Abstract
Rationale
Neuropathic pain is associated with significant co-morbidities, including depression, which impact considerably on the overall patient experience. Pain co-morbidity symptoms are rarely assessed in animal models of neuropathic pain. Neuropathic pain is characterized by hyperexcitability within nociceptive pathways and remains difficult to treat with standard analgesics.
Objectives
The present study determined the effect of bis selenide and conventional antidepressants (fluoxetine, amitriptyline, and bupropion) on neuropathic pain using mechanical allodynic and on depressive-like behavior.
Methods
Male mice were subjected to chronic constriction injury (CCI) or sham surgery and were assessed on day 14 after operation. Mice received oral treatment with bis selenide (1–5 mg/kg), fluoxetine, amitriptyline, or bupropion (10–30 mg/kg). The response frequency to mechanical allodynia in mice was measured with von Frey hairs. Mice were evaluated in the forced swimming test (FST) test for depression-like behavior.
Results
The CCI procedure produced mechanical allodynia and increased depressive-like behavior in the FST. All of the drugs produced antiallodynic effects in CCI mice and produced antidepressant effects in control mice without altering locomotor activity. In CCI animals, however, only the amitriptyline and bis selenide treatments significantly reduced immobility in the FST.
Conclusion
These data demonstrate an important dissociation between the antiallodynic and antidepressant effects in mice when tested in a model of neuropathic pain. Depressive behavior in CCI mice was reversed by bis selenide and amitriptyline but not by the conventional antidepressants fluoxetine and buproprion. Bis selenide was more potent than the other drugs tested for antidepressant-like and antiallodynic effects in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal models of chronic pain have demonstrated the importance of serotonergic, dopaminergic, and noradrenergic systems in regulating pain (Fishbain 2000; Millan 2002). Descending pathways serve to inhibit input from the gut and muscle skeletal system to a greater or lesser extent depending on the level of stress and the input from painful stimuli (Millan 2002; Benarroch 2008). A dysfunction of these descending pathways can engender a heightened sensitivity to pain and even a painful reaction to normally non-noxious stimuli. Serotonin (5-HT), dopamine (DA), and noradrenaline (NA) are implicated in enhancing endogenous analgesic mechanisms via the descending inhibitory pain pathways in the brain and spinal cord (Fishbain 2000).
Compounds enhancing 5-HT, DA, and NA neurotransmission would be expected to be effective in the control of chronic pain. Clinical guidelines rank antidepressants, together with anticonvulsants, as first-line drugs for neuropathic pain treatment (Moulin et al. 2007). Neuropathic pain is generally a chronic condition, severe, and resistant to most analgesics (Attal et al. 2006). These antidepressant drugs increase extracellular concentrations of 5-HT, DA, and NA by blocking reuptake transporters (Berton and Nestler 2006). It has been clinically and preclinically demonstrated that specific serotonin reuptake inhibitors (SSRIs) (fluoxetine, paroxetine, and reboxetine), tricylic antidepressants (TCAs) (imipramine, desipramine, and amitriptyline), and atypical antidepressants (AA) (bupropion, trazodone, and venlafaxine) alleviate neuropathic pain (Mico et al. 2006; Benbouzid et al. 2008).
Rodent models are valuable in identifying pathophysiological mechanisms and therapeutic targets for emotional (Cryan and Holmes 2005) and pain disorders (Seltzer et al. 1990). However, recently, a few studies have investigated the potential effects of neuropathic pain on measure of depression-related behavior in rodents (Wang and Wang 2003). The current study examined whether the chronic constriction injury (CCI) model of neuropathic pain causes depression-like behavior in animals and whether this behavior could be reversed by antidepressant drugs. The CCI model (Bennett and Xie 1988) was selected because, compared with other peripheral nerve injury models, a robust mechanical hypersensitivity has been reported (Dowdall et al. 2005; Roeska et al. 2008). Moreover, in different models of traumatic peripheral nerve injury, the measure in the forced swimming test (FST) reflects a depression-like behavior. Since the FST evaluates motivated behavior, it provides possible correlates of the affective-motivational component of ongoing pain in animals.
Low selenium status (low selenium diet contains 32–36 μg/day) has been associated with a significant increased incidence of depression, anxiety, confusion, and hostility (Rayman 2000). Besides, some studies have reported the role of selenium in the management of nociception (Nogueira et al. 2004; Savegnago et al. 2007a, b, 2008). In this context, bis selenide, an organoselenium compound with antinociceptive, anti-inflammatory, and antidepressant-like properties (Savegnago et al. 2006; Jesse et al. 2008, 2009, 2010), could be an attractive target for the treatment of depression and chronic pain.
The first objective of the present study was to examine if rodents display depressive-like behavior induced by nerve injury to better understand the relationship between chronic pain and depression. The second objective of this study was to compare the effect of bis selenide with conventional antidepressants (fluoxetine, amitriptyline, and bupropion) in a mechanical allodynia, with von Frey hair filaments, and depressive-like behavior, in the FST, in mice with neuropathic pain.
Materials and methods
Animals
The behavioral experiments were conducted using male adult Swiss mice (25–35 g) maintained at 22–25°C with free access to water and food, under a 12:12 h light/dark cycle, with lights on at 6:00 a.m. All manipulations were carried out between 8:00 a.m. and 4:00 p.m. Animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources, the Federal University of Santa Maria, Brazil. Experiments were conducted in accordance with the current guidelines for the care of laboratory animals and ethical guidelines for the investigation of experimental pain in conscious animals suggested by Zimmerman (1983). The number of animals and intensities of noxious stimuli used were minimum necessary to demonstrate the consistent effects of the drug treatments. At the end of the experimental procedure, the mice were killed by decapitation.
Animal surgery
CCI was performed based on the original description by Bennett and Xie (1988) under pentobarbital sodium (60 mg/kg, intraperitoneal) anesthesia. Briefly, the left sciatic nerve was exposed after the incision of skin and blunt separation of the muscle. The sciatic nerve was freed of the adhering tissue gently for about 7 mm, and four ligatures (4/0 Ethicon GmbH, Norderstedt, Germany) were made loosely with 1.0–1.5 mm interval between each. Great care was taken to tie the ligatures so that the diameter of the nerve was just barely constricted. Sham operation was performed by exposing sciatic nerve except for nerve ligation. Operated mice were routinely tested for the presence of pain-like behavior for up to 14 days after surgery, according to previously described methods (Blackburn-Munro et al. 2004; Bomholt et al. 2005). Mice with CCI model of neuropathic pain did not present paw drooping or autotomy.
Experimental procedure
CCI-injured and sham-operated animals were repeatedly tested for mechanical allodynia with von Frey hairs (VFH). The response frequency to VFH stimulation (percent) was determined before nerve injury (baseline) to obtain data purely derived from CCI-induced allodynia. In order to determine the basal response frequency, all groups were submitted to pre-surgical evaluation. The response frequency was recorded immediately before (0) and after (1, 2, 4, 8, and 12 h) treatment. Same groups of mice were repeatedly scored in different times for mechanical allodynia.
Other groups of mice were utilized to examine the locomotor activity and the depression-like behavior in the mouse OFT and FST, respectively. For these experiments, CCI-injured and sham-operated animals were examined in behavioral tests at one of six time points, 0, 1, 2, 4, 8, and 12 h after treatment with bis selenide or antidepressants (n = 7 per group, i.e., separate groups of animals were used at each time point). The locomotor activity was evaluated in the open field test (OFT), and immediately after that, the same animals were assessed in the FST.
Chemicals and drug treatment
Bis selenide [(Z)-2,3-bis(4-chlorophenylselanyl)prop-2-en-1-ol] was prepared and characterized in our laboratory by the method previously described (Moro et al. 2005). Analysis of the 1H NMR and 13C NMR spectra showed analytical and spectroscopic data in full agreement with its assigned structure. The chemical purity of bis selenide (99.9%) was determined by GC/HPLC. The following drugs were used: fluoxetine, amitriptyline, and bupropion (Sigma Chemical Co, USA). All other chemicals were of analytical grade and obtained from standard commercial suppliers. Bis selenide was dissolved in canola oil. Antidepressants were dissolved in saline solution (0.9% NaCl). Mice received all drugs in a constant volume of 10 ml/kg body weight.
Treatments with doses of bis selenide (1 and 5 mg/kg, p.o.), fluoxetine, amitriptyline, bupropion (10 or 30 mg/kg, p.o.), or vehicle were carried out 2 weeks after the surgical procedure in mice (groups: operated and sham operated). Mice were evaluated 1, 2, 4, 8, and 12 h subsequent to oral administration. Doses and the schedule of administration were chosen on the basis of experiments previously performed (Pedersen et al. 2005; Jesse et al. 2008, 2009, 2010; Sawynok et al. 2008).
Mechanical allodynia induced by CCI injury
The response frequency was measured after ten applications (duration of 1–2 s each) of VFH (Stoelting, Chicago, IL). To this end, mice were further habituated in individual clear Plexiglas boxes (9 × 7 × 11 cm) on an elevated wire mesh platform to allow access to the ventral surface of the hind paws. A previous study indicated that 0.6 g VFH produced a mean withdrawal frequency of approximately 15%, which is considered to be an adequate value for the measurement of mechanical allodynia (Savegnago et al. 2007a). Therefore, 0.6 g VFH alone was used in these experiments. The filament was applied for a period of 1–2 s. Both the ipsilateral (right hind paw) and the contralateral hind paw were tested in order to evaluate the presence of “mirror pain”, described elsewhere as present in neuropathic pain pathologies (Tal and Bennett 1994). In order to obtain data purely derived from the CCI model-induced allodynia, maximal inhibition (MI) values were represented as the difference between the basal values of vehicle- or drug-treated animals and respective controls.
Open-field test
The OFT was carried out to exclude the possibility that the lesion of the CCI model affects the ambulation of the animals. The open field was made of plywood and surrounded by walls 30 cm in height. The floor of the open field, 45 cm in length and 45 cm in width, was divided by masking tape markers into 9 squares (3 rows of 3). Each animal was placed individually at the center of the apparatus and observed for 6 min to record the locomotor (number of segments crossed with the four paws) (Walsh and Cummins 1976).
Motor coordination for swimming—motivational test
This test was performed to further exclude the possibility that the lesion of the CCI model affects locomotion of the animals, since the motor coordination for swimming is different of those necessary for the open field test. The motor coordination for swimming was carried out in an iron pool (86 × 17 × 37 cm) filled with clear water to a depth of 20 cm. Water temperature was maintained at 20 ± 0.5°C. At the end of the pool, there was a red platform on which the mice could climb. The location of the platform was made visible by a blue-colored picture mounted above the platform. During the test, the mouse was put into the water at the start point and swam to the end of the pool to climb onto the platform. The swimming time was recorded (Li et al. 2001).
Depressive-like behavior—forced swimming test)
The test was performed as in the original method described elsewhere (Porsolt et al. 1977, 1978). Briefly, mice were individually forced to swim in open cylinders (25 cm height × 10 cm diameter) containing 19 cm of water at 25 ± 1°C. The duration of immobility was scored during the 6-min test period as described previously. The immobility time was determined when no additional activity was observed other than the movements necessary to keep the mice head above the water.
Statistical analysis
All experimental results are given as the mean ± S.E.M. The withdrawal response frequency, number of crossings and depression-like behavior in sham and CCI animals was done by analyzing Newman–Keuls test for post hoc comparison when appropriate. Comparisons between experimental and control groups were analyzed with two-way ANOVA, including the factors surgery (sham and CCI), treatment (bis selenide, fluoxetine, amitriptyline, and bupropion), and interaction. A value of P < 0.05 was considered to be significant. MI values were represented as the difference between the basal values of vehicle- or drug-treated animals and respective controls.
Results
Mechanical allodynia
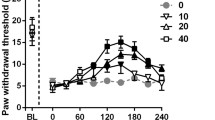
Mechanical sensitivity of the animal hind paws was evaluated by VHF. CCI model produced a marked and long-lasting development of mechanical allodynia in the ipsilateral (injured) side in mice (Fig. 1a–d), but not in the contralateral side (results not shown). Before surgery, no significant difference was observed between operated and sham-operated groups for the baseline of response frequency. Sham surgery did not influence the mechanical threshold in mice (Fig. 1a–d).
Effect of oral treatment with bis selenide (1 and 5 mg/kg) (a), fluoxetine (10 and 30 mg/kg) (b), amitriptyline (10 and 30 mg/kg) (c), and bupropion (10 and 30 mg/kg) (d) on the response frequency to VFH stimulation (%) in ipsilateral paw in CCI (closed points) and sham-operated mice (open points). Each point represents the mean of seven animals and the error bars indicate the S.E.M. *P < 0.05, **P < 0.01, and ***P < 0.001 when compared to the CCI control, and # P < 0.001 versus baseline (B) values
Animals that received bis selenide (1 and 5 mg/kg, p.o.) showed a significant reduction in the response frequency induced by CCI model (Fig. 1a). This reduction started 1 h after bis selenide (1 and 5 mg/kg) administration (MI was 82 ± 5%). CCI-injured mice that received 1 and 5 mg/kg of bis selenide demonstrated a significant reduction in the response frequency, which was maintained for up to 4 and 8 h, respectively (Fig. 1a).
Acute treatment with fluoxetine (10 and 30 mg/kg, p.o.) significantly decreased the response frequency in the ipsilateral side 1 h after antidepressant administration (MI values were 35 ± 2% and 51 ± 2%). This antiallodynic effect was kept for 2 h after fluoxetine treatment (Fig. 1b).
Treatment with amitriptyline at doses of 10 and 30 mg/kg (p.o.) was markedly effective in reducing the response frequency induced by CCI model in mice. The reduction in the response frequency started 1 h after amitriptyline administration (30 mg/kg), and the MI was 62 ± 5%. The significant decrease in the response frequency was maintained for up to 2 and 8 h when CCI-injured mice received 10 and 30 mg/kg of amitriptyline, respectively (Fig. 1c).
CCI-injured mice treated orally with bupropion (30 mg/kg, p.o.) showed a significant reduction in the response frequency. Antiallodynic effect was observed 1, 2, and 4 h after bupropion administration (30 mg/kg) in CCl-injured mice (MI was 78 ± 5% at 1 h; Fig. 1d).
Acute treatment with bis selenide and conventional antidepressants had no influence in the response frequency in sham-operated mice (Fig. 1a–d).
Depression-like behavior
CCI-injured mice showed a marked prolongation of the immobility time compared to sham-operated mice in the FST (Fig. 2a–d), which indicates that CCI animals displayed depression-like behavior.
Effect of oral treatment with bis selenide (1 and 5 mg/kg) (a), fluoxetine (10 and 30 mg/kg) (b), amitriptyline (10 and 30 mg/kg) (c), and bupropion (10 and 30 mg/kg) (d) on immobility time (s) in the FST in CCI (closed points) and sham-operated (open points) mice. Each point represents the mean of seven animals and the error bars indicate the S.E.M. *P < 0.05, **P < 0.01, and ***P < 0.001 when compared to the CCI or sham-operated group, and # P < 0.001 versus sham-operated control group
Bis selenide administration (1 and 5 mg/kg, p.o.) showed a reduction in depression-like behavior in CCI-injured mice. This effect started 1 h after bis selenide administration (MI was 35 ± 3% at 5 mg/kg). The significant reduction in the immobility time was maintained for up to 2 and 8 h when CCI-injured mice received 1 and 5 mg/kg of bis selenide, respectively (Fig. 2a). Bis selenide administration (1 and 5 mg/kg, p.o.) caused a reduction in the immobility time in sham-operated mice. The antidepressant-like effect of bis selenide (5 mg/kg) reached its peak at 1 h (MI was 43 ± 3%) and remained significant up to 4 h after administration in sham-operated mice (Fig. 2a).
Fluoxetine, at doses of 10 and 30 mg/kg, did not attenuate the depression-like behavior in CCI-injured mice at all time points. It can be seen that fluoxetine, given by oral route at the doses of 10 and 30 mg/kg, decreased the immobility time in the FST in sham-operated mice (Fig. 2b). The anti-immobility effect started 1 h after fluoxetine (30 mg/kg) administration in sham-operated mice (MI was 40 ± 2%). The significant reduction in the immobility time was maintained for up to 2 and 4 h when mice received 10 and 30 mg/kg of fluoxetine, respectively (Fig. 2b).
Treatment with amitriptyline, at the dose of 30 mg/kg (p.o.), significantly reduced the depression-like behavior in CCI-injured mice (Fig. 2c). The anti-immobility effect started 1 h after amitriptyline (30 mg/kg) administration, and the MI observed was 34 ± 4%. The significant decrease in the immobility time induced by amitriptyline (30 mg/kg) was maintained for up to 2 h in CCI-injured mice. Amitriptyline administration (10 and 30 mg/kg, p.o.) produced a reduction in the immobility time in sham-operated mice. The antidepressant-like effect of amitriptyline, at the dose of 30 mg/kg, reached its peak at 1 h (MI was 33 ± 3%) and remained significant up to 4 h after p.o. administration (Fig. 2c).
The effects of bupropion administered orally in mice are illustrated in Fig. 2d. Acute treatment with bupropion (10 and 30 mg/kg) did not modify the depression-like behavior in CCI-injured mice at any time point. The anti-immobility effect was observed 1, 2, and 4 h after bupropion administration (30 mg/kg) in sham-operated mice, with a peak effect in 1 h (MI was 29 ± 5%) (Fig. 2d).
Locomotor activity
There was no significant difference between sham-operated and CCI animals in the number of crossings in the OFT. Bis selenide and antidepressants had no significant effect on the number of crossings in the OFT, at any time point monitored (0, 1, 2, 4, 8, and 12 h), when compared to respective controls (Table 1).
There was no significant difference in the latency for swimming in the motor coordination test between sham-operated and CCI groups. Treatment with bis selenide, at the dose of 5 mg/kg (p.o.), did not influence the motor function (Fig. 3).
Discussion
The present study demonstrated that mice with CCI of the sciatic nerve developed depression-like behavior as reflected in an increase in the time of immobility in the FST and mechanical allodynia demonstrated by the increase of baseline values in the response frequency to VFH. The main original pharmacological finding of the present study was that bis selenide diminished both the mechanical hypersensitivity and depressive-like behavior in CCI animals, without having an effect on locomotor activity.
The CCI model (Bennett and Xie 1988) was selected for this study due to a significant mechanical hypersensitivity in comparison with other animal neuropathic pain models (Dowdall et al. 2005; Roeska et al. 2008). We used a pharmacologically validated test for depression-like behavior, the FST (Porsolt et al. 1977, 1978). This test is frequently used to assess depression-like behavior and is validated by its sensitivity to clinically effective antidepressants that cause in mice to actively and persistently engage in escape-directed behavior compared with non-treated controls. Moreover, the fact that animals with neuropathic pain exhibit depression-like behavior is in agreement with the clinical evidence reporting a relationship between chronic pain and depression (Attal et al. 2006; Mico et al. 2006).
The current study also demonstrated that fluoxetine, at the dose of 30 mg/kg, generated an antiallodynic effect. Additionally, fluoxetine reduced the immobility time in sham animals but did not modify the depressive-like behavior in CCI animals in the FST. It has been reported that fluoxetine, commonly used to treat major depression in humans (Holtzheimer and Nemeroff 2006), is modestly active in a test of tactile allodynia and nociceptive tests (Sindrup et al. 1992; Pedersen et al. 2005). The SSRI employed in this study, fluoxetine, displays distinct selectivity for 5-HT neuronal uptake sites (Hyttel 1994). Thus, fluoxetine produces its pharmacological action via the blockade of 5-HT reuptake, enhancing the efficacy of the 5-HT transmission (Weiss et al. 1986). Taken together, these findings indicated that fluoxetine treatment may cause the attenuation of pain but did not modify the depression-like behavior induced by CCI.
In this study, treatment with amitriptyline produced a significant antiallodynia effect, which is in accordance with other reports (Bomholt et al. 2005; Vissers et al. 2006). However, to the best of our knowledge, this is the first time that the anti-immobility effect of amitriptyline in a CCI neuropathic pain-like state was demonstrated. Treatment with amitriptyline also showed antidepressant-like effect in sham-operated mice. These results were corroborated by Hu et al. (2009) who demonstrated that desipramine (20 mg/kg) reduced the depression-like behavior in rats with mononeuropathy. In a number of neuropathic pain conditions, their analgesic action has been demonstrated to be independent of mood alteration (Max et al. 1987; Wolfe and Trivedi 2004; Sindrup et al. 2005). Acute treatment with amitriptyline has been shown to decrease thermal and chemical hyperalgesia (Casas et al. 1995). Amitriptyline is well known as a dual NA and 5-HT reuptake inhibitor, and its ability to inhibit the uptake of both amines can be implicated in its action demonstrated in this study. In a previous study, it was reported that chronic treatment with the TCAs, nortriptyline, and amitriptyline, but not with the SSRI, fluoxetine, alleviated cuff-induced mechanical allodynia (Benbouzid et al. 2008). This suggests that chronic inhibition of the 5-HT reuptake site alone does not relieve allodynia and that the inhibition of NA uptake plays a critical role in the management of allodynia. A variety of mechanisms have been suggested to explain amitriptyline antinociceptive actions, including block of uptake of NA and 5-HT, engagement of opioid mechanisms, inhibition of ion channel activity, block of N-methyl-d-aspartic acid receptors, increased γ-aminobutyric acid receptor activity, and modulation of immune function (Mico et al. 2006; Dick et al. 2007). Adenosine mechanisms are also implicated in antinociception produced by systemic (Ulugol et al. 2002) and peripherally administered amitriptyline (Sawynok et al. 1999; Ulugol et al. 2002) in models of chronic pain. Adenosine A1 receptors (A1Rs) mediate suppression of nociception, and methylxanthines block adenosine analog effects on pain mediated via A1Rs (Sawynok and Liu 2003). Amitriptyline has been demonstrated to inhibit uptake of adenosine (Phillis and Wu 1982).
Bupropion administration produced an antidepressant-like effect in sham-operated mice, while causing an antiallodynic action in the CCI model in mice. The antiallodynic action of bupropion has been reported (Pedersen et al. 2005). Acute administration of bupropion, an AA, decreases the reuptake of DA and NA into rat and mouse synaptosomes, reduces the firing rate of central NA- and DA-containing neurons, and increases extracellular DA levels (Cooper et al. 1994). Thus, the ability of bupropion to reduce mechanical allodynia suggests that inhibition of NA and DA reuptake accounted for this action. Different from sham-operated mice, bupropion did not alleviate the depressive-like behavior in the FST in CCI animals.
Although, bupropion is a dopamine reuptake inhibitor and increases dopamine levels in the brain, it had no significant effect on the locomotor activity. In this context, Pedersen et al. (2005) demonstrated that bupropion (3–30 mg/kg, i.p.) administered in rats had no significant effect on motor performance at any of the doses tested at 30 or up to 120 min after administration when compared with corresponding vehicle treatment. Although some studies showed that there was no change in locomotor activity, other studies reported alterations (Redolat et al. 2005; Mitchell et al. 2006). However, most of the changes in locomotor activity have been observed when the route of administration is intraperitoneal (Sekihashi et al. 2001). Thus, it is possible that the results we found with bupropion could be explained by the administration route used, the oral route.
In the current study, we showed that the depression-like behavior was diminished by bis selenide in both sham and CCI animals. These data further support the interpretation that the increased time of immobility of CCI mice measured in the FST reflects a depression-like behavior. Bis selenide was more potent than amitriptyline, the only antidepressant that demonstrated anti-immobility effect in CCI mice. In addition, bis selenide was also more potent than fluoxetine, amitriptyline, and bupropion when the immobility time in sham-operated mice was compared. The present study demonstrated the efficacy of bis selenide in the model of CCI neuropathic pain and a rank order of potency was bis selenide > bupropion > amitriptyline > fluoxetine.
Taking together the results, all of the drugs studied reduced immobility in the sham condition, yet only bis selenide and amitriptyline reduced immobility after nerve injury. Bis selenide seems to have a comparable action under both conditions, but amitriptyline has less effect after nerve injury as only the highest dose is active. It seems that the FST reflects presumptive antidepressant activity in the sham state, but the presence of nerve injury (and chronic pain) decreases their ability to produce an antidepressant effect. The suppression of the immobility after nerve injury by amitriptyline could be explained by the dual inhibition of NA and 5-HT reuptake (Benarroch 2008). Conversely, the absence of fluoxetine and bupropion effects on the immobility response after nerve injury in mice could be attributed to the fact that fluoxetine has a distinct selectivity for 5-HT neuronal uptake sites (Hyttel 1994), and bupropion modulates central NA- and DA-containing neurons and increases extracellular DA levels (Cooper et al. 1994).
It has previously been shown that bis selenide elicits antinociceptive, anti-inflammatory, and antidepressant-like effects in mice. The mechanisms through which this organoselenium compound exerts its action involve an interaction with ATP-sensitive and voltage-gated K+ channels, kainate, trans-ACDP, serotonergic (5-HT2A and 5-HT3), and histamine H2 receptors (Jesse et al. 2008, 2009, 2010). Thus, we postulate that the efficacy of amitriptyline and bis selenide in a neuropathic pain model and in the depressive-like behavior can be attributed to the modulation of channels and receptors previously mentioned. Based on the results, one could speculate that 5-HT and DA systems are less contributory to the profile observed.
It is important to point out that the therapeutic latency is much shorter in treating neuropathic pain compared to depression (Benbouzid et al. 2008). There has been report that the neuropathic allodynia was suppressed by antidepressants at doses two to four times lower than the ones classically used on mice in models predictive of depression. Moreover, this analgesic effect was only seen after 10–14 days of chronic treatment. Given that, it is possible that the analgesic effect seen acutely with the antidepressants in the CCI model neither reflects an effect on the emotional aspect of pain nor contributes to the alleviation of the co-morbid pain depression.
The results demonstrated in this study confirmed previous data (Gonçalves et al. 2008; Hu et al. 2009) in which rodents display depressive-like behavior induced by nerve injury. The data demonstrate an important dissociation between the antiallodynic and antidepressant effects in mice when tested in a model of neuropathic pain. Depressive behavior in CCI mice was reversed only by bis selenide and amitriptyline but not by the conventional antidepressants fluoxetine and buproprion. Bis selenide was more potent than the other drugs tested for antidepressant-like and antiallodynic effects in mice.
References
Attal N, Cruccu G, Haanpää M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P (2006) EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol 13:1153–1169
Benarroch EE (2008) Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology 71:217–221
Benbouzid M, Choucair-Jaafar N, YalcinI WE, Muller A, Freund-Mercier MJ, Barrot M (2008) Chronic, but not acute, tricyclic antidepressant treatment alleviates neuropathic allodynia after sciatic nerve cuffing in mice. Eur J Pain 12:1008–1017
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151
Blackburn-Munro G, Bomholt SF, Erichsen HK (2004) Behavioural effects of the novel AMPA/GluR5 selective receptor antagonist NS1209 after systemic administration in animal models of experimental pain. Neuropharmacology 47:351–362
Bomholt SF, Mikkelsen JD, Blackburn-Munro G (2005) Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animals models of acute, persistent and neuropathic pain. Neuropharmacology 48:252–263
Casas J, Gibert-Rahola J, Chover AJ, Mico JA (1995) Test-dependent relationship of the antidepressant and analgesic effects of amitriptyline. Methods Find Exp Clin Pharmacol 17:583–588
Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM (1994) Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology 11:133–141
Cryan JF, Holmes A (2005) Model organisms: the ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790
Dick IE, Brochu RM, Purohit Y, Kaczorowski GJ, Martin WJ, Priest BT (2007) Sodium channel blockade may contribute to the analgesic efficacy of antidepressants. J Pain 8:315–324
Dowdall T, Robinson I, Meert TF (2005) Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav 80:93–108
Fishbain D (2000) Evidence-based data on pain relief with antidepressants. Ann Med 32:305–316
Gonçalves L, Silva R, Pinto-Riberio F, Pêgo F, Bessa JM, Pertovaara A, Sousa N, Almeida A (2008) Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol 213:48–56
Holtzheimer PE, Nemeroff CB (2006) Advances in the treatment of depression. J NeuroRx 3:42–56
Hyttel J (1994) Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int Clin Psychopharmacol 1:19–26
Hu B, Doods H, Treede RD, Ceci A (2009) Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selecetive agonist GW405833. Pain 143:206–212
Jesse CR, Savegnago L, Nogueira CW (2008) Spinal mechanisms of antinociceptive effect caused by oral administration of bis-selenide in mice. Brain Res 1231:25–33
Jesse CR, Savegnago L, Nogueira CW (2009) Mechanisms involved in the antinociceptive and anti-inflammatory effects of bis selenide in mice. J Pharm Pharmacol 61:623–630
Jesse CR, Wilhelm EA, Bortolatto CF, Nogueira CW (2010) Evidence for the involvement of the serotonergic 5-HT2A/C and 5-HT3 receptors in the antidepressant-like effect caused by oral administration of bis selenide in mice. Prog Neuropsychopharmacol Biol Psychiatry 34:294–302
Li Z, Wua CF, Peib G, Xua NJ (2001) Reversal of morphine-induced memory impairment in mice by withdrawal in Morris water maze. Possible involvement of cholinergic system. Pharmacol Biochem Behav 68:507–513
Max MB, Culnane M, Schafer SC, Gracely RH, Walther DJ, Smoller B, Dunbar R (1987) Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 37:589–596
Mico JA, Ardid D, Berrocoso E, Eschalier A (2006) Antidepressants and pain. Trends Pharmacol Sci 7:348–354
Millan MJ (2002) Descending control of pain. Prog Neurobiol 66:355–474
Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D (2006) The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biol Psychiatry 60:1046–1052
Moro AV, Nogueira CW, Barbosa NBV, Menezes PH, Rocha JBT, Zeni G (2005) Highly stereoselective one-pot producers to prepare bis- and tris-chalcogenide alkenes via addition of disulfides and diselenides to terminal alkynes. J Org Chem 70:5257–5268
Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, Coderre T, Morley-Forster PK, Stinson J, Boulanger A, Peng P, Finley GA, Taenzer P, Squire P, Dion D, Cholkan A, Gilani A, Gordon A, Henry J, Jovey R, Lynch M, Mailis-Gagnon A, Panju A, Rollman GB, Velly A (2007) Pharmacological management of chronic neuropathic pain—consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag 12:13–21
Nogueira CW, Zeni G, Rocha JB (2004) Organoselenium and organotellurim compounds: toxicology and pharmacology. Chem Rev 104:6255–6285
Pedersen LH, Nielsen AN, Blackburn-Munro G (2005) Anti-nociception is selectively enhanced by parallel inhibition of multiple systems of monoamine transporters in rat models of persistent and neuropathic pain. Psychopharmacology 182:551–556
Phillis J, Wu PH (1982) The effect of various centrally active drugs on adenosine uptake by the central nervous system. Comp Biochem Pharmacol 72:179–187
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391
Rayman MP (2000) The importance of selenium to human health. Lancet 356:23–41
Redolat R, Vidal J, Gomez MC, Carrasco MC (2005) Effects of acute bupropion administration on locomotor activity in adolescent and adult mice. Behav Pharmacol 16:59–62
Roeska K, Doods H, Arndt K, Treede RD, Ceci A (2008) Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain 139:349–357
Savegnago L, Jesse CR, Moro AV, Borges VC, Santos FW, Rocha JB, Nogueira CW (2006) Bis selenide alkene derivatives. A class of potential antioxidant and antinociceptive agents. Pharmacol Biochem Behav 83:221–229
Savegnago L, Jesse CR, Pinto LG, Rocha JB, Nogueira CW (2007a) Diphenyl diselenide attenuates acute thermal hyperalgesia and persistent inflammatory and neuropathic pain behavior in mice. Brain Res 1175:54–59
Savegnago L, Pinto LG, Jesse CR, Alves D, Rocha JB, Nogueira CW, Zeni G (2007b) Antinociceptive properties of diphenyl diselenide: evidences for the mechanisms of action. Eur J Pharmacol 555:129–138
Savegnago L, Jesse CR, Nogueira CW (2008) Caffeine and a selective adenosine A(2B) receptor antagonist but not imidazoline receptor antagonists modulate antinociception induced by diphenyl diselenide in mice. Neurosci Lett 436:120–123
Sawynok J, Liu XJ (2003) Adenosine in the spinal cord and periphery, release and regulation of pain. Prog Neurobiol 69:313–340
Sawynok J, Reid AR, Esser MJ (1999) Peripheral antinociceptive action of amitriptyline in the rat formalin test: involvement of adenosine. Pain 80:45–55
Sawynok J, Reid AR, Fredholm BB (2008) Caffeine reverses antinociception by amitriptyline in wild type mice but not in those lacking adenosine A1 receptors. Neurosci Lett 440:181–184
Sekihashi K, Sasaki T, Yamamoto A, Kawamura K, Ikka T, Tsuda S, Sasaki YF (2001) A comparison of intraperitoneal and oral gavage administration in comet assay in mouse eight organs. Mutat Res 493:39–54
Seltzer Z, Dubner R, Shir Y (1990) A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43:205–218
Sindrup SH, Brøsen K, Gram LF (1992) Antidepressants in pain treatment: antidepressant or analgesic effect. Clin Neuropharmacol 15(Suppl 1 Pt A):636A–637A
Sindrup SH, Otto M, Finnerup NB, Jensen TS (2005) Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 96:399–409
Tal M, Bennett GJ (1994) Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain 57:375–382
Ulugol A, Karadag HC, Tamer M, Firat Z, Aslantas A, Dokmeci I (2002) Involvement of adenosine in the anti-allodynic effect of amitriptyline in streptozotocin-induced diabetic rats. Neurosci Lett 328:129–132
Vissers KCP, Geenen F, Biermans R, Meert TH (2006) Pharmacological correlation between the formalin test and the neuropathic pain behavior in different species with chronic constriction injury. Pharmacol Biochem Behav 84:479–486
Wang LX, Wang ZJ (2003) Animal and cellular models of chronic pain. Adv Drug Deliv Rev 55:949–965
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504
Weiss GF, Papadakos P, Knudson K, Leibowitz SF (1986) Medial hypothalamic serotonin: effects on deprivation and norepinephrine-induced eating. Pharmacol Biochem Behav 25:1223–1230
Wolfe GI, Trivedi JR (2004) Painful peripheral neuropathy and its nonsurgical treatment. Muscle Nerve 30:3–19
Zimmerman M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jesse, C.R., Wilhelm, E.A. & Nogueira, C.W. Depression-like behavior and mechanical allodynia are reduced by bis selenide treatment in mice with chronic constriction injury: a comparison with fluoxetine, amitriptyline, and bupropion. Psychopharmacology 212, 513–522 (2010). https://doi.org/10.1007/s00213-010-1977-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-1977-6