Abstract
Rationale
In humans, the retrieval of memories associated with an alcohol-related experience frequently evokes alcohol-seeking behaviour. The reconsolidation hypothesis states that a consolidated memory could again become labile and susceptible to disruption after memory retrieval.
Objectives
The aim of our study was to examine whether retrieval of alcohol-related memories undergoes a reconsolidation process.
Methods
For this purpose, male Wistar rats were trained to self-administer ethanol in the presence of specific conditioned stimuli. Thereafter, animals were left undisturbed in their home cages for the following 21 days. Memory retrieval was performed in a single 5-min exposure to all alcohol-associated stimuli. The protein synthesis inhibitor anisomycin, the non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist MK-801 and acamprosate, a clinically used drug known to reduce a hyper-glutamatergic state, were given immediately after retrieval of alcohol-related memories. The impact of drug treatment on cue-induced alcohol-seeking behaviour was measured on the following day and 7 days later.
Results
Administration of both anisomycin and MK-801 reduced cue-induced alcohol-seeking behaviour, showing that memory reconsolidation was disrupted by these compounds. However, acamprosate had no effect on the reconsolidation process, suggesting that this process is not dependent on a hyper-glutamatergic state but is more related to protein synthesis and NMDA receptor activity.
Conclusions
Pharmacological disruption of reconsolidation of alcohol-associated memories can be achieved by the use of NMDA antagonists and protein synthesis inhibitors and may thus provide a potential new therapeutic strategy for the prevention of relapse in alcohol addiction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Relapses contribute considerably to the maintenance of addiction and are a major challenge in the treatment of addictive diseases (Cami and Farre 2003). One fundamental problem in the treatment of alcoholism and drug addiction is the ability of drug-associated environmental cues to evoke drug-seeking behaviour leading to relapse even after years of abstinence (O'Brien et al. 1992). Consistent with the long-lasting risk of relapse, several recent studies indicate that long-term memory formation and development of drug addiction are sharing common neural circuitries and molecular mechanisms (Kelley 2004; Hyman et al. 2006). Thus, understanding learning and memory processes in the addicted brain is an important key for understanding the persistence of addiction, and it is reasonable to hypothesise that selective disruption of drug-related memories might help to prevent relapses.
Several earlier studies have shown that newly acquired memories are initially labile but then are stabilised through a process called memory consolidation (McGaugh 2000; Dudai 2004). Within the first minutes to hours, this process is susceptible to interference. Following this stabilisation period, the consolidation theory proposes that memories, once stored, are resistant to interference (McGaugh 2000). In contrast to this idea, Misanin et al. (1968) proposed that reactivation of a consolidated memory trace returns it to an unstable state again. More than 30 years later, Nader et al. (2000) confirmed this assumption in a fear-conditioning paradigm with targeted infusions of the protein synthesis inhibitor anisomycin into the lateral and basal nuclei of the amygdala—sites known to play an important role in fear learning—after retrieving previously conditioned fear memories. The study demonstrated that infusion of anisomycin shortly after memory reactivation produced amnesia on later tests, whilst anisomycin application in the absence of re-exposure to the conditioned cue left the memory intact. Thus, reactivation of a consolidated memory can return to a labile state in which the memory trace has to undergo reconsolidation for which, like consolidation, new protein synthesis is required. Subsequently, further studies have provided further evidence for the reconsolidation theory and its dependence on renewed protein synthesis (Debiec et al. 2002; Duvarci and Nader 2004), whilst some others did not find disruption of reconsolidation by protein synthesis inhibition (Lattal and Abel 2004; Power et al. 2006). Antagonism of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor has been shown to be effective in disruption of memory reconsolidation as well (Przybyslawski and Sara 1997; Torras-Garcia et al. 2005; Tronson and Taylor 2007; Lee and Everitt 2008), likely because of the crucial role of these receptors in learning and memory (Riedel et al. 2003).
Whilst the majority of research on memory reconsolidation has used conditioned aversion paradigms, Lee et al. (2005) and Miller and Marshall (2005) were the first to demonstrate that retrieved appetitive drug-related memories also undergo reconsolidation. Those results were followed by several other studies demonstrating disruption of the reconsolidation of cocaine-, heroine- and morphine-related memories (Hellemans et al. 2006; Lee et al. 2006a; Milekic et al. 2006; Valjent et al. 2006; Bernardi et al. 2007; Robinson and Franklin 2007). However, even though alcohol addiction is one of the major neuropsychiatric diseases with a profound public health impact (Spanagel 2009), disruption of alcohol-associated memories was never tested in animals trained to self-administer alcohol.
Several studies have shown that in experimental animals, a conditioned stimuli (CS) paired repeatedly with the self-administration of alcohol is able to reinstate alcohol-seeking behaviour after an alcohol-free period (Sanchis-Segura and Spanagel 2006). Here, we investigated whether the behavioural impact of conditioned alcohol cues could be reduced by blocking the reconsolidation of these alcohol associations after the memory retrieval procedure. For this purpose, we employed a behavioural model of cue-induced alcohol seeking in Wistar rats (Sanchis-Segura and Spanagel 2006). The protein synthesis inhibitor anisomycin and the non-competitive NMDA receptor antagonist MK-801 were given after retrieval of alcohol-related memories to test whether these memories undergo a protein synthesis- and NMDA receptor-dependent reconsolidation. Additionally, we used acamprosate as an abstinence-promoting drug that is widely used in the treatment of alcohol addiction. Although the primary site of action is still not known, it has been demonstrated that acamprosate dampens a hyper-glutamatergic state in the alcohol-dependent brain and thereby reduces the risk of relapse (Spanagel et al. 2005; Spanagel and Kiefer 2008). Due to this interference with the glutamatergic system, we hypothesised that acamprosate may also have an impact on the memory reconsolidation processes.
Materials and methods
Subjects
One hundred and thirty-two 2-month-old male Wistar rats (Charles River, Sulzfeld, Germany) were used. All animals were housed individually in standard rat cages (Ehret, Emmendingen, Germany) under a 12-h artificial light–dark cycle (lights on at 5:00 p.m.). Room temperature was kept constant (temperature 22 ± 1°C, humidity 55 ± 5%). Standard laboratory rat food (Ssniff, Soest, Germany) and tap water were provided ad libitum throughout the experimental period (unless stated otherwise). All experimental procedures were approved by the Committee on Animal Care and Use (Regierungspräsidium Karlsruhe) and carried out in accordance with the local Animal Welfare Act and the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drugs
Ethanol drinking solutions were prepared from 96% ethanol (Sigma-Aldrich, Taufkirchen, Germany) and then diluted with tap water. Acamprosate (Lipha, France) and MK-801 (Sigma-Aldrich, Taufkirchen, Germany) and chloral hydrate (Sigma-Aldrich, Taufkirchen, Germany) were dissolved in isotonic saline. All solutions were freshly prepared and injected as a volume of 2 ml/kg intraperitoneally. Anisomycin (Sigma-Aldrich, Taufkirchen, Germany) was dissolved in equimolar HCl, diluted with isotonic saline and adjusted to pH 7 with NaOH to a final concentration of anisomycin 100 μg/μl. The solution was freshly prepared and infused as a volume of 4 μl per animal intracerebroventricularly (ICV). Control experiments were performed following administration of the respective vehicle.
Surgery and intracerebroventricular infusions
For the cannula implantation, animals were anaesthetised using chloral hydrate (360 mg/kg) and mounted in a Stoelting® stereotaxic instrument (Stoelting Co., Wood Dale, IL, USA). Rats were implanted unilaterally with 26-gauge stainless steel guide cannulas (Plastics One Inc., Roanoke, USA) aiming at the left or right ventricle (coordinates were from bregma AP −0.9 mm, ML ±1.4 mm and from dura DV −3.2 mm). Guide cannulas were fixed to the brain using three anchor screws and dental cement and filled with stylets to maintain patency. After surgery, rats were placed back in their home cages and were allowed to recover from surgery for 10 days before submitting them to behavioural studies (see below).
For drug delivery, a 33-gauge infusion cannula (Plastics One Inc.) was tightly fitted into the guide cannula. ICV infusion of anisomycin was made at a 2-µl/min flow rate using a PHD2000 microinfusion pump (Harvard Apparatus, USA). Infusions were carried out over 2 min, and the infusion cannulas were left in place for an additional 15 s to minimise backflow.
Cannula placement was verified post-mortem immediately after the last behavioural test. Thus, at the end of the experiment, rats were sacrificed, brains were frozen in isopentane solution and probe placements were verified histologically on microsections of 50 µm. Only data from animals with correct cannula implants were used.
Behavioural studies
Operant ethanol self-administration apparatus
All ethanol-seeking experiments were carried out in operant chambers (MED Associates Inc., St. Albans, VT, USA) enclosed in ventilated sound-attenuating cubicles. The chambers were equipped with a response lever on each side panel of the chamber. A loudspeaker (65 dB, ‘beep’) was positioned above the left lever of the self-administration chamber. Responses at the left (active) lever activated a syringe pump that delivered a ~30-µl drop of fluid into a liquid receptacle next to it. Responses on the right (inactive) lever were recorded but did not result in activation of the pump. The delivery of ethanol during the conditioning phase (see below) was accompanied by a 5-s auditory stimulus, which served as a ‘time-out’, during which responses were recorded but not reinforced. An IBM compatible computer controlled the delivery of fluids, presentation of stimuli and data recording.
Ethanol self-administration training
All animal training and testing sessions were performed during the dark phase of their light cycle. Animals were trained to self-administer 10% (v/v) ethanol in daily 30-min sessions under a fixed ratio 1 schedule using Samson's sucrose-fading procedure (Samson 1986). During the first 3 days of training, animals were deprived of fluid for 20 h per day. Responses at the left lever were reinforced by the delivery of 0.2% (w/v) saccharin solution. For the next 3 days, animals underwent the same procedure without fluid deprivation. Following the acquisition of saccharin-reinforced responding, rats were trained to self-administer ethanol. Thus, rats had access to 0.2% saccharin with 5% ethanol for 1 day, 5% ethanol for 1 day, 0.2% saccharin with 8% ethanol for 1 day, 8% ethanol for 1 day, 0.2% saccharin with 10% ethanol for 1 day and 10% ethanol for 1 day.
Conditioning to ethanol discriminative stimuli
The purpose of the conditioning phase was to train the animals to associate the availability of ethanol with the presence of specific discriminative stimuli. This phase started after the completion of the saccharin-fading procedure. Discriminative stimuli predicting ethanol (10%) availability were presented during each subsequent daily 30-min session. An orange flavour extract served as the cue stimulus (S) for ethanol. This olfactory stimulus was generated by depositing six drops of an orange extract into the bedding of the operant chamber before each session. In addition, each lever press resulting in ethanol delivery was accompanied by a 5-s conditioned auditory stimulus (CS). At the end of each session, the bedding of the chamber was changed and trays were thoroughly cleaned. The animals received a total of ten ethanol conditioning sessions.
Memory reactivation and pharmacological studies
After completing the conditioning phase, animals were left undisturbed in their home cages for the following 3 weeks (as described above, in case of anisomycin study, cannulas were implanted in left or right ventricle 10 days before the start of the memory retrieval session). During the single memory retrieval session, rats were exposed to the same conditions as during the conditioning phase (i.e. presented with S/CS), except that the ethanol was not made available. Additionally, the first two presses on the formerly active lever were reinforced by 30 µl of ethanol, it served as an additional olfactory/gustatory ethanol cue (Vengeliene et al. 2007). The duration of a memory retrieval session was assessed in a pilot study in which two animal groups were subjected to either a 5- or 10-min memory reactivation session and their behaviour during subsequent cue-induced ethanol-seeking testing (see below) was compared with the behaviour of animals that had not received memory reactivation procedure (‘no-retrieval’ control group). Memory retrieval duration of 5 min was chosen for the following experiments (see “Results”, Fig. 1).
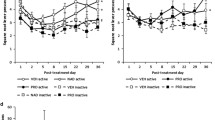
Effect of memory retrieval of 0 min (i.e. no-retrieval), 5 min, and 10 min duration (n = 8 per group) on the cue-induced ethanol-seeking behaviour testing 24 h later (test 1). Data are shown as number of lever presses on ethanol-associated (active) and inactive levers after the presentation of a stimulus previously paired with ethanol in combination with two drops (60 µl) of ethanol as an additional olfactory/gustatory cue. Data are presented as means ± SEM. Asterisk indicates significant differences from the ‘no-retrieval’ control group lever responses, p < 0.05
For the drug testing, animals were grouped into 12 groups according to their behaviour during the conditioning phase and retrieval session (n = 8–11 per treatment group). The first six animal groups received either vehicle vs. 200 mg/kg of acamprosate, or vehicle vs. 0.1 mg/kg of MK-801, or vehicle vs. 400 μg of anisomycin (note: the standard doses of these compounds are well established, as all of them are commonly used in behavioural pharmacological studies, see e.g. Biała and Kotlińska 1999; Bachteler et al. 2005; Füllgrabe et al. 2007; Spanagel et al. 1996; Robinson and Franklin 2007; Sadler et al. 2007). All drugs were administered immediately after the memory reactivation session. To ensure that drug treatment did not directly influence animal performance during cue-induced ethanol-seeking testing, which was performed 24 h later (see below), six additional groups of rats received the same treatment in their home cages (i.e. vehicle vs. drug) without a memory reactivation session (‘no-retrieval’ control groups).
Cue-induced ethanol-seeking testing
A single cue-induced ethanol-seeking testing of 30 min duration was performed 24 h after the memory reactivation session (test 1) in order to see whether previous drug treatment disrupted memory reconsolidation. In this test, rats were exposed to the same conditions as during the memory reactivation session. Thus, animals were presented with ethanol discriminative stimuli (S/CS) combined with the two drops of ethanol as an additional olfactory/gustatory ethanol cue. Responses at both the active and inactive levers were recorded.
Seven days later, animals underwent a second session of cue-induced ethanol-seeking testing of 30 min duration (test 2).
Locomotor activity measurements using the E-motion system
In order to test for any sedative effects resulting from the drug treatment, locomotor activity during test 1 was monitored by the use of an infrared sensor connected to a recording and data storing system (Mouse-E-Motion by Infra-e-motion, Henstedt-Ulzburg, Germany). A Mouse-E-Motion device was placed above the operant ethanol self-administration chamber so that the rat could be detected at any position inside the chamber. The device sampled whether the rat was moving or not every second. The sensor could detect body movement of the rat of at least 1.5 cm from one sample point to the successive one. The data measured by each Mouse-E-Motion device were downloaded onto a personal computer and processed with Microsoft Excel.
Statistics
Data obtained from ethanol conditioning sessions were analysed by the use of a one-way analysis of variance (ANOVA) for repeated measures [factor: treatment group (vehicle vs. drug)], data from memory reactivation sessions were analysed by the use of a two-way ANOVA [factors were: treatment group (vehicle vs. drug), lever (active vs. inactive)] and data from ethanol-seeking experiments were analysed by the use of a three-way ANOVA for repeated measures [(factors were: treatment group (vehicle vs. drug), lever (active vs. inactive) and test (test 1 vs. test 2)]. Whenever significant differences were found, post hoc Student–Newman–Keuls tests were performed. Data derived from locomotor activity measurements were analysed using an unpaired t test (factor: treatment group). The chosen level of significance was p < 0.05.
Results
At the end of the conditioning phase, rats exhibited 155 ± 14 lever presses reinforced by delivery of 30 μl of 10% ethanol (that corresponds to ~4.65 ml/session of 10% ethanol intake or ~0.8 g/kg of body weight/session of pure ethanol intake).
Memory reactivation and cue-induced ethanol-seeking (the pilot study)
Following the 3-week home cage period, animals re-exposed to ethanol-associated cues in the operant conditioning chambers (‘no-retrieval’ group) exhibited 39 ± 6 responses on the previously reinforced lever in a 30-min test session (test 1). The second animal group that received one memory retrieval session of 5 min 24 h before test 1 session exhibited a significantly higher number of lever presses during this test, demonstrating that the retrieval of the memory increased ethanol seeking in these animals [factor group: F(2, 42) = 3.7, p < 0.05] (Fig. 1). However, in the third animal group subjected to 10-min-long memory retrieval, a slight extinction of lever-pressing behaviour was already seen during test 1 compared to the ‘no-retrieval’ group (Fig. 1). No differences between groups were observed 1 week later during test 2 (p = 0.49; data not shown). Due to these findings, 5 min was chosen as the length of memory retrieval sessions for the following pharmacological studies.
Pharmacological studies
Animals were assigned into different treatment groups on the basis of their performance during the last four conditioning sessions and during the 5-min memory retrieval session. Table 1 demonstrates that no significant differences in lever responses between control and drug-treated animal groups were seen during either the last conditioning sessions or during memory retrieval session (Table 1).
With respect to the pharmacological treatment, a three-way ANOVA for repeated measures revealed that 200 mg/kg of acamprosate administered directly after the memory retrieval session did not affect memory reconsolidation significantly when compared to the vehicle-treated animal group (p = 0.59). Thus, acamprosate-treated animals exhibited the same level of lever responses as seen in the vehicle control animal group during either test 1 or test 2 performed 1 week later (Fig. 2a). However, administration of 0.1 mg/kg of the NMDA receptor antagonist MK-801 reduced responding on the active lever during cue-induced ethanol-seeking testing as compared to vehicle-treated animals [factor group: F(1, 32) = 5.1, p < 0.05]. The following post hoc analysis showed that this reduction was significant only during test 1. During test 2, MK-801-treated animals exhibited a reduced, however, non-significant (p = 0.17) responding on the active lever, showing an effect of this NMDA receptor antagonist on memory reconsolidation after a single memory retrieval session to ethanol-associated cues (Fig. 3a). Similarly, ICV administration of 400 μg of the protein synthesis blocker anisomycin caused a significant reduction of responses on the active lever during both test 1 and test 2 [factor group: F(1, 36) = 4.1, p < 0.05], again showing the long-lasting effect of the treatment on the cue-induced alcohol seeking (Fig. 4a).
Effect of either vehicle (n = 8–10) or 200 mg/kg of acamprosate (n = 8–10) on memory reconsolidation of ethanol-seeking behaviour (a); ‘no-retrieval’ control groups received the same procedures except a memory reactivation session (b). Test 1 was performed 24 h after a 5-min memory retrieval session and test 2 was performed 7 days after the memory retrieval session. The administration of all drugs was performed immediately after the retrieval session. Data are shown as the number of lever presses on ethanol-associated (active) and inactive levers after the presentation of a stimuli previously paired with ethanol in combination with two drops (60 µl) of ethanol as an additional olfactory/gustatory cue. Data are presented as means ± SEM
Effect of either vehicle (n = 8–10) or 0.1 mg/kg of MK-801 (n = 8–10) on memory reconsolidation of ethanol-seeking behaviour (a); ‘no-retrieval’ control groups received the same procedures except a memory reactivation session (b). Test 1 was performed 24 h after a 5-min memory retrieval session and test 2 was performed 7 days after the memory retrieval session. The administration of all drugs was performed immediately after the retrieval session. Data are shown as the number of lever presses on ethanol-associated (active) and inactive levers after the presentation of a stimuli previously paired with ethanol in combination with two drops (60 µl) of ethanol as an additional olfactory/gustatory cue. Data are presented as means ± SEM. Asterisk indicates significant differences from the vehicle control group, p < 0.05
Effect of either vehicle (n = 8–11) or 400 μg of protein synthesis inhibitor anisomycin (n = 8–9) on memory reconsolidation of ethanol-seeking behaviour (a); ‘no-retrieval’ control groups received the same procedures except a memory reactivation session (b). Test 1 was performed 24 h after a 5-min memory retrieval session and test 2 was performed 7 days after the memory retrieval session. The administration of all drugs was performed immediately after the retrieval session. Data are shown as the number of lever presses on ethanol-associated (active) and inactive levers after the presentation of a stimuli previously paired with ethanol in combination with two drops (60 µl) of ethanol as an additional olfactory/gustatory cue. Data are presented as means ± SEM. Asterisk indicates significant differences from the vehicle control group, p < 0.05
None of the treatments were found to have an effect on locomotor activity measured during test 1 by the use of infrared sensor Mouse-E-Motion (Table 2). In addition, there were no differences seen on responding on the inactive lever (Figs. 2, 3, 4), showing the absence of sedation caused by treatment.
In non-retrieved control animal groups, administration of either acamprosate (p = 0.37), or MK-801 (p = 0.66), or anisomycin (p = 0.65) had no effect on either the first or the second testing (Figs. 2b, 3b and 4b).
Discussion
Previous studies have shown that retrieved appetitive, cocaine- or opiate-related memories undergo reconsolidation which can be disrupted by either protein synthesis inhibition or by NMDA receptor blockade (Milekic et al. 2006; Bernardi et al. 2007; Kelley et al. 2007; Robinson and Franklin 2007; Sadler et al. 2007; Milton et al. 2008). Here, we provide evidence that ethanol-associated memories can also become unstable and liable to disruption after their reactivation. Both the protein synthesis blocker anisomycin as well as the NMDA receptor antagonist MK-801 given immediately after re-exposure of animals to alcohol-paired conditioned stimuli impaired the ability of these stimuli to induce alcohol-seeking behaviour in subsequent test sessions. Our findings demonstrate that the administration of anisomycin and MK-801 specifically disrupted reconsolidation, as the administration of these agents without the reactivation of alcohol-related memory had no effect on the responsiveness of the animals to the alcohol-paired CS during alcohol-seeking tests. To our knowledge, this is the first study investigating the reconsolidation of appetitive alcohol-related memories in animals.
In the present experiments, we used cue-induced alcohol-seeking behaviour (Sanchis-Segura and Spanagel 2006) in order to study reconsolidation processes. During a single memory retrieval session, rats were exposed to the same cues, as during the conditioning phase, except that ethanol was not made available. One may argue that non-reinforced retrieval trials are extinction trials. A critical parameter that determines whether amnesic-like treatment will block reconsolidation or extinction seems to be the length of the re-exposure sessions (Pedreira and Maldonado 2003; Lee et al. 2006b; Power et al. 2006; Bernardi et al. 2007). Therefore, we conducted a pilot study in which two animal groups were subjected to either 5 or 10 min of memory reactivation, and 24 h later, their behaviour was compared with that of animals that had not received memory reactivation. We found that, compared to the non-retrieved group, 10-min retrieval led to the slight extinction of ethanol-seeking behaviour. The second animal group that received one 5-min memory retrieval session exhibited a significantly higher number of active lever presses 24 h later, demonstrating that the retrieval of the memory increased ethanol seeking in these animals. Due to these findings, 5 min was chosen as the duration for the alcohol-related memory reactivation session. These findings have two implications. First, retrieval is effective and relevant for behaviour as it increases ethanol-seeking behaviour after a short memory reactivation. Second, whether retrieval is causing extinction or not is strongly dependent on the duration of the retrieval session. This is in line with other studies suggesting that reconsolidation processes are dominant with cue exposure of shorter duration, whereas extinction processes are dominant following cue exposure of longer duration (Pedreira and Maldonado 2003; Lee et al. 2006b; Bernardi et al. 2007). Thus, Bernardi et al. (2007) showed that systemic administration of anisomycin following a 5-min re-exposure to a previously cocaine-conditioned context disrupted reconsolidation, whereas this effect was not seen when anisomycin was administered following a 30-min re-exposure session. Lee et al. (2006b) investigated the effects D-cycloserine and MK-801 on reconsolidation and extinction of a conditioned fear memory. They found that memory reconsolidation can be both disrupted and enhanced, and extinction can be both potentiated and impaired, depending on the duration and number of CS re-exposures presented during the memory reactivation/extinction session.
We applied the broad protein synthesis blocker anisomycin and found that ethanol-memory reconsolidation was disrupted by ICV administration of this compound immediately after memory reactivation. Robinson and Franklin (2007) found that reconsolidation of a previously morphine-induced conditioned place preference (Tzschentke 2007) can only be blocked by the post-reactivation ICV infusions of anisomycin when selectively paired with morphine re-exposure, whereas ICV infusions of anisomycin without morphine application failed to block reconsolidation. On the contrary, we found that re-exposure to the ethanol-associated cues alone rendered the memory susceptible to disruption of anisomycin and led thereby to a significant reduction of cue-induced ethanol-seeking behaviour.
Furthermore, we demonstrate that disruption of ethanol-related memory reconsolidation can be achieved by NMDA receptor blockade. Systemic administration of MK-801 significantly reduced alcohol-seeking behaviour 24 h later as compared to vehicle-treated animals after. The impairment of alcohol-related memory reconsolidation demonstrated in the present study is consistent with the findings showing that administration of MK-801 disrupts the reconsolidation of cocaine-conditioned contexts. Thus, MK-801 was shown to impair the reconsolidation of a cocaine-conditioned place preference (Kelley et al. 2007). Similarly, disruption of drug memory reconsolidation with NMDA receptor antagonists was demonstrated using an animal model of cue-induced drug-seeking (Milton et al. 2008) and sucrose-seeking behaviour (Lee and Everitt 2008). Taken together, these results demonstrate that NMDA receptor-mediated signalling is required for appetitive memory reconsolidation. In contrast, systemic administration of acamprosate following a memory retrieval session did not reduce ethanol-seeking behaviour, suggesting the specific involvement of NMDA receptor in memory reconsolidation processes. Moreover, the lack of effect of acamprosate on the reconsolidation process suggests that this process is not due to enhanced glutamatergic activity.
The amnesic-like effect of MK-801 was somewhat decreased in the second test performed 7 days later. However, ethanol-seeking behaviour was still lower when compared to the vehicle group. In the anisomycin group, the significant effect of treatment was seen during both the first and the second tests, demonstrating a long-lasting effect of memory reconsolidation on alcohol-seeking behaviour. Research on the duration of effect on the memory reconsolidation disruption yielded conflicting results. Thus, several reports indicate that anisomycin permanently disrupts reactivated memories (Duvarci and Nader 2004). Other studies indicate that anisomycin can produce amnesic-like effect for a reactivated memory by only temporarily preventing access to a reactivated memory (Lattal and Abel 2004; Power et al. 2006). One study by Lattal and Abel (2004) showed that behavioural impairments in mice caused by anisomycin administration immediately following contextual fear conditioning were present for 21 days, whereas similar effects of anisomycin administration following context re-exposure lasted for only 24 h. It is not yet clear whether these discrepant results reflect procedural differences between the studies or different cellular and molecular mechanisms involved in memory retrieval. Therefore, one central question remains: is the disruption of reactivated memories temporary or permanent?
The systemic application of the drugs in our study involves some limitations. Thus, studies with systemic administration of pharmacological agents are likely to be of greater translational utility; however, this type of studies provides little information about the brain regions involved in memory reconsolidation. At present, the neural sites involved in drug-associated memory reconsolidation has not yet been defined, although the basolateral amygdala (Lee et al. 2005; Milton et al. 2008) and the core of the nucleus accumbens (Miller and Marshall 2005) seem to play a crucial role in this process.
In conclusion, we have shown that ethanol-associated memories can be disrupted pharmacologically after their reactivation by both protein synthesis inhibition and NMDA receptor antagonism. These findings have important clinical implications because they show that it is possible to selectively reduce long-lasting alcohol-associated memories. Hence, the disruption of alcohol-related memory reconsolidation may be an effective treatment strategy for the reduction of relapse in abstinent alcoholics.
References
Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R (2005) The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacology 30:1104–1110
Bernardi RE, Lattal KM, Berger SP (2007) Anisomycin disrupts a contextual memory following reactivation in a cocaine-induced locomotor activity paradigm. Behav Neurosci 121:156–163
Biała G, Kotlińska J (1999) Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol 34:175–182
Cami J, Farre M (2003) Drug addiction. N Engl J Med 349(10):975–986
Debiec J, LeDoux JE, Nader K (2002) Cellular and systems reconsolidation in the hippocampus. Neuron 36:527–538
Dudai Y (2004) The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55:51–86
Duvarci S, Nader K (2004) Characterization of fear memory reconsolidation. J Neurosci 24:9269–9275
Füllgrabe MW, Vengeliene V, Spanagel R (2007) Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav 86:320–326
Hellemans KG, Everitt BJ, Lee JL (2006) Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci 26:12694–12699
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598
Kelley AE (2004) Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44:161–179
Kelley JB, Anderson KL, Itzhak Y (2007) Long-term memory of cocaine-associated context: disruption and reinstatement. NeuroReport 18:777–780
Lattal KM, Abel T (2004) Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci USA 101:4667–4672
Lee JL, Everitt BJ (2008) Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiol Learn Mem 90:147–154
Lee JL, Di Ciano P, Thomas KL, Everitt BJ (2005) Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47:795–801
Lee JL, Milton AL, Everitt BJ (2006a) Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26:5881–5887
Lee JL, Milton AL, Everitt BJ (2006b) Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci 26:10051–10056
McGaugh JL (2000) Memory—a century of consolidation. Science 287:248–251
Milekic MH, Brown SD, Castellini C, Alberini CM (2006) Persistent disruption of an established morphine conditioned place preference. J Neurosci 26:3010–3020
Miller CA, Marshall JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47:873–884
Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ (2008) Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci 28:8230–8237
Misanin JR, Miller RR, Lewis DJ (1968) Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160:554–555
Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726
O'Brien CP, Childress AR, McLellan AT, Ehrman R (1992) Classical conditioning in drug-dependent humans. Ann N Y Acad Sci 654:400–415
Pedreira ME, Maldonado H (2003) Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38:863–869
Power AE, Berlau DJ, McGaugh JL, Steward O (2006) Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: the role of re-exposure duration. Learn Mem 13:27–34
Przybyslawski J, Sara SJ (1997) Reconsolidation of memory after its reactivation. Behav Brain Res 84:241–246
Riedel G, Platt B, Micheau J (2003) Glutamate receptor function in learning and memory. Behav Brain Res 140:1–47
Robinson MJ, Franklin KB (2007) Effects of anisomycin on consolidation and reconsolidation of a morphine-conditioned place preference. Behav Brain Res 178:146–153
Sadler R, Herzig V, Schmidt WJ (2007) Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behav Pharmacol 18:699–703
Samson HH (1986) Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10:436–442
Sanchis-Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11:2–38
Spanagel R (2009) Alcoholism—a systems approach from molecular physiology to behavior. Physiol Rev 89:649–705
Spanagel R, Kiefer F (2008) Drugs for relapse prevention of alcoholism—10 years of progress. Trends Pharmacol Sci 29:109–115
Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U (2005) The circadian clock gene Period2 alters the glutamatergic system and thereby modulates alcohol consumption. Nat Med 11:35–42
Spanagel R, Zieglgänsberger W, Hundt W (1996) Acamprosate and alcohol: III. Effects on alcohol discrimination in the rat. Eur J Pharmacol 305:51–56
Torras-Garcia M, Lelong J, Tronel S, Sara SJ (2005) Reconsolidation after remembering an odor-reward association requires NMDA receptors. Learn Mem 12:18–22
Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8:262–275
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA (2006) Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA 103:2932–2937
Vengeliene V, Heidbreder CA, Spanagel R (2007) The effect of lamotrigine on alcohol seeking and relapse. Neuropharmacology 53:951–795
Acknowledgements
We thank Sabrina Koch and Elisabeth Röbel for excellent technical assistance. This work was supported by two SFB636 grants—B1 given to RS and D6 given to FK.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. von der Goltz and V. Vengeliene contributed equally to this work.
Rights and permissions
About this article
Cite this article
von der Goltz, C., Vengeliene, V., Bilbao, A. et al. Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology 205, 389–397 (2009). https://doi.org/10.1007/s00213-009-1544-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1544-1