Abstract
Studies with mice and rats have demonstrated that nicotine induces a Pavlovian conditioning denominated conditioned place preference (CPP). This behavioral paradigm is performed by exposing an animal to a drug in a particular environment. If the animal associates the drug (unconditioned stimulus) with the place where the drug is administrated (conditioned stimulus), a CPP is established. Similarly, zebrafish have also been used as a model system to identify factors influencing nicotine-associated reward. The protocol described here was designed to establish nicotine-CPP in zebrafish by using a biased approach. Moreover, pros and cons of using biased vs. unbiased design are also discussed. The protocol design is based in the establishment of nicotine/environment associations (nicotine-paired group). Since nicotine exerts anxiolytic effects, we used a counterbalanced nicotine-exposed control group, which did not show a significant place preference shift, providing evidence that the preference shift in the nicotine-paired group was not due to a reduction of aversion for the initially aversive compartment. Nicotine-induced place preference in zebrafish was corroborated by behavioral analysis of several indicators of drug preference, such as time spent in the drug-paired side, number of entries to the drug paired side, and distance traveled. This method provided further evidence that zebrafish actually develop a preference for nicotine, although the drug was administrated in an aversive place for the fish. This methodology offers an incremental value to the drug addiction field, because it describes behavioral features associated to nicotine-induced CPP in zebrafish. Therefore, this model is useful to screen for exogenous and endogenous molecules involved in nicotine-associated reward in vertebrates.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Tobacco is one of the most commonly used addictive substances, and nicotine is its principal psychoactive compound. Nicotine binds to nicotinic acetylcholine receptors (nAChR), ion channels that bind acetylcholine and can induce a cooperative effect with other neurotransmitter systems to modulate synaptic plasticity [1, 2]. As all addictive drugs, nicotine stimulates strongly the midbrain mesolimbic dopaminergic system, increasing excitability and synaptic strength in several brain areas such as the substantianigra-ventral tegmental area; dorsal and ventral striatum, amygdala, sensory cortex, and hippocampus [3–5]. The highly conserved nature of the rewarding pathway and the universal ability of drugs of abuse to stimulate the nervous system allow drug-associated reward to be modeled in nonmammalian species [6–8]. One of the major challenges in the drug addiction field is the identification of factors and structures involved in drug reward and relapse. Nevertheless, the behavioral screening of drug of abuse effects represents a real bottleneck in this field [9] and to find good animal behavior models for nervous system diseases is a present challenge. A vertebrate model for the rapid assessment of cognitive behaviors could be a good solution to find out the rewarding effects of nicotine. The zebrafish (Danio rerio) is a good model to evaluate behavior. The zebrafish brain is able to control a variety of complex behaviors such as learning, addiction, aggression, as well as social interactions. This species has been used as an animal model for identifying molecules involved in the rewarding effects of drugs [6, 10, 11]. Previous results demonstrated that the dopaminergic system in zebrafish participates in cocaine reward [6], suggesting that this pathway responds similarly in zebrafish and mammals.
There are two main behavioral paradigms to evaluate drug addiction, conditioned place preference (CPP) and self-administration (SA). The first evaluates the association between a drug and the environment where the drug is consumed [12]; the second examines the motivation of an animal to obtain the drug. To the present, no SA paradigm is developed for zebrafish. The CPP paradigm is a classical conditioning model that is widely used to investigate the mechanisms underlying context-dependent learning associated with drugs of abuse [13, 14]. The association between nicotine and environmental cues constitutes a form of conditioning which occurs in humans and other animals. On the other hand, zebrafish have shown Pavlovian conditioning in several tasks including CPP [15]. Zebrafish showed CPP responses to cocaine [6], amphetamine [11], opiates [16], ethanol [2], and nicotine [10, 17, 18]. Particularly, nicotine CPP in zebrafish can be established from 3 to 32 conditioning sessions [10, 17]. In case of determining CPP after conditioning with nicotine during few days or sessions, the rewarding properties of the drug are evaluated. Experimental designs based on long lasting conditioning sessions, i.e., exposure to nicotine in association with the environment for at least 4 weeks, are more related to long-term effects of the drug which is further associated with addiction [10]. Zebrafish showed a strong rewarding behavior to nicotine as it was demonstrated by a significant preference shift to an initially aversive compartment, which was associated with the drug [17]. Moreover, repetitive exposure of adult zebrafish to nicotine led to a robust CPP that persisted following 3 weeks of abstinence and in an environment with adverse stimuli, a behavioral indicator of the establishment of dependence [10].
An important factor to consider in CPP is the “biased” vs. “unbiased” apparatus design [12]. A biased apparatus is one in which animals show a significant preference for one compartment over the other prior to conditioning. In an unbiased apparatus, animals do not show a significant preference for one compartment over the other. Both can be used, although some researchers prefer one over the other. The drug and the question under assessment are fundamental factors for using biased or unbiased designs.
Here we discuss two types of conditioned place preference assays based on that previously described by Kily et al. in 2008 [10] and Kedikian et al. in 2013 [17]. The CPP assessment is accompanied by a detailed exploration of behavioral measurements [19], in experimental animals and their corresponding control groups, which are useful to study the rewarding properties of nicotine in adult zebrafish. Furthermore, postmortem brain tissue can be used to quantify several molecular markers to evaluate at the molecular level the effects of nicotine and nicotine-environment association reward in the brain.
2 Materials and Setup Conditions
2.1 Nicotine Concentration and Preparation
For the studies two types of nicotine salts are available: nicotine hydrogen tartrate and nicotine hemisulfate (Sigma-Aldrich, St. Louis, USA; Tocris Bioscience, Bristol, UK; Santa Cruz, CA, USA). Nicotine is prepared in clean tank water. Data obtained in our laboratory suggest that 15 μM of nicotine tartrate [17] and 5 μM of nicotine hemisulfate (20; data not published) are sufficient to induce CPP. Nicotine hemisulfate has not been used in CPP with rodents; however, it has been effectively used in zebrafish [20]. Nicotine hemisulfate is significantly less expensive than nicotine tartrate and it can be used at lower concentrations therefore is appropriate to be diluted in the relatively high volumes of water in experimental tanks.
Nicotine diluted in the CPP tank should be changed every 6 exposures (approximately twice a day). This should be done to clean water in the tank from fish excretions because the CPP tank is devoid of a filtration system. The half-life of diluted nicotine in water has been estimated to be approximately of 3 days [21]. It is important to remark that in ours as well as other laboratory protocols, nicotine is directly dissolved in the water tank (1.5 l) [2, 10, 11, 17, 19, 20], while other authors inject the fish intraperitoneally (i.p.) by using a Hamilton syringe [22]. This method is likely cheaper, due to the amount of drug that must be used (2 μl (0.001 mg/kg) against 1.5 l with 15 mg/l of nicotine). However, we consider that i.p. injections are not appropriate for nicotine CPP. Establishing nicotine CPP is very difficult therefore every stressful stimulus can induce changes that could set reproducibility at risk. Injections are stressful for rodents and we consider them to also be disturbing for fish. Moreover, chemical anesthesia or chilly water is also a stressful stimulus considering effects of anesthetics and that zebrafish are warm water fish.
2.2 Holding Tank and Experimental Tanks
Adult zebrafish (Danio rerio), approximately 6–9 months old, are kept a 100 per tank (filled with 90 l of carbon activated-filtered tap water) with a constant 14:10 h light–dark cycle at 26–28 °C, with aquatic plants and stone floor (enriched environment) filtered with an external canister filter (Eheim Eccopro 130, Germany) and fed twice a day with Artemia sp. and dry food. Carbon activated-filtered tap water is further filtered and aerated for at least 2 days with the external canister which contains organic as well as carbon activated filters, before placing zebrafish in the tank. All fish are acclimatized to the laboratory facility for at least 20 days in the tank and conditions described above. Afterwards, the animals are moved to the behavioral room and housed in floating acrylic chambers (12 cm height × 16 cm top × 14 cm bottom × 14 cm width) with two animals per chamber (recently we observed that it is possible to house four fish per tank). Ten floating chambers are placed in a 60 l tank. All experiments are conducted between 9:00 a.m. and 4:00 p.m.
Behavioral tanks were designed according to Ninkovic and Bally-Cuif [11] (biased) and to Kily et al. [10] (unbiased) with some modifications. The conditioning tank dimensions are 13 cm in length, 20 cm in width and 20 cm in depth. The CPP tank dimensions are 26.5 cm in length, 20 cm in width and 20 cm in depth. For the biased tank, distinct visual cues divide the experimental tank into two halves: one half is colored light-brown and the other half colored white with two black spots placed at the bottom of the tank (more recent experiments showed that six black spots work better) (see Figs. 1a and 2). Zebrafish prefer the light-brown compartment and avoid the white; therefore it is considered a biased tank. For the unbiased tank, the walls of one half are colored white with several black spots and the other half walls are colored white with black vertical striped lines [10]. The water level must be kept at 12–14 cm from the bottom of the tank to minimize stress. Fish are transported between tanks carefully using a net thus minimizing handling stress.
Diagram of CPP biased (white and light brown) tanks used during pretest, conditioning and test. (a) Pretest and CPP test tank, (b) conditioning tanks and (c) representative computer-generated behavioral traces produced by system water (left) or nicotine (right) diluted in system water in the nicotine CPP test session
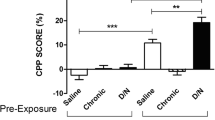
Conditioned place preference (CPP). (a) CPP can be established at different nicotine concentrations: 0 (control), 15, 30, and 50 mg/l. CPP score was calculated as % of time spent in the drug-paired side after drug exposure (test) minus % of time spent on the drug paired side before drug exposure (pretest) over a 300 s time period. (b) This graph shows 15 mg/l nicotine-CPP scores for nicotine-paired, nicotine-unpaired (counterbalanced control) and saline control groups at different time points following a 5 min interval of habituation
2.3 Behavioral Room
All conditioning and analysis are performed in a dedicated behavioral room with uniform lighting and neutral decoration. A camera connected to a computer is placed approximately 1.2 m above CPP tanks. The behavioral room contains: the home tank with ten floating chambers housing two or four zebrafish each and in the opposite corner of the room, the CPP and conditioning tanks .
2.4 Biased vs. Unbiased Procedure
Biased and unbiased protocols offer different alternatives. We chose a biased protocol with zebrafish considering that in previous studies, a biased tank was used to test the rewarding effects of stimulants such as amphetamine and cocaine [6, 11]. Moreover, in biased protocols, following the establishment of CPP, animals after conditioning spend a substantial amount of time in the initially non-preferred chamber (they stay even longer than in the naturally preferred side). This likely indicates the strength of the rewarding properties of a particular drug, since drug–environment associations force the animal’s permanence in an aversive environment. Finally, some authors have suggested that nicotine-CPP is more effectively induced by using a biased protocol in rodents [13, 23, 24].
On the other hand, however, unbiased protocols were used satisfactorily demonstrating nicotine CPP in adult zebrafish. The authors by using an unbiased design showed that CPP persisted following prolonged periods of abstinence (see [10]; Kedikian and Bernabeu’s unpublished data).
3 CPP Protocol
3.1 Basal Preference
At least 3 days before beginning the procedure, zebrafish must be moved to the floating chambers in the behavioral room to allow acclimation to the new conditions. After 3–5 days in the floating chambers, the experimenter ought to familiarize the fish to the environmental cues and conditioning procedure. This habituation step is important to ensuring an accurate determination of the baseline preference of each individual fish for the environmental cues used during conditioning.
3.2 Procedure
-
1.
Place the fish into the CPP tank.
-
2.
Allow the fish to settle for at least 5 min (the exact interval of time is not critical, but should be the same for all fish).
-
3.
After an initial 5 min habituation period in the CPP tank, allow the fish to freely explore the tank for 10 min more (15 min approximately in the CPP tank).
-
4.
Transfer the fish back to its floating chamber in the home tank.
-
5.
Repeat the above procedure during three consecutive days. However, more than 3 days of pre-exposure can induce latent inhibition (see below) [25]. In the last pre-exposure day the basal preference for each fish must be determined. Each fish is tested for baseline place preference by measuring the time spent in a given side of the tank over a 10 min period after 5 min habituation. The preferred compartment is defined as the compartment in which a fish spends most of the time during the pretest. In the case of a biased protocol, as the one described in this chapter, the preferred side corresponds to the brown half and in the unbiased device, the half of the tank where the fish spends most of the time.
3.3 Basal Preference Considerations
-
1.
Transfer the fish to be tested to the CPP tank and turn the camera on.
-
2.
Determine the time spent on a given side of the tank over a 10 min period after the 5 min habituation interval. Preference testing can be done manually by using a stopwatch or using motion detection software (Ethovision, Viewpoint, Panlab, Anymaze, or any other system of the kind). The software is easy to use and offer the possibility to measure some parameters which are not possible to analyze manually, such as distance traveled, velocity, and angles between head and tail.
-
3.
Take real care to stay far away from the tank and move softly while recording because the presence of the observer can influence the behavior of the fish. Randomize the orientation of the visual cues relative to the observer across the population being tested.
-
4.
The use of tracking software offers advantages over manual quantification, but we suggest using both procedures, because some behavioral parameters are difficult to assess with the software. Moreover, software offer the possibility to analyze several tanks at the same time and some specific parameters, such as mean velocity and distance traveled, can be determined with precision (see below). The use of the software removes also the possibility of the experimenter bias; and if extended time periods are used, once the program is set up, the observer can leave the room ensuring he/she will not influence fish behavior. Furthermore, by using the software it is possible to analyze the behavior of each fish minute by minute (see Fig. 3c) giving a more detailed analysis of the selected parameters.
Fig. 3 Total distance swum in the brown or the white compartment during conditioning. (a) Shows the total distance swum in the brown compartment on days 1, 2, and 3 of the conditioning session by each of the three groups of zebrafish (saline, nicotine-unpaired, nicotine-paired). (b) Displays the total distance swum in the white compartment on days 1, 2, and 3 during conditioning also depicting the three groups of zebrafish. (c) The total distance swum was measured and plotted minute-to-minute during the whole conditioning session (20 min) on day 1 in the white chamber as well as in the brown chamber (upper right inset in c). Throughout the 3 conditioning days, the distance swum changed in days 2 and 3 according with the habituation to the chamber and the effect of repetitive nicotine exposure (for further analysis see ref. [17])
-
5.
Determine the basal preference at most in three separate occasions. Two or three occasions guarantee the preference for one compartment, but sometimes one exposure is sufficient to determine the basal preference, principally when using biased tanks [17].
-
6.
In the case of unbiased protocols, any fish showing more than 75 % preference for one side should not be used further, because the tank for a fish with a side preference is biased. In the case of a biased protocol, preference for one side between 65 and 95 % are usual. Animals that show a preference inferior to 60 % for the brown side should be re-exposed to evaluate if this was due to stress or exposure to a novel environment effect. Nevertheless, if the low percentage preference persists, the animal should not be used for further analysis. The reason for this choice is because the preference value in such a case is closer to unbiased scores and therefore that particular fish perceives the tank as unbiased. Therefore, all fish used in a biased design should show a measurable preference (fish should spent 65–95 % of the pretest time in the preferred compartment) for one of the sides often the one considered the safe side .
4 Conditioning
4.1 Determining the Reinforcing Properties of Nicotine
-
1.
One day following the pretest, fish are randomly assigned to one of three treatment groups (at least 9 fish per group should be used for statistical accurateness).
-
2.
Transfer the fish, carefully with a transparent white net, from the floating (home) chamber to the conditioning tank.
-
3.
The conditioning is run for three consecutive days:
-
(a)
Experimental (CPP) group:
For the nicotine-paired group, transfer the fish first to the preferred side for 20 min (light-brown or the preferred side) and then transfer the fish to the non-preferred side (white or the least preferred) where the fish is exposed to a single dose of nicotine (15 mg/l) for 20 min [17]. Several nicotine concentrations should be tested by experimenters in cases that weak CPPs are obtained. We tested 15, 30, and 50 mg/l and all of these concentrations produced a high CPP score. We selected 15 mg/l because it seems always appropriate to use the lowest effective concentration to avoid possible side effects. We and other labs checked different exposure times to nicotine and 20 min worked well, so as in the previous case with nicotine doses, the lowest effective time with the drug was selected, not only to avoid possible side effects, but also, because behavior must be determined between 9 a.m. and 5 p.m. If zebrafish are exposed for longer periods, the number of animals that can be used per session in a day and by experiment ought to be reduced. Alternatively, a bigger room with more tanks would be necessary, which can unnecessarily complicate fish manipulation, recording and care.
-
(b)
Control groups in the conditioning phase :
-
Counterbalanced or nicotine-unpaired group: this control is very important when using the biased protocol. Animals in this group are first restricted for 20 min to either the white or the brown compartment. Then, fish are exposed for 20 min to a single dose of nicotine (15 mg/l) on the first and third day in the brown compartment and on the second day in the white chamber, thus the fish will not be able to associate a particular environment with nicotine availability. A freshly prepared nicotine solution (at a final concentration of 15 mg/l of clean tank water) was added to the tank daily at the beginning of each session. We did not measured nicotine concentration in the tank, but we diluted a concentrated stock that gave the indicated final concentration in a volume that oversized by many times the volume of the fish. We can safely assume that nicotine concentration was stable throughout conditioning sessions.
-
Saline group: zebrafish of the saline-treated control group are exposed during the three conditioning days to both sides alternately (20 min in each compartment) without nicotine.
-
-
(a)
-
4.
CPP test:
On the next day after the three conditioning days, CPP for each zebrafish is tested in a drug free environment like it was performed in the pretest (using the same tank that during pretest, for biased or unbiased procedures). Zebrafish are allowed to freely swim between compartments and after a 5 min habituation period, the percentage of time spent on each side of the tank is determined for 10 min (denominated the test session). During analysis of results, data from the 10 min period of the test session are compared with the same interval of the pretest session to evaluate changes in place preference between both sessions.
Changes in place preference are determined by using the following scores:
Score % = percentage of the time spent in the non-preferred side during test—percentage of the time spent in the non-preferred side during pretest.
Another score also used is:
Score (s) = time spent in the least preferred side during test (after conditioning)—time spent in the least preferred side during pretest (before conditioning).
Nicotine induced CPP is assessed on the nicotine-paired group as well as saline and counterbalanced nicotine control groups .
5 Behavioral Analysis
-
1.
At approximately 1.2–1.5 m above the CPP tanks a high resolution (HD) camera is connected to a computer by an USB port (LifeCam Microsoft or similar). It is important to use a HD camera to improve video quality for detailed analysis, and a USB port to connect the camera to any computer (CPU, laptop, notebook, ultrabook). During pretest, conditioning as well as CPP test, zebrafish behavior is recorded and videos are analyzed first by direct observation and then with any video tracking software available (as described above).
-
2.
It is important to set up a good contrast between the fish and the background of the tank in the video to ensure that the tracking software can follow fish movements.
-
3.
The analysis of videos should include the following measurements for behavior recordings:
-
(a)
Time spent in the drug-paired side: the amount of time zebrafish spend in the least preferred side. The camera is set in order to record both sides of the tank, therefore the same measurement in the preferred side may help to evaluate if the tracking is correct, because the sum of both periods needs to be equal to the total time of the recording .
-
(b)
Number and duration of motionless positions (stillness for 3 s or longer).
-
(c)
Total distance swum.
-
(d)
Average entry duration to the least preferred side (time spent in the white or least preferred side divided by the number of entries to the white or least preferred side).
-
(e)
Number of transitions to the drug-paired side (number of times the fish entered to the white or least preferred side).
-
(f)
Average velocity (distance swum in the brown compartment divided by the time spent in the brown side).
For further and detailed description of the behavior to be analyzed with the parameters described here please see the reviews [26, 27].
-
(a)
-
4.
During conditioning sessions, zebrafish behavior may also be recorded to analyze locomotor activity (LA) in both chambers in the presence or absence of nicotine or other drugs of interest, evaluating the effect of the drug during all conditioning phases .
6 Data Analysis and Results
In our experience, using the biased protocol, treatments with different doses of nicotine were assayed considering a range of concentrations based in previous results [10]. Therefore, fish exposure for 3 days to nicotine concentrations of 15, 30, or 50 mg/l for 20 min induced a significant increase in the time spent in the drug paired-side (which was initially the non-preferred side for the fish) and gave a change in preference of around 20 % for the nicotine paired-side (see Fig. 2).
It is noteworthy that these findings are not valid for other species, because doses two times higher than the one that induces CPP in rats provoke aversion (conditioning place aversion or CPA [14]). Therefore, by using nicotine CPP in zebrafish one can assume that a wider range of nicotine concentrations may be evaluated without aversive effects observed in rodents.
Once the concentration and time of exposure to the drug are determined, the characterization of several specific responses to identify preference-related behaviors to nicotine-conditioning in zebrafish helps to evaluate deeper the rewarding properties of nicotine or any drug. The first parameter to evaluate is the locomotor activity (distance swum) of the animal induced by nicotine. This parameter should be measured for each conditioning day in both compartments in all the experimental groups (Fig. 3a–c). Locomotor activity is recorded and determined by the tracking system and is usually expressed in cm. It is important to check that the fish swimming in the tank is at any time and place detected by the software, to be sure that its trajectory is tracked during the whole 20 min session. To corroborate this after tracking, the software produces information that indicates if at any time during recordings the software lost the objective (the fish swimming in the tank).
Once evaluated the effect of nicotine per se on locomotor activity during conditioning, it is advisable to evaluate the effect of nicotine on CPP by analyzing behavioral changes before (pretest) and after (test) conditioning (Fig. 4a–c). Parameters such as time spent in the least preferred side (Fig. 4a), number of transitions to that side (Fig. 4b) and average entry duration to the least preferred side (Fig. 4c) are appropriate to evaluate the power of the CPP protocol .
Baseline (pretest) and test values of behavioral parameters in the non-preferred compartment in nicotine-CPP. CPP was performed by using 15 mg/l of nicotine. Panel (a) shows the time spent in the white compartment, and (b) the number of transitions to the white compartment. (c) Shows the average entry duration to the white compartment. *p: 0.05 and **p: 0.01 between pretest and test and #: p < 0.05, ##: p < 0.01 and ###: p < 0.001 between controls (saline and Nic-unpaired) and Nic-paired. Control: saline; Nic-unpaired: counterbalanced nicotine treatment, and Nic-paired: nicotine treatment associated to the white compartment
7 Trouble Shooting
7.1 Determining Preference
-
1.
Basal preference could show high variance.
No more than 3 days of pretest sessions is suggested. More days of pretesting increase the probability of inducing latent inhibition, which will reduce the association between the drug and the environment.
The experimenter must not stay near the tank when preference measurement is in progress. The experimenter must keep a safe distance from the test tank or if possible leave the behavioral room to avoid any influence on fish behavior due to human presence. Avoiding any sharp noise and the implementation of a white noise in the behavioral room is advisable; fish have an excellent sense of hearing.
-
2.
To be able to establish nicotine CPP is necessary, like in rodents, to work with adolescent or young adult fish (6–9 months old).
-
3.
Fish freeze in the tank.
When the fish freezes in the bottom of the tank, it could be due to stress. Stress can be generated by transfer from the home tank, the new environment or any other unidentified stressful stimulus. In this case, the experimenter must give time for habituation and wait till the fish start moving. If the fish freezes for more than 2 min, the experimenter can move the fish to a new tank with fresh water for 10 min and then transfer it back to the CPP tank. If the stressful behavior continues, the fish should not be used further.
-
4.
Fish are hyperactive.
Hyperactivity could be a consequence of similar factors to the ones described in item 3. Under stress, some animals freeze whereas some animals swim faster. The procedure should be similar to the one described in the previous condition (item 3) to minimize either freezing or hyperactivity.
-
5.
Fish remain for a long period of time close to the side of the tank, touching the glass with their mouth. This behavior may be due to reflection of the fish or to any mark on the side of the tank. Adjust lighting intensity to minimize reflection or place visual cues inside the tank to prevent reflection (such as an opaque screen) .
7.2 Determining Conditioned Place Preference
-
1.
CPP could show high variance. This could be due to different reasons: Use fish from same age and weight, avoiding excessive variability.
-
2.
Increasing the number of conditioning sessions is convenient, since this can induce stronger associations between drug and environment (previous studies have used until 20 conditioning sessions (4 weeks) [10].
-
3.
Increasing the number of experimental animals also proves to be beneficial.
-
4.
Keeping the temperature of the CPP tank constant and similar to the home tank temperature is very important, because zebrafish are extremely sensitive to temperature changes.
8 Conclusion
We describe here conditioned place preference assays that can be used to evaluate the rewarding or reinforcing properties of nicotine in zebrafish, which are also suitable for performing CPP with other drugs of abuse or drugs with potential rewarding effects that could be administered in the tank water (specific setup conditions will probably be necessary for each drug to be tested).
Pharmacological studies in zebrafish offer the advantage, in contrast to mammals, that they can be performed without invasive stressful interventions, such as i.p. injections. Moreover, exposure and systemic levels of the drug can be continuous and stable. In fact, the concentration of a drug in fish tissues after a while (sec to min), for a rapidly diffusible substance (such as nicotine),can be considered equal to its concentration in the tank water. Experiments with other drugs with a rapid and evident locomotor activity effect, such as convulsive drugs or strong stimulants, showed that drug clearance in zebrafish is quick (around 1 min) when fish are moved to a tank with system water (unpublished data from our laboratory).
On the other hand, the animal can be exposed to the drug for several minutes to hours, helping to determine the pharmacokinetic values of the drug [28, 29]. In the protocols described here zebrafish were exposed for 20 min to nicotine, which could be considered acute. However, they could be exposed for longer times (hours, days, or weeks), i.e., more chronically to the drug. For chronic exposures, half of the volume in the tank is daily replaced with a freshly prepared nicotine solution. Chronic drug delivery in rodents is generally stressful and invasive because is performed throughout osmotic minipumps which requires surgery or, alternatively, it requires repetitive injections for several days. A treatment is considered to be chronic when animals receive a drug for a minimum of 10 days. However, determining chronicity of a treatment is specifically dependent on the drug tested.
The results and considerations showed and described here indicate that zebrafish is an excellent model for screening the rewarding properties of nicotine. We demonstrated that these animals showed a clear preference for the aversive environment associated with the drug, which was indicated and supported by several behavioral parameters. Furthermore, biochemical and molecular analysis of some markers associated with nicotine addiction in mammals showed that zebrafish can be used to determine the effects of nicotine on an addicted brain [17]. This protocol can be further used to screen exogenous and endogenous molecules involved in nicotine-associated reward in vertebrates.
References
Miwa JM, Freedman R, Lester HA (2011) Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron 70(1):20–33
Mathur P, Lau B, Guo S (2011) Conditioned place preference behavior in zebrafish. Nat Protoc 6(3):338–345
Changeux JP (2010) Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11(6):389–401
Gotti C, Zoli M, Clementi F (2006) Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27(9):482–491
Mansvelder HD, Keath JR, McGehee DS (2002) Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33(6):905–919
Darland T, Dowling JE (2001) Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci U S A 98(20):11691–11696
Feng Z et al (2006) A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell 127(3):621–633
Wolf FW, Heberlein U (2003) Invertebrate models of drug abuse. J Neurobiol 54(1):161–789
Robinson TE, Berridge KC (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363(1507):3137–3146
Kily LJ et al (2008) Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. J Exp Biol 211(Pt 10):1623–1634
Ninkovic J, Bally-Cuif L (2006) The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods 39(3):262–274
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12(3–4):227–462
Le Foll B, Goldberg SR (2005) Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology (Berl) 178(4):481–492
Pascual MM, Pastor V, Bernabeu RO (2009) Nicotine-conditioned place preference induced CREB phosphorylation and Fos expression in the adult rat brain. Psychopharmacology (Berl) 207(1):57–71
Collier AD et al (2014) Zebrafish and conditioned place preference: a translational model of drug reward. Prog Neuropsychopharmacol Biol Psychiatry 55:16–25
Bretaud S et al (2007) A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience 146(3):1109–1116
Kedikian X, Faillace MP, Bernabeu R (2013) Behavioral and molecular analysis of nicotine-conditioned place preference in zebrafish. PLoS One 8(7):e69453
Klee EW et al (2011) Zebrafish for the study of the biological effects of nicotine. Nicotine Tob Res 13(5):301–312
Cachat J et al (2010) Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat Protoc 5(11):1786–1799
Parker MO et al (2013) Behavioural phenotyping of casper mutant and 1-pheny-2-thiourea treated adult zebrafish. Zebrafish 10(4):466–471
Seckar JA et al (2008) Environmental fate and effects of nicotine released during cigarette production. Environ Toxicol Chem 27(7):1505–1514
Ponzoni L et al (2014) The cytisine derivatives, CC4 and CC26, reduce nicotine-induced conditioned place preference in zebrafish by acting on heteromeric neuronal nicotinic acetylcholine receptors. Psychopharmacology (Berl) 231(24):4681–4693
Brielmaier JM, McDonald CG, Smith RF (2008) Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav 89(1):94–100
Calcagnetti DJ, Schechter MD (1994) Nicotine place preference using the biased method of conditioning. Prog Neuropsychopharmacol Biol Psychiatry 18(5):925–933
Weiss KR, Brown BL (1974) Latent inhibition: a review and a new hypothesis. Acta Neurobiol Exp (Wars) 34(2):301–316
Kalueff AV et al (2013) Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10(1):70–86
Stewart AM et al (2014) Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 37(5):264–278
Brennan CH (2011) Zebrafish behavioural assays of translational relevance for the study of psychiatric disease. Rev Neurosci 22(1):37–4829
Guo S (2004) Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav 3(2):63–74
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Faillace, M.P., Bernabeu, R.O. (2016). Conditioned Place Preference and Behavioral Analysis to Evaluate Nicotine Reinforcement Properties in Zebrafish. In: Li, M. (eds) Nicotinic Acetylcholine Receptor Technologies. Neuromethods, vol 117. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3768-4_3
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3768-4_3
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3766-0
Online ISBN: 978-1-4939-3768-4
eBook Packages: Springer Protocols