Abstract
Rationale
Psychiatric disorders such as anxiety and depression are frequently observed in neuropathic pain patients, and negatively impact their quality of life. Mirogabalin is a novel ligand for the α2δ subunit of voltage-gated calcium channels and has unique binding characteristics to α2δ subunits and potent and long-lasting analgesic effects in neuropathic pain models.
Objectives
To provide further information on the pharmacological profile of mirogabalin and its utility for chronic pain therapy, we investigated its anxiolytic effects in an experimental animal model for neuropathic pain.
Methods
In chronic constriction injury (CCI) model rats, mechanical hypersensitivity was determined by the von Frey test. Anxiety- and depression-related behaviours were evaluated using the elevated plus maze test and forced swimming test, respectively.
Results
CCI model rats showed sustained tactile allodynia followed by anxiety-related behaviours, not depression-related behaviours. The tactile allodynia (significant decreases in paw withdrawal threshold) developed within 2 weeks after model preparation, whereas the anxiety-related behaviours (significant decreases in the number of entries and time spent in open arms and significant increases in time spent in closed arms) were observed at 5 weeks but not 4 weeks after model preparation. Single oral administration of mirogabalin (3 or 10 mg/kg) dose-dependently alleviated the above-mentioned anxiety-related behaviours and tactile allodynia.
Conclusions
CCI model rats showed anxiety-related behaviours in a time-dependent manner in the elevated plus maze test. Mirogabalin alleviated both the anxiety-related behaviours and tactile allodynia in CCI model rats. Mirogabalin may provide effective anxiety relief as well as pain relief in patients with neuropathic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuropathic pain is defined as ‘pain caused by a lesion or disease of the somatosensory nervous system’. It is a complicated syndrome underlain by multiple mechanisms, and the current therapies for it are not completely satisfactory (Jensen et al. 2011; Colloca et al. 2017). Some neuropathic pain patients suffer from various comorbid symptoms, such as sleep disturbances and psychiatric disorders (e.g. anxiety and depression). Both chronic pain and these comorbid symptoms lead to a vicious cycle that seriously affects the quality of life (Gustorff et al. 2008; Attal et al. 2011; Radat et al. 2013; Schaefer et al. 2014; Failde et al. 2018). For the management of neuropathic pain, several sets of international and regional guidelines recommend gabapentin and pregabalin as the first-line therapy (O’Connor and Dworkin 2009; Attal et al. 2010; Cruccu and Truini 2017). Gabapentin and pregabalin are selective ligands for the α2δ subunit of voltage-gated calcium channels (Gee et al. 1996; Li et al. 2011) and exert various pharmacological effects, including anticonvulsant, anxiolytic and antidepressant activities, as well as analgesia (Dooley et al. 2007; Stahl et al. 2013).
Mirogabalin {[(1R,5S,6S)-6-(aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetic acid} is a newly synthesised potent and selective α2δ ligand. It has been licenced for the treatment of peripheral neuropathic pain including painful diabetic peripheral neuropathy and postherpetic neuralgia in Japan (Deeks 2019). We previously reported that mirogabalin has unique binding characteristics to α2δ subunits (Domon et al. 2018a) and shows potent and long-lasting analgesic effects in neuropathic pain models (Domon et al. 2018a, b) and fibromyalgia models (Saeki et al. 2019). Here, to provide further information on the pharmacological profile of mirogabalin and its utility for chronic pain therapy, we investigated its anxiolytic effects in a rat model of chronic constriction injury (CCI) as an experimental animal model for neuropathic pain.
Materials and methods
Animals
Male Crl:CD(SD) rats at 6 weeks of age were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). The animals were housed under standard laboratory conditions of temperature (21–27 °C), relative humidity (35–70%) and a 12-h light/dark cycle (lights on 06:00–18:00 h), with a commercial diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water available ad libitum. The animals were randomly allocated to the study groups using a computed randomisation procedure (IBUKI; Nihon Bioresearch Inc., Gifu, Japan) based on pain response and body weight.
All experiments were performed in accordance with the Guidelines for the Management and Welfare of Experimental Animals (Hashima Laboratory, Nihon Bioresearch Inc.; April 2, 2007, modified on August 27, 2010), and the Guidelines of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co., Ltd.
Chemicals
Mirogabalin besylate (code number DS-5565) was synthesised by Daiichi Sankyo Co., Ltd. (Tokyo, Japan). The drug was dissolved in distilled water (Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) and administered orally at a volume of 10 mL/kg. Control groups received an equal amount of distilled water. The dose levels (expressed as free form) and pretreatment time of mirogabalin were determined based on our previous reports (Domon et al. 2018a, b; Saeki et al. 2019) and preliminary study (Supplementary material). The chemical structure of mirogabalin besylate is shown in Fig. 1.
Preparation of CCI model
The CCI model was prepared in accordance with a previous report (Bennett and Xie 1988) with minor modifications. Under isoflurane anaesthesia (Isoflurane Inhalation Solution [Pfizer]; Pfizer Japan Inc., Tokyo, Japan), the left femoral skin was incised and the sciatic nerve was exposed. The sciatic nerve was loosely ligated at mid-thigh level with two surgical 4–0 silk threads at an interval of 1 mm. The surgical area was sutured with surgical threads and the animals received subcutaneous injection of ampicillin sodium (50 mg/0.25 mL/body, Viccillin® 1 g for injection; Meiji Seika Pharma Co., Ltd., Tokyo, Japan).
Assessment of pain response (von Frey test)
The plantar surface of the left hind paw was stimulated with von Frey filaments (bending force: 0.4, 0.6, 1, 2, 4, 6, 8 and 15 g; North Coast Medical Inc., Gilroy, CA, USA), and the 50% paw withdrawal threshold was determined by the up-down method (Dixon 1980; Chaplan et al. 1994). The von Frey test was conducted before and 7, 14 and 21 days after model preparation. CCI model rats with 50% paw withdrawal threshold of 6 g or lower on Day 21 were selected and assigned to the study groups. The actual success rate of CCI model was 90%.
Assessment of anxiety-related behaviour (elevated plus maze test)
The elevated plus maze test was conducted using a maze elevated 60 cm above the floor level, composed of two open arms and two closed ones (42 cm in length, 15 cm in width, wall height 30 cm) that crossed in the middle perpendicularly, and a central platform. The rats were placed individually in the central platform facing an open arm and allowed to explore for 5 min. The behaviour was analysed with a video tracking system (SMART; Panlab Inc., Holliston, MA, USA) and the following parameters were measured: (1) time spent in each arm and platform, (2) number of entries into each arm and platform, and (3) distance travelled in each arm and platform.
Assessment of depression-related behaviour (forced swimming test)
The forced swimming test, originally reported by Porsolt et al. (1977), was conducted using an automated analysis system (Shimamura et al. 2007) with minor modifications. Briefly, a small magnet (3 mm in width, 2 mm in depth, 3 mm in height) was fixed on each forelimb using surgical tape. Then, the rats were placed individually in a vertical acrylic cylinder (20 cm in diameter, 50 cm in height) containing water up to 25 cm at 24±2 °C. The duration of immobility was detected and analysed using an automated behaviour analytical system (MicroAct™; Neuroscience, Inc., Tokyo, Japan). Visual observation using video recordings was also conducted as a supplement. There was no distinction between the automated analysis and supplemental visual observation. The forced swimming test was conducted on two consecutive days (first trial for 15 min and second trial for 5 min).
Experimental schedule
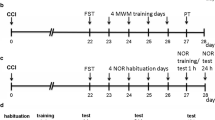
This study consisted of a series of two experiments, namely, confirmation of anxiety- and depression-related behaviours (experiment 1), followed by the evaluation of drug effects (experiment 2). In experiment 1, the elevated plus maze test was conducted at 4 or 5 weeks after model preparation, and the forced swimming test was conducted at 6 or 8 weeks after model preparation. Based on the results of experiment 1, experiment 2 focused on the effects of mirogabalin on the anxiety-related behaviours observed at 5 weeks after model preparation. The drug was administered 2 h before undergoing the elevated plus maze test at 5 weeks after model preparation. The von Frey test was conducted after the elevated plus maze test (i.e., 4 h after drug administration). The study design and experimental schedule are summarised in Fig. 2.
Statistical analysis
All data are expressed as the mean ± standard error. Statistical analysis was performed using the F-test followed by Student’s or Aspin–Welch’s t test for two-group comparisons, and Bartlett’s test followed by Dunnett’s or Steel’s test for multiple-group comparisons. In the von Frey test, Wilcoxon’s rank sum test was used for two-group comparisons. Differences were considered significant when p<0.05. The SAS System (SAS Software Release 9.1.3; SAS Institute Japan Ltd., Tokyo, Japan) was used for these analyses.
Results
Experiment 1
In the von Frey test, CCI model rats showed sustained decreases in the 50% paw withdrawal threshold compared with sham rats (Fig. 3). In the elevated plus maze test, there were no statistically significant differences in any anxiety parameters between CCI model rats and sham rats at 4 weeks after model preparation (Fig. 4, top, middle and bottom). At 5 weeks after model preparation, CCI model rats exhibited significant decreases in time spent in the open arms and platform and significant increases in time spent in the closed arms (Fig. 4, top). The number of entries into the open arms significantly decreased in CCI model rats (Fig. 4, middle). The distance travelled in the open arms and platform significantly decreased in CCI model rats (Fig. 4, bottom).
Anxiety parameters in elevated plus maze test in CCI model rats. Top: time spent in platform, open arms and closed arms. Middle: numbers of entries into platform, open arms and closed arms. Bottom: distance travelled in platform, open arms and closed arms. Each value represents the mean ± standard error (n=10). *p<0.05, **p<0.01: Significantly different from the sham control group with Student’s t test or the Aspin–Welch t test.
In the forced swimming test, there were no statistically significant differences in the immobility time between CCI model rats and sham rats at 6 or 8 weeks after model preparation (Fig. 5).
Experiment 2
As in experiment 1, CCI model rats showed sustained decreases in the 50% paw withdrawal threshold (data not shown). At 5 weeks after model preparation, a single oral administration of mirogabalin (3 or 10 mg/kg) dose-dependently increased the 50% paw withdrawal threshold and the effect of 10 mg/kg was statistically significant upon comparison with the CCI control group (Fig. 6).
Analgesic effects of mirogabalin in CCI model rats. Mirogabalin besylate was administered orally (10 mL/kg), while the control group received distilled water. The dose levels are expressed as the levels of the free form of the drug. Each value represents the mean ± standard error (n=10). **p<0.01: Significantly different from the sham control group with Wilcoxon’s rank sum test. #p<0.05: Significantly different from the CCI control group with Steel’s test.
In the elevated plus maze test, anxiety-related behaviours were confirmed in a similar fashion to that in experiment 1. Namely, CCI model rats exhibited significant decreases in time spent in the open arms, significant increases in time spent in the closed arms (Fig. 7, top), significant decreases in the number of entries into each arm and distance travelled in each arm (Fig. 7, middle and bottom). The decreased number of entries into each arm reflected that CCI model rats stayed in the closed arms for a long time and rarely moved to the other areas. A single oral administration of mirogabalin (3 or 10 mg/kg) alleviated these anxiety-related behaviours in a dose-dependent manner (Fig. 7, top, middle and bottom). In particular, mirogabalin at 10 mg/kg significantly recovered the decreased time spent in the open arms and the increased time spent in the closed arms, compared with those in the CCI control group (Fig. 7, top). Mirogabalin also recovered the decreased number of entries into each arm and the decreased distance travelled in each arm (Fig. 7, middle and bottom).
Anxiolytic effects of mirogabalin in elevated plus maze test in CCI model rats. Top: time spent in platform, open arms and closed arms. Middle: numbers of entries into platform, open arms and closed arms. Bottom: distance travelled in platform, open arms and closed arms. Mirogabalin besylate was administered orally (10 mL/kg), while the control group received distilled water. The dose levels are expressed as the levels of the free form of the drug. Each value represents the mean ± standard error (n=10). *p<0.05, **p<0.01: Significantly different from the sham control group with Student’s t test. #p<0.05, ##p<0.01: Significantly different from the CCI control group with Dunnett’s test
Discussion
The present study indicated neuropathic pain-induced anxiety- or depression-related behaviours in a time-dependent manner. Indeed, CCI model rats developed sustained tactile allodynia within 2 weeks after model preparation, whereas anxiety-related behaviours were observed in the elevated plus maze test at 5 weeks but not at 4 weeks after model preparation. Furthermore, a prolonged immobility time as one of the depression-related behaviours was not observed in the forced swimming test even 6 or 8 weeks after model preparation. Similar phenomena have been reported in sciatic nerve cuffing model mice (Benbouzid et al. 2008) and partial sciatic nerve ligation model rats (Wang et al. 2015), in which anxiety-related behaviours but not depression-related behaviours developed under conditions of chronic neuropathic pain. On the other hand, it has been reported that depression-related behaviours tend to develop later than anxiety-related behaviours in sciatic nerve cuffing model mice (Yalcin et al. 2011). Therefore, it remains possible that depression-related behaviours might be observed more than 8 weeks after model preparation under our experimental conditions. In addition to the above findings, some studies have demonstrated the time-dependent development of anxiety- or depression-related behaviours in neuropathic pain models (Narita et al. 2006; Suzuki et al. 2007; Sawada et al. 2014; Yalcin et al. 2014; Alba-Delgado et al. 2016) and chronic inflammatory pain models (Narita et al. 2006; Wu et al. 2017), indicating that time might be one of the critical points in chronic pain-induced psychiatric disorders.
In the pharmacological evaluation, mirogabalin alleviated tactile allodynia in CCI rats as in the other experimental neuropathic pain models, such as streptozotocin-induced diabetes, partial sciatic nerve ligation and spinal cord injury rats (Domon et al. 2018a, b). Furthermore, mirogabalin alleviated anxiety-related behaviours in CCI rats in the elevated plus maze test. In addition, an interesting finding in this study was the decreased locomotor activity in CCI rats (i.e., decreases in the number of entries into each arm and distance travelled in each arm) and the effect of mirogabalin on that. In spite of sustained neuropathic pain state from 2 weeks to 5 weeks after model preparation, the decreased locomotor activity was observed at week 5 but not at week 4. Furthermore, the decreased locomotor activity was only observed in the open arms and platform but not in the closed arms even at week 5 in experiment 1. These results suggest that the decreased locomotor activity in CCI rats in the elevated plus maze test is one of anxiety-related behaviours, not pain-related behaviours, and that the recovery effect of mirogabalin on decreased locomotor activity depends on its anxiolytic activity rather than analgesic activity.
The standard α2δ ligands, gabapentin and pregabalin, have been reported to have anxiolytic effects in normal animals (Field et al. 2001; Belliotti et al. 2005; Lotarski et al. 2011) and experimental neuropathic pain models, such as CCI (Roeska et al. 2008; Grégoire et al. 2012; Harte et al. 2016), spinal nerve transection (Hasnie et al. 2007), partial sciatic nerve ligation (La Porta et al. 2016), spinal cord injury (Baastrup et al. 2011) and anti-retroviral-associated neuropathy (Wallace et al. 2008). In fact, pregabalin has been licenced and used for the treatment of generalised anxiety disorder in the EU (Montgomery 2006; Frampton 2014). In our preliminary study (Supplementary material), mirogabalin showed an anxiolytic effect in normal rats in the elevated plus maze test, and the effect of mirogabalin 10 mg/kg was comparable to that of diazepam 3 mg/kg. Interestingly, the anxiolytic effect of mirogabalin was relatively short-lasting compared with its sustained analgesic effects. The anxiolytic effect of mirogabalin 10 mg/kg peaked at 2 h after administration and returned to the control level after 4 h (Supplementary Fig. 1, top). By contrast, the analgesic effects of mirogabalin peaked at 4 h after administration and remained until 8 h or more after administration (Domon et al. 2018a, b). Therefore, time-course changes of the anxiolytic effects of mirogabalin were different from those of the analgesic effects. Similar findings were obtained for pregabalin. The analgesic effects of pregabalin peaked at 4 h after administration (Domon et al. 2018a, b), whereas the maximum anxiolytic effect of pregabalin 30 mg/kg was observed 1 h after administration and diminished after 2 h (Supplementary Fig. 1, bottom). Taken together, the discrepancy in time-course changes between anxiolytic and analgesic effects suggests that the anxiolytic effects of α2δ ligands are not dependent on their analgesic effects. It is not clear yet whether the anxiolytic effects of mirogabalin in CCI model rats depend on the primary anxiolytic activity or secondary effects via the analgesic activity. However, at least in part, the primary anxiolytic activity of mirogabalin is considered to contribute to the alleviation of anxiety-related behaviours in CCI rats. Anxiety disorders are highly prevalent in neuropathic pain patients and negatively influence the quality of life (Gustorff et al. 2008; Attal et al. 2011; Radat et al. 2013; Schaefer et al. 2014; Failde et al. 2018). The analgesics with anxiolytic effects might offer a significant benefit for neuropathic pain therapy.
In conclusion, CCI model rats showed anxiety-related behaviours in a time-dependent manner in the elevated plus maze test. Mirogabalin alleviated both the anxiety-related behaviours and tactile allodynia in CCI model rats. Mirogabalin may thus provide effective anxiety relief as well as pain relief in patients with neuropathic pain.
References
Alba-Delgado C, Cebada-Aleu A, Mico JA, Berrocoso E (2016) Comorbid anxiety-like behavior and locus coeruleus impairment in diabetic peripheral neuropathy: a comparative study with the chronic constriction injury model. Prog Neuro-Psychopharmacol Biol Psychiatry 71:45–56. https://doi.org/10.1016/j.pnpbp.2016.06.007
Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T (2010) EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 17:1113–1123. https://doi.org/10.1111/j.1468-1331.2010.02999.x
Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D (2011) The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain 152:2836–2843. https://doi.org/10.1016/j.pain.2011.09.014
Baastrup C, Jensen TS, Finnerup NB (2011) Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res 1370:129–135. https://doi.org/10.1016/j.brainres.2010.11.008
Belliotti TR, Capiris T, Ekhato IV, Kinsora JJ, Field MJ, Heffner TG, Meltzer LT, Schwarz JB, Taylor CP, Thorpe AJ, Vartanian MG, Wise LD, Zhi-Su T, Weber ML, Wustrow DJ (2005) Structure-activity relationships of pregabalin and analogues that target the α2-δ protein. J Med Chem 48:2294–2307. https://doi.org/10.1021/jm049762l
Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, Freund-Mercier MJ, Barrot M (2008) Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain 12:591–599. https://doi.org/10.1016/j.ejpain.2007.10.002
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107. https://doi.org/10.1016/0304-3959(88)90209-6
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. https://doi.org/10.1016/0165-0270(94)90144-9
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN (2017) Neuropathic pain. Nat Rev Dis Primers 3:17002. https://doi.org/10.1038/nrdp.2017.2
Cruccu G, Truini A (2017) A review of neuropathic pain: from guidelines to clinical practice. Pain Ther 6(Suppl 1):S35–S42. https://doi.org/10.1007/s40122-017-0087-0
Deeks ED (2019) Mirogabalin: first global approval. Drugs. 79:463–468. https://doi.org/10.1007/s40265-019-01070-8
Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462. https://doi.org/10.1146/annurev.pa.20.040180.002301
Domon Y, Arakawa N, Inoue T, Matsuda F, Takahashi M, Yamamura N, Kai K, Kitano Y (2018a) Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. J Pharmacol Exp Ther 365:573–582. https://doi.org/10.1124/jpet.117.247551
Domon Y, Kitano Y, Makino M (2018b) Analgesic effects of the novel α2δ ligand mirogabalin in a rat model of spinal cord injury. Pharmazie 73:659–661. https://doi.org/10.1691/ph.2018.8550
Dooley DJ, Taylor CP, Donevan S, Feltner D (2007) Ca2+ channel α2δ ligands: novel modulators of neurotransmission. Trends Pharmacol Sci 28:75–82. https://doi.org/10.1016/j.tips.2006.12.006
Failde I, Dueñas M, Ribera MV, Gálvez R, Mico JA, Salazar A, de Sola H, Pérez C (2018) Prevalence of central and peripheral neuropathic pain in patients attending pain clinics in Spain: factors related to intensity of pain and quality of life. J Pain Res 11:1835–1847. https://doi.org/10.2147/JPR.S159729
Field MJ, Oles RJ, Singh L (2001) Pregabalin may represent a novel class of anxiolytic agents with a broad spectrum of activity. Br J Pharmacol 132:1–4. https://doi.org/10.1038/sj.bjp.0703794
Frampton JE (2014) Pregabalin: a review of its use in adults with generalized anxiety disorder. CNS Drugs 28:835–854. https://doi.org/10.1007/s40263-014-0192-0
Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN (1996) The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem 271:5768–5776. https://doi.org/10.1074/jbc.271.10.5768
Grégoire S, Michaud V, Chapuy E, Eschalier A, Ardid D (2012) Study of emotional and cognitive impairments in mononeuropathic rats: effect of duloxetine and gabapentin. Pain 153:1657–1663. https://doi.org/10.1016/j.pain.2012.04.023
Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, Rieder A (2008) Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand 52:132–136. https://doi.org/10.1111/j.1399-6576.2007.01486.x
Harte SE, Meyers JB, Donahue RR, Taylor BK, Morrow TJ (2016) Mechanical conflict system: a novel operant method for the assessment of nociceptive behavior. PLoS One 11:e0150164. https://doi.org/10.1371/journal.pone.0150164
Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, Kinchington PR, Dickenson AH, Pheby T, Rice AS (2007) Further characterization of a rat model of varicella zoster virus-associated pain: relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience 144:1495–1508. https://doi.org/10.1016/j.neuroscience.2006.11.029
Jensen TS, Baron R, Haanpää M, Kalso E, Loeser JD, Rice AS, Treede RD (2011) A new definition of neuropathic pain. Pain 152:2204–2205. https://doi.org/10.1016/j.pain.2011.06.017
La Porta C, Lara-Mayorga IM, Negrete R, Maldonado R (2016) Effects of pregabalin on the nociceptive, emotional and cognitive manifestations of neuropathic pain in mice. Eur J Pain 20:1454–1466. https://doi.org/10.1002/ejp.868
Li Z, Taylor CP, Weber M, Piechan J, Prior F, Bian F, Cui M, Hoffman D, Donevan S (2011) Pregabalin is a potent and selective ligand for α2δ-1 and α2δ-2 calcium channel subunits. Eur J Pharmacol 667:80–90. https://doi.org/10.1016/j.ejphar.2011.05.054
Lotarski SM, Donevan S, El-Kattan A, Osgood S, Poe J, Taylor CP, Offord J (2011) Anxiolytic-like activity of pregabalin in the Vogel conflict test in α2δ-1 (R217A) and α2δ-2 (R279A) mouse mutants. J Pharmacol Exp Ther 338:615–621. https://doi.org/10.1124/jpet.111.180976
Montgomery SA (2006) Pregabalin for the treatment of generalised anxiety disorder. Expert Opin Pharmacother 7:2139–2154. https://doi.org/10.1517/14656566.7.15.2139
Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T (2006) Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 31:739–750. https://doi.org/10.1038/sj.npp.1300858
O’Connor AB, Dworkin RH (2009) Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 122(10 Suppl):S22–S32. https://doi.org/10.1016/j.amjmed.2009.04.007
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Radat F, Margot-Duclot A, Attal N (2013) Psychiatric co-morbidities in patients with chronic peripheral neuropathic pain: a multicentre cohort study. Eur J Pain 17:1547–1557. https://doi.org/10.1002/j.1532-2149.2013.00334.x
Roeska K, Doods H, Arndt K, Treede RD, Ceci A (2008) Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain 139:349–357. https://doi.org/10.1016/j.pain.2008.05.003
Saeki K, Yasuda SI, Kato M, Kano M, Domon Y, Arakawa N, Kitano Y (2019) Analgesic effects of mirogabalin, a novel ligand for α2δ subunit of voltage-gated calcium channels, in experimental animal models of fibromyalgia. Naunyn Schmiedeberg's Arch Pharmacol 392:723–728. https://doi.org/10.1007/s00210-019-01628-z
Sawada A, Niiyama Y, Ataka K, Nagaishi K, Yamakage M, Fujimiya M (2014) Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain 155:1762–1772. https://doi.org/10.1016/j.pain.2014.05.031
Schaefer C, Mann R, Sadosky A, Daniel S, Parsons B, Nieshoff E, Tuchman M, Nalamachu S, Anschel A, Stacey BR (2014) Burden of illness associated with peripheral and central neuropathic pain among adults seeking treatment in the United States: a patient-centered evaluation. Pain Med 15:2105–2119. https://doi.org/10.1111/pme.12502
Shimamura M, Kuratani K, Kinoshita M (2007) A new automated and high-throughput system for analysis of the forced swim test in mice based on magnetic field changes. J Pharmacol Toxicol Methods 55:332–336. https://doi.org/10.1016/j.vascn.2006.11.003
Stahl SM, Porreca F, Taylor CP, Cheung R, Thorpe AJ, Clair A (2013) The diverse therapeutic actions of pregabalin: is a single mechanism responsible for several pharmacological activities? Trends Pharmacol Sci 34:332–339. https://doi.org/10.1016/j.tips.2013.04.001
Suzuki T, Amata M, Sakaue G, Nishimura S, Inoue T, Shibata M, Mashimo T (2007) Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg 104:1570–1577. https://doi.org/10.1213/01.ane.0000261514.19946.66
Wallace VC, Segerdahl AR, Blackbeard J, Pheby T, Rice AS (2008) Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci Lett 448:153–156. https://doi.org/10.1016/j.neulet.2008.10.005
Wang XQ, Zhong XL, Li ZB, Wang HT, Zhang J, Li F, Zhang JY, Dai RP, Xin-Fu Z, Li CQ, Li ZY, Bi FF (2015) Differential roles of hippocampal glutamatergic receptors in neuropathic anxiety-like behavior after partial sciatic nerve ligation in rats. BMC Neurosci 16:14. https://doi.org/10.1186/s12868-015-0150-x
Wu Y, Yao X, Jiang Y, He X, Shao X, Du J, Shen Z, He Q, Fang J (2017) Pain aversion and anxiety-like behavior occur at different times during the course of chronic inflammatory pain in rats. J Pain Res 10:2585–2593. https://doi.org/10.2147/JPR.S139679
Yalcin I, Barthas F, Barrot M (2014) Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci Biobehav Rev 47:154–164. https://doi.org/10.1016/j.neubiorev.2014.08.002
Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, Barrot M (2011) A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry 70:946–953. https://doi.org/10.1016/j.biopsych.2011.07.017
Acknowledgements
We thank Asuka Kawamura and Kousei Shimada for help with the chemical synthesis. We also thank Miki Sugiyama, Katsuhiro Higuchi and Yuri Noda for their expertise in the experiments. In addition, we thank Masami Kato, Mayumi Kano, Jun Harada, Mitsuhiro Makino, Kaori Ito, Yuki Domon, Naohisa Arakawa and Kazufumi Kubota for all of their support in this study. Finally, we wish to express our gratitude to Scientific Language Co., Ltd. (Ibaraki, Japan), for reviewing and editing this manuscript.
Author information
Authors and Affiliations
Contributions
HM and YK conceived and designed the research. HM, HK and KS performed the experiments and analysed the data. HM and YK wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
All animal experiments were conducted in accordance with the Guidelines for Management and Welfare of Experimental Animals (Hashima Laboratory, Nihon Bioresearch Inc.; April 2, 2007, modified on August 27, 2010), and the Guidelines of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co., Ltd. This article does not describe any studies with human participants performed by any of the authors.
Conflict of interest
HM, HK and KS are employees of Nihon Bioresearch Inc., while YK is an employee of Daiichi Sankyo Co., Ltd. This study was sponsored by Daiichi Sankyo Co., Ltd.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 55.7 kb)
Rights and permissions
About this article
Cite this article
Murasawa, H., Kobayashi, H., Saeki, K. et al. Anxiolytic effects of the novel α2δ ligand mirogabalin in a rat model of chronic constriction injury, an experimental model of neuropathic pain. Psychopharmacology 237, 189–197 (2020). https://doi.org/10.1007/s00213-019-05356-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05356-3