola G (2002) Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology 27:212–224
Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV (2003) Evidence for enhanced vulnerability to nicotine during periadolescence in rats. J Neurosci 23:4712–4716
Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc, C, Carlezon, WA (2002) Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci 5:13–14
Belluzzi JD, Lee AG, Oliff HS, Leslie FM (2004) Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology 174:389–395
Belluzzi JD, Wang R, Leslie FM (2005) Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 30:705–712
Breslau N, Peterson EL (1996) Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. Am J Public Health 86:214–220
Brandon CL, Marinelli M, Baker LK, White FJ (2001) Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology 25:651–661
Calcagnetti DJ, Schechter MD (1994) Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry 18:925–933
Carr GD, White NM (1983) Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sci 33:2551–2557
Carr GD, White NM (1986) Anatomical disassociation of amphetamine’s rewarding and aversive effects: an intracranial microinjection study. Psychopharmacology 89:340–346
Campbell A, Baldessarini RJ, Teicher MH (1988) Decreasing sensitivity to neuroleptic agents in developing rats; evidence for a pharmacodynamic factor. Psychopharmacology 94:46–51
Chen J, Millar WJ (1998) Age of smoking initiation: implications for quitting. Health Rep 9:39–46
Chester JA, Cunningham CL (1999) GABA(A) receptors modulate ethanol-induced conditioned place preference and taste aversion in mice. Psychopharmacology 144:363–372
Clark DB, Kirisci L, Tarter RE (1998) Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend 49:115–121
Clarke PB, Fibiger HC (1987) Apparent absence of nicotine-induced conditioned place preference in rats. Psychopharmacology 92:84–88
Compechot C, Baulieu E, Robel P (1981) Testosterone, dihydrotestosterone and andresanediols in plasma testis and prostrates of rats during development. Acta Endocrinol (Copenh) 96:127–135
Cruz FC, DeLucia R, Planeta CS (2005) Differential behavioral and neuroendocrine effects of repeated nicotine in adolescent and adult rats. Pharmacol Biochem Behav 80:411–417
Douglas LA, Varlinskaya EI, Spear LP (2004) Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol 45:153–162
Eissenberg T, Balster RL (2000) Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend 59:S41–S60
Elliott BM, Faraday MM, Phillips JM, Grunberg NE (2005) Adolescent and adult female rats differ in sensitivity to nicotine’s activity effects. Pharmacol Biochem Behav 80:567–575
Etscorn F, Moore GA, Scott EP, Hagen LS, Caton TM, Sanders DL, Divine KK (1987) Conditioned saccharin aversions in rats as a result of cutaneous nicotine or intraperitoneal nicotine administered in divided doses. Pharmacol Biochem Behav 28:495–502
Fudala PJ, Teoh KW, Iwamoto ET (1985) Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav 22:237–241
Grant BF (1998) Age at smoking onset and its association with alcohol consumption and DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 10:59–73
Grigson PS (1997) Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci 111:129–136
Hanna EZ, Grant BF (1999) Parallels to early onset alcohol use in the relationship of early onset smoking with drug use and DSM-IV drug and depressive disorders: findings from the National Longitudinal Epidemiologic Survey. Alcohol Clin Exp Res 23:513–522
Higgins GA, Nguyen P, Sellers EM (1992) The NMDA antagonist dizocilpine (MK801) attenuates motivational as well as somatic aspects of naloxone precipitated opioid withdrawal. Life Sci 50:PL167–PL172
Hunt T, Amit Z (1987) Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci Biobehav Rev 11:107–130
Kelley BM, Middaugh LD (1999) Periadolescent nicotine exposure reduces cocaine reward in adult mice. J Addict Dis 18:27–39
Kosowski AR, Liljequist S (2005) Behavioural sensitization to nicotine precedes onset of nicotine-conditioned locomotor stimulation. Behav Brain Res 156:11–17
Ksir C, Hakan RL, Kellar KJ (1987) Chronic nicotine and locomotor activity: influences of exposure dose and test dose. Psychopharmacology 92:25–29
Kumar RJ, Pratt A, Stolerman IP (1983) Characteristics of conditioned taste aversion produced by nicotine in rats. Br J Pharmacol 79:245–253
Laviolette SR, van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55–65
Le Foll B, Goldberg SR (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology 178:481–492
Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzi JD (2004) Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci 1021:148–159
Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS (2003) Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology 169:141–149
Mayer LA, Parker LA (1993) Rewarding and aversive properties of IP and SC cocaine: assessment by place and taste conditioning. Psychopharmacology 112:189–194
McBride WJ, Murphy JM, Ikemoto S (1999) Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101:129–152
Mucha RF, Iversen SD (1984) Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology 82:241–247
Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P (1982) Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243:91–105
Parker LA (1995) Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev 19:143–157
Paredes RG, Alonso A (1997) Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav Neurosci 111:123–128
Pratt JA, Stolerman IP (1984) Pharmacologically specific pretreatment effects on apomorphine-mediated conditioned taste aversions in rats. Pharmacol Biochem Behav 20:507–511
Rezvani AH, Levin ED (2004) Adolescent and adult rats respond differently to nicotine and alcohol: motor activity and body temperature. Int J Dev Neurosci 22:349–354
Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2002a) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res 26:1632–1641
Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2002b) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res 26:1642–1652
Schochet TL, Kelley AE, Landry CF (2004) Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology 175:265–273
Sellings LHL, McQuade L, Clarke PBS (2005) Different nucleus accumbens subregions mediate the rewarding and aversive effects of nicotine. Poster presented at the 2nd Annual Invitational Symposium for Research to Inform Tobacco Control, Toronto, Ontario
Shoaib M, Stolerman IP (1995) Conditioned taste aversions in rats after intracerebral administration of nicotine. Behav Pharmacol 6:375–385
Shoaib M, Gommans Morley A, Stolerman IP, Grailhe R, Changeux JP (2002) The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology 42:530–539
Slotkin TA (2002) Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol 24:369–384
Smith AM, Kelly RB, Chen WJ (2002) Chronic continuous nicotine exposure during periadolescence does not increase ethanol intake during adulthood in rats. Alcohol Clin Exp Res 26:976–979
Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463
Spear LP, Brake SC (1983) Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol 16:83–109
Stanwood GD, McElligot S, Lu L, McGonigle P (1997) Ontogeny of dopamine D3 receptors in the nucleus accumbens of the rat. Neurosci Lett 223:13–16
Tirelli E, Laviola G, Adriani W (2003a) Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev 27:163–178
Tirelli E, Tambour S, Michel A (2003b) Sensitised locomotion does not predict conditioned locomotion in cocaine-treated mice: further evidence against the excitatory conditioning model of context-dependent sensitisation. Eur Neuropsychopharmacol 13:289–296
Torrella TA, Badanich KA, Philpot RM, Kirstein CL, Wecker L (2004) Developmental differences in nicotine place conditioning. Ann N Y Acad Sci 1021:399–403
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
van der Kooy D, O’Shaughnessy M, Mucha RF, Kalant H (1983) Motivational properties of ethanol in naive rats as studied by place conditioning. Pharmacol Biochem Behav 19:441–445
Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP (2002) Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77:107–114
Wilmouth CE, Spear LP (2004) Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann N Y Acad Sci 1021:462–464
Wise RA, Yokel RA, DeWit H (1976) Both positive reinforcement and conditioned aversion from amphetamine and from apomorphine in rats. Science 191:1273–1275
Acknowledgements
This work was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism to A.D. Lê. M.J. Shram was supported by a Natural Sciences and Engineering Research Council postgraduate scholarship and a Canadian Institute for Health Research Strategic Training Programme for Tobacco Use in Special Populations scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shram, M.J., Funk, D., Li, Z. et al. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology 186, 201–208 (2006). https://doi.org/10.1007/s00213-006-0373-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0373-8