Abstract
Rationale
The endocannabinoid system plays a crucial role in the control of emotionality and recent clinical findings have shown that heavy prenatal exposure to cannabis is significantly associated with self-reported anxiety symptoms in exposed children. However, the long-term neurobehavioral consequences of in utero exposure to low–moderate doses of cannabinoid compounds have never been investigated.
Objective
The objective of this study was to investigate whether perinatal exposure to moderate doses of the active constituent of cannabis, the CB1 cannabinoid receptor agonist delta-9-tetrahydrocannabinol (THC), influences the emotional reactivity of rat offspring.
Methods
Primiparous Wistar rats were treated during pregnancy and lactation with doses of THC equivalent to the current estimates of moderate cannabis consumption in humans (2.5–5 mg kg−1, per os, from gestational day 15 to postnatal day 9). The emotional reactivity of infant, adolescent, and adult offspring was investigated using the isolation-induced ultrasonic vocalization, social interaction, and elevated plus-maze tests, respectively.
Results
Perinatal THC treatment did not affect parameters of reproduction; however, at the dose of 5 mg kg−1, it increased the number of ultrasounds emitted by rat pups removed from the nest, inhibited social interaction and play behavior in the adolescent offspring, and induced an anxiogenic-like profile in the adult offspring tested in the elevated plus-maze test.
Conclusion
These results suggest that the endocannabinoid system is involved in the control of emotionality since early developmental stages. Thus, even moderate doses of cannabinoid compounds, when administered during the perinatal period, can have profound consequences for brain maturation, leading to long-lasting neurodevelopmental alterations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several findings support the hypothesis of an important role of the endocannabinoid system in the modulation of emotional states (Millan 2003; Witkin et al. 2005). Firstly, CB1 cannabinoid receptors are highly expressed in brain areas involved in the modulation of emotionality (Ameri 1999; Davies et al. 2002). Secondly, endocannabinoids, produced by activated neurons, act presynaptically modulating the release of several neurotransmitters and neuropeptides that play a key role in anxiety (Hermann et al. 2002; Katona et al. 2001; Schlicker and Kathmann 2001; Tsou et al. 1998; Ameri 1999; Rodriguez de Fonseca et al. 1997). Thirdly, several studies in cannabis users have shown that the consumption of marijuana produces a wide range of subjective emotional effects (Tournier et al. 2003; Wachtel et al. 2002). These observations have their counterpart in animal studies, showing that cannabinoids elicit dose-dependent and environment-dependent anxiolytic and/or anxiogenic effects in rodent models of anxiety (Martin et al. 2002; Onaivi et al. 1990; Rodriguez de Fonseca et al. 1997; Rodriguez de Fonseca et al. 1996).
The endocannabinoid system plays specific roles during early developmental stages (Fernandez-Ruiz et al. 2000). Several studies have described the presence of the CB1 receptor (Rodriguez de Fonseca et al. 1993) and its endogenous ligands (Paria and Dey 2000; Berrendero et al. 1999) in the developing brain. The atypical, transient localization of CB1 receptors during the perinatal period suggests a specific involvement of the endocannabinoid system in brain development (Fernandez-Ruiz et al. 2000). However, despite this evidence, the effects of cannabis exposure during critical developmental periods are still poorly understood. From a clinical point of view, this lack of information is of particular concern because, among the social problems related to marijuana abuse, the increasing consumption of cannabis derivatives in pregnant women is noteworthy (Fried and Smith 2001; Goldschmidt et al. 2000; Sloan et al. 1992). This increases the urgency to understand the effects of cannabis exposure on the fetus.
Cannabinoids can be transferred from the mother to the offspring through the placental blood during gestation and through the maternal milk during lactation (Fernandez-Ruiz et al. 2004; Hutchings et al. 1989; Jakubovic et al. 1977). In this way, as CB1 receptors are already present during development (Buckley et al. 1998), marijuana exposure during pregnancy and/or lactation could interfere with the sequence of events occurring during the ontogeny of the central nervous system (CNS), thus, possibly, leading to the onset of neurodevelopmental alterations.
Some clinical studies have investigated the effects of in utero exposure to cannabis in humans (Fried 1980; 1989a, b). Only few of them, however, focused on children past the age of four or five (Fried 2002a, b; Fried et al. 2003). This is due to the different confounding factors and many socioeconomic variables that make follow-up studies of children prenatally exposed to drugs of abuse until adulthood particularly difficult.
In this scenario, animal models may provide a useful tool for examining the potential long-term effects of in utero exposure to cannabis derivates in the offspring. Animal studies of prenatal exposure to cannabinoids have revealed long-term effects on functional regulation of motor behaviors, learning and memory processes, as well as nociception (Antonelli et al. 2005; Fride and Mechoulam 1996; Mereu et al. 2003; Moreno et al. 2003; Navarro et al. 1995; Rubio et al. 1995). Furthermore, it has been shown that prenatal exposure to a moderate dose of WIN55,212-2, a synthetic cannabinoid agonist, alters emotional reactivity in 10-day-old pups (Antonelli et al. 2005). However, the long-term changes of emotionality induced by in utero exposure to moderate doses of cannabinoids have not been studied yet. This observation prompted us to investigate, in a longitudinal behavioral study, the long-term consequences of perinatal exposure to moderate doses of delta-9-tetrahydrocannabinol (THC) on the emotional reactivity of the offspring. To this aim, primiparous Wistar rats were treated during pregnancy (from day 15 of gestation) and lactation (until day 9 after parturition) with doses of THC equivalent to the current estimates of low to moderate cannabis consumption in humans (Molina-Holgado et al. 1996). This temporal window of THC exposure was chosen because in terms of brain development this time period roughly corresponds to the second half of pregnancy in humans (Maier et al. 1999). The emotional reactivity of infant, adolescent, and adult offspring was then investigated using the isolation-induced ultrasonic vocalization (USV), social interaction, and elevated plus-maze tests.
Materials and methods
Animals and exposure conditions
Experiments were performed in accordance with the “principles of laboratory animal care” promulgated by the Italian Ministry of Health (Decreto Legislativo 116/92 and Decreto Legislativo 111/94-B), the Declaration of Helsinki, and the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health (USA).
Primiparous pregnant female Wistar rats weighing 250–280 g were purchased from Harlan (Milan, Italy) and were individually housed in 42 × 27 × 14-cm Plexiglas boxes in air-conditioned rooms (temperature 21 ± 1°C, relative humidity 60 ± 10%; lights on from 8:00 a.m. to 8:00 p.m.). Pellet food (Morini, San Polo D’Enza, Italy) and tap water were available ad libitum.
Pregnant rats received a daily dose of THC (2.5–5 mg kg−1) orally administered through a buccopharyngeal cannula from gestational day (GD) 15 to postnatal day (PND) 9. The highest dose administered is equivalent to the current estimates of moderate exposure to THC in humans, correcting for differences in route of administration and body surface area (Molina-Holgado et al. 1996). THC (Sigma, Milan, Italy) was dissolved in sesame oil and prepared as described before (Molina-Holgado et al. 1993). Control pregnant rats received the same volume of vehicle.
Newborn litters found up to 5.00 p.m. were considered to be born on that day (PND 0). On PND 1, all litters were reduced to a standard size of eight pups per litter (six males and two females). On PND 21, pups were weaned and housed in groups of three in 42 × 27 × 14-cm Plexiglas boxes in air-conditioned rooms (temperature 21 ± 1°C, relative humidity 60 ± 10%; lights on from 8:00 a.m. to 8:00 p.m.). One male pup per litter from different litters per treatment group was used in each experiment. Each male rat was tested only once.
Reproduction data
Body weights of the dams were taken daily throughout pregnancy (from GD 0 to GD 20). The number of dams giving birth and the length of pregnancy were determined. Litter size at birth, body weights of male pups, and postnatal mortality (the number of male pups that died before weaning) were also measured.
Isolation-induced ultrasonic vocalizations
USVs are emitted by rodent pups when removed from the nest and play an important communicative role in mother–offspring interactions (Branchi et al. 2006). The rate of USVs follows an ontogenetic profile. In rats, it increases during the first days of life, reaching a peak around PND 10. It then starts to decrease, completely disappearing around PND 17 to 20 (Branchi et al. 2006).
On PND 12, USVs of vehicle- and THC-treated pups were recorded according to the procedure described by Calamandrei et al. (1999) and Tattoli et al. (2001).
Apparatus
Subjects were tested in a Plexiglas arena (30 × 30 × 30 cm) placed inside a temperature-controlled room. USVs were detected by an ultrasonic microphone fixed at 15 cm above the arena (SM2, Ultrasound Advice) connected to a Bat Detector (US 30 Ultrasound Advice) tuned to 30 ± 10 kHz and connected to a high-speed tape recorder (Racal Store).
Procedure
One male per litter for each treatment group was randomly removed from the nest and placed in the center of the arena. The recording session lasted 3 min. Number of USVs was manually and independently recorded by three independent experimenters blind to the treatment, by listening to the audible output of the tape recorder through headphones (Philips HI-FI stereo SHP9000). Moreover, crossings of square limits with both forepaws were recorded for 3 min (Laviola et al. 1988). Axillary temperature was measured at the end of the test by means of a digital thermometer provided with a rat probe.
Social interaction
The social interaction test was carried out at PND 35 because a high degree of social interactions characterizes adolescent rodents (Vanderschuren et al. 1997). Briefly, 24 h prior to the test, all subjects were weighed and individually housed in cages identical to the home cage, containing some of their own sawdust. At the time of test, each unfamiliar pair (perinatal THC-treated experimental rat and same sex, age, and strain naïve unfamiliar partner) was placed in a test cage identical to the home cage, with new sawdust as bedding, and allowed to interact for 15 min. The behaviors of the animals were videotaped using a video camera, videotape recorder, and television monitor. Analysis from the videotape recordings was performed afterwards by the same observer, who was unaware of animal treatment, using the Observer 3.0 software (Noldus Information Technology B.V., Wageningen, The Netherlands). The following behavioral elements were scored per 15 min:
-
Play-related behaviors: pouncing (i.e., when one animal attempts to nose or rub the nape of the neck of the play partner), which is the clearest index of play solicitation; boxing–wrestling (a group of playful activities including boxing, wrestling, and charging); pinning (i.e., the most common terminal component of a play bout, in which one animal stands over a supine partner), which is the consummatory measure of play;
-
Social behaviors unrelated to play: social exploration (sniffing any part of the body of the test partner, including the anogenital area); social grooming (chewing and licking the fur of the partner, during which the animal that grooms mostly places its forepaws on the back of the neck of the partner); crawling over and under the test partner.
Elevated plus maze
The elevated plus-maze apparatus comprised two open arms (50 × 10 × 0 cm) and two closed arms (50 × 10 × 40 cm) that extended from a common central platform (10 × 10 cm). The apparatus, made of Plexiglas (gray floor, clear walls), was elevated to a height of 60 cm above the floor level. A video camera above the maze was connected to a television monitor connected to a video recorder.
The elevated plus-maze test was performed following the procedure described by Pellow and File (1986) and modified by Bortolato et al. (2006). Briefly, on PND 80, rats were individually placed on the central platform facing a closed arm and a 5-min test period was recorded on videotape for subsequent analysis. Immediately after each session, the apparatus was thoroughly cleaned with cotton pads wetted with 70% ethanol–water solution and dried. Behavioral analyses were carried out by the same observer, who was unaware of animal treatment, using the Observer 3.0 software (Noldus Information Technology B.V., Wageningen, The Netherlands). The following parameters were analyzed:
-
(a)
% Time spent on the open arms (% TO), calculated as the amount of time spent on the open arms of the maze per 5 min. Time on the open arms was timed from the moment that all four paws of the rat were placed on an open arm;
-
(b)
% Open entries (% OE), calculated as the number of entries into the open arms of the maze per number of entries into open + closed arms;
-
(c)
Number of total arm entries (open + closed arm entries);
-
(d)
Number of exploratory head dippings (HDIPS) made over the edge of the open arms;
-
(e)
Number of stretched-attend postures (SAP) made from the exit of a closed towards an open arm. This exploratory posture can be described as a forward elongation of the body, with static hindquarters, followed by a retraction to the original position.
Statistical analysis
All data are presented as mean ± SEM (n = 20–26 for vehicle group; n = 12 for THC 2.5 group; n = 12 for THC 5 group). Reproduction data and all parameters measured in the isolation-induced ultrasonic vocalization, social interaction, and elevated plus-maze tests were analyzed using one-way analysis of variance (Anova), followed by Tukey’s post hoc test where appropriate.
Results
Reproduction data
No differences were observed in the body weight gains of THC-treated dams during gestation as compared to the control-treated animals (Table 1). Moreover, perinatal exposure to THC did not affect pregnancy length, litter size at birth, pup weight gain, and postnatal mortality (Table 1).
Isolation-induced ultrasonic vocalizations
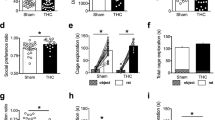
One-way Anova revealed that 12-day-old pups exposed to THC during pregnancy and lactation displayed increased anxiety in the isolation-induced ultrasonic vocalization test (F (2, 47) = 4.43, p < 0.05). Tukey’s post hoc comparison revealed that perinatal exposure to THC (5 mg kg−1) significantly increased the number of ultrasounds emitted by rat pups removed from the nest (Fig. 1a). This effect was not due to changes in body temperature, as one-way Anova for axillary’s temperature revealed no significant differences among groups. Moreover, the increased rate of ultrasonic emission in 12-day-old offspring was not accompanied by any changes in locomotor activity, as one-way Anova for the number of arena crossings showed no significant differences between the treatment groups (Fig. 1b).
Results obtained in the isolation-induced ultrasonic vocalization test carried out in 12-day-old offspring. Perinatal THC exposure significantly increased the number of ultrasounds emitted by 12-day-old rat pups removed from the nest (a). This effect was not due to any change in locomotor activity because the number of crossings was unaffected by perinatal THC treatment (b). Data represent mean values ± SEM. *p < 0.05 vs. control (one-way Anova followed by Tukey’s post hoc test)
Social interaction
Exposure to THC during pregnancy and lactation altered social play behavior in the adolescent offspring. Adolescent rats exposed to THC during pregnancy and lactation were significantly less engaged in play behavior than controls, as pinning (F (2, 41) = 3.70, p < 0.05; p < 0.05 for post hoc comparisons between vehicle and THC 5 mg kg−1; Fig. 2a), pouncing (F (2, 41) = 11.55, p < 0.001; p < 0.01 for post hoc comparisons between vehicle and THC 5 mg kg−1; Fig. 2b), and boxing–wrestling (F (2, 41) = 6.24, p < 0.01; p < 0.01 for post hoc comparisons between vehicle and THC 5 mg kg−1; Fig. 2c) frequencies were significantly reduced in animals treated with THC 5 mg kg−1. Perinatal THC exposure did not affect frequency (Fig. 2d) of social behaviors unrelated to play.
Results obtained in the social interaction test carried out in 35-day-old offspring. Adolescent rats exposed to THC during pregnancy and lactation were significantly less engaged in pinning behavior than controls (a). Pouncing (b) and boxing–wrestling (c) were also reduced in THC-exposed offspring, while social behaviors unrelated to play were unaffected (d). Data represent mean values ± SEM. *p < 0.05, **p < 0.01, vs. control (one-way Anova followed by Tukey’s post hoc test)
Overall, the total time spent in social activity, calculated as the sum of social behaviors both related and unrelated to play, was reduced in adolescent rats treated with THC 5 mg kg−1 (F (2, 41) = 7.59, p < 0.01; data not shown).
Elevated plus maze
The increased emotionality found in the infant and adolescent offspring perinatally exposed to THC was long lasting. In fact, THC perinatal treatment affected the performance of adult rats in the elevated plus-maze test (Fig. 3), with THC 5 mgkg−1 decreasing the percentage of time spent on the open arms (% TO, F (2, 38) = 6.35, p < 0.01; p < 0.01 for post hoc comparisons between vehicle and THC 5 mg kg−1; Fig. 3a) and the percentage of entries in the open arms (% OE, F (2, 38) = 6.47, p < 0.01; p < 0.01 for post hoc comparisons between vehicle and THC 5 mg kg−1; Fig. 3b). Moreover, perinatal treatment with THC 5 mg kg−1 decreased the number of HDIPS (F (2, 38) = 5.5, p < 0.01; p < 0.01 for post hoc comparisons between vehicle and THC 5 mg kg−1; Fig. 3c) and increased the number of SAP (F (2, 38) = 11.78, p < 0.001; p < 0.01 for post hoc comparisons between vehicle and THC 5 mg kg−1; Fig. 3d). The increased emotional reactivity displayed by THC-exposed adult offspring was not secondary to changes in locomotor activity, as the number of total entries was unaffected (Fig. 3e).
a–e Results obtained in the elevated plus-maze test carried out in 80-day-old offspring. Perinatal exposure to THC decreased the percentage of time spent on the open arms (% TO, a) and the percentage of entries in the open arms (% OE, b). Moreover, perinatal THC treatment decreased the number of HDIPS (c) and increased the number of SAP (d). Data represent mean values ± SEM. **p < 0.01 vs. control (one-way Anova followed by Tukey’s post hoc test)
Discussion
The present study provides new evidence that perinatal exposure to THC, at a dose (5 mg kg−1) that is not associated with overt signs of toxicity, produces subtle and enduring neurobehavioral changes in the emotional behavior of the rat offspring. An increased emotionality has been found, indeed, at neonatal, adolescent, and adult ages in rats exposed to THC.
Concerning the neonatal age, we found that 12-day-old pups exposed to THC during the perinatal period display an increased rate of USVs compared to the control group. The USV test has been extensively validated and widely used to investigate the ontogeny of emotionality (Insel et al. 1986). Furthermore, it is considered a useful test, among the few available, in detecting subtle effects of adverse treatment during development (Cuomo et al. 1987; Branchi et al. 2006). USVs are emitted by rodent pups in response to separation from the mother and the nest and play an important communicative role in mother–offspring interaction. They are, indeed, a potent stimulus for maternal retrieval and elicit caregiving behaviors in the dam (Farrell and Alberts 2002; Noirot 1972). As high rates of USVs are generally indicative of an anxiety-like state, the present results show that perinatal exposure to THC induces an increased emotional reactivity of the offspring. Whether this could be due to an effect of the drug on the development of brain areas controlling emotionality, or to a possible direct pharmacological effect of circulating THC in the pup blood remains to be clarified. Anyway, the persistence of increased emotionality up to 80 days of age (71 days after the end of THC exposure) supports the first hypothesis.
The increased USV emission displayed by THC-exposed pups could also be the consequence of an altered maternal responsiveness, which is one of the factors tuning the rate of USV emission of the offspring (D’Amato et al. 2005). It has been suggested that THC disrupts all components of maternal behavior in the postpartum rat (Bromley et al. 1978). Conversely, other authors failed to detect changes in maternal care in rhesus monkeys exposed to THC during pregnancy and lactation (Golub et al. 1981). Furthermore, we should take into account that alterations in the USVs emitted by rat pups influence maternal behavior which, in turn, might affect the behavior of the offspring. Thus, whether the altered emotionality found in THC-exposed rats could be due to a direct effect of the drug on brain areas involved in emotional behavior and/or to an indirect effect of the drug on maternal behavior is an interesting issue which deserves more investigation.
Interestingly, our previous findings (Antonelli et al. 2005) showed a reduction of USVs in rat pups prenatally exposed to the synthetic cannabinoid agonist WIN55,212-2, thus highlighting how different time windows of exposure to a psychotropic agent can induce even opposite neurofunctional effects (Costa et al. 2004). However, differences in cannabinoid agonist used, tested dose (cannabinoids can induce either anxiolytic- or anxiogenic-like behaviors depending on the dose, Millan 2003), and treatment schedule (acute vs. chronic treatment) could also account for the apparent discrepancies between the present study and previous reports (Antonelli et al. 2005; McGregor et al. 1996).
It has been suggested that ultrasonic emission in rodent pups may be related to human infant crying (Elsner et al. 1990) and that particular changes in the acoustic features of the neonatal cry may be an indicator of long-term neurobehavioral alterations caused by adverse pre and postnatal events (Lester 1987; Michelsson et al. 1977).
The alterations observed in the emotional reactivity of THC-exposed pups seem to be long lasting. In fact, in our study, an anxiogenic-like behavior was detected also in the adolescent offspring (PND 35). At this time point, as the pharmacological treatment was stopped more than 20 days before testing, a direct effect of the drug on behavior could be excluded. THC-exposed rats displayed lower social activity than controls in the social interaction test. In particular, all aspects of social play behavior were affected, with a decrease of pinning, pouncing, and boxing–wrestling, without any alteration of other social behaviors unrelated to play. Our results are in agreement with the finding that the synthetic cannabinoid agonist CP 55,940, repeatedly administered during the postnatal period, reduced social interaction in 60-day-old rats (O’Shea et al. 2006).
It has been proven that play deprivation in juvenile rats causes abnormal patterns of social, sexual, and aggressive behaviors in adulthood (Van den Berg et al. 1999). Social play might function to establish social organization, to develop the ability to express and understand intraspecific communicative signals, and to cope with social conflicts. As a consequence, the disturbances of adolescent social play behavior induced by perinatal THC treatment may have profound effects on the development of communicative skills and appropriate behavioral patterns later in life (Vanderschuren et al. 1997).
It has been suggested that three forms of behaviors are associated with the transition from reptiles to mammals: nursing, audiovocal communication necessary to maintain mother–offspring contact and play (MacLean 1990). Nursing, vocalization, and play all share a common motivation for social interaction and, under appropriate circumstances, may lead to social attachment (Insel 2003). Taken together, then, the increased emotional distress displayed by THC-treated pups during acute periods of separation from the nest and the reduced play behavior displayed by THC-treated adolescent offspring highlight how perinatal THC treatment can disrupt affiliative behavior and social attachment in the exposed offspring.
From these results, however, it is not possible to assess whether the altered behavioral profile observed in THC-exposed offspring reflects only a disruption of affiliative behavior and social attachment or whether it is, at the same time, the consequence of increased emotionality caused by perinatal THC exposure. To further address this issue, we tested the offspring in the elevated plus-maze test at adulthood. This test is one of the most popular animal tests of anxiety currently in use. Although it has been frequently used as a tool to screen anxioselective effects of drugs (Handley and Mithani 1984; Pellow et al. 1985; Pellow and File 1986), nowadays its usefulness has spread towards the understanding of the biological basis of emotionality (Adamec et al. 1998; Carobrez et al. 2001; Lamprea et al. 2000; Rasmussen et al. 2001). It has been suggested that the social interaction and the elevated plus-maze tests measure distinct facets of anxiety (i.e., social anxiety in the social interaction test and generalized anxiety in the elevated plus-maze test) that may be differentially susceptible to drug treatments (File and Hyde 1978). In the present study, THC-exposed adult rats showed increased anxiety-like behaviors in the elevated plus-maze test, with respect to control rats. They spent, indeed, more time in the closed arms of the maze, exhibited a significantly lower number of head dippings, and a higher number of SAP than vehicle-exposed rats. The number of total entries, however, was not affected by perinatal treatment, thus confirming that the locomotor activity of the offspring was not compromised by THC exposure. The altered emotional reactivity displayed by THC-exposed adult offspring in the elevated plus-maze test shows that the deleterious neurobehavioral effects of perinatal THC exposure seem to be irreversible.
In the rodent CNS, CB1 receptors have been detected as early as GD 11, where they mediate endocannabinoid regulation of proliferation, migration, specification, and survival of neural progenitors, dictate the phenotypic differentiation of neurons, and control the establishment of synaptic communication (Harkany et al. 2007). The long-term alterations found in the brain of rats perinatally exposed to THC likely reflect alterations at these sites, although other possible molecular targets cannot be excluded.
On the whole, the findings from this longitudinal behavioral study are in accordance with the hypothesis that the endocannabinoid system might be involved in the control of emotional states since early developmental stages and are in line with clinical evidence showing that prenatal exposure to cannabis is associated with child’s self-reported anxiety symptoms (Goldschmidt et al. 2004; Gray et al. 2005; Leech et al. 2006).
References
Adamec R, Kent P, Anisman H, Shallow T, Merali Z (1998) Neural plasticity, neuropeptides and anxiety in animals—implications for understanding and treating affective disorder following traumatic stress in humans. Neurosci Biobehav Rev 23:301–318
Ameri A (1999) The effects of cannabinoids on the brain. Prog Neurobiol 58:315–348
Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, Mazzoni E, Trabace L, Steardo L, Cuomo V, Ferraro L (2005) Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex 15:2013–2020
Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ (1999) Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse 33:181–191
Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D (2006) Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology 31:2652–2659
Branchi I, Santucci D, Alleva E (2006) Analysis of ultrasonic vocalizations emitted by infant rodents. In: Costa LG, Hodgson E, Lawrence DA, Reed DJ (eds) Current protocols in toxicology. Wiley, Hoboken
Bromley BL, Rabii J, Gordon JH, Zimmerman E (1978) Delta-9-tetrahydrocannabinol inhibition of suckling-induced prolactin release in the lactating rat. Endocr Res Commun 5:271–278
Buckley NE, Hansson S, Harta G, Mezey E (1998) Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 82:1131–1149
Calamandrei G, Venerosi A, Branchi I, Valanzano A, Puopolo M, Alleva E (1999) Neurobehavioral effects of prenatal lamivudine (3TC) exposure in preweaning mice. Neurotoxicol Teratol 21:365–373
Carobrez AP, Teixeira KV, Graeff FG (2001) Modulation of defensive behavior by periaqueductal gray NMDA/glycine-B receptor. Neurosci Biobehav Rev 25:697–709
Costa LG, Steardo L, Cuomo V (2004) Structural effects and neurofunctional sequelae of developmental exposure to psychotherapeutic drugs: experimental and clinical aspects. Pharmacol Rev 56:103–147
Cuomo V, De Salvia MA, Maselli MA, Santo L, Cagiano R (1987) Ultrasonic calling in rodents: a new experimental approach in behavioural toxicology. Neurotoxicol Teratol 9:157–160
D’Amato FR, Scalera E, Sarli C, Moles A (2005) Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet 35:103–112
Davies SN, Pertwee RG, Riedel G (2002) Functions of cannabinoid receptors in the hippocampus. Neuropharmacology 42:993–1007
Elsner J, Suter D, Alder S (1990) Microanalysis of ultrasound vocalizations of young rats: assessment of the behavioral teratogenicity of methylmercury. Neurotoxicol Teratol 12:7–14
Farrell WJ, Alberts JR (2002) Stimulus control of maternal responsiveness to Norway rat (Rattus norvegicus) pup ultrasonic vocalizations. J Comp Psychol 116:297–307
Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA (2000) The endogenous cannabinoid system and brain development. Trends Neurosci 23:14–20
Fernandez-Ruiz J, Gomez M, Hernandez M, de Miguel R, Ramos JA (2004) Cannabinoids and gene expression during brain development. Neurotox Res 6:389–401
File SE, Hyde JR (1978) Can social interaction be used to measure anxiety? Br J Pharmacol 62:19–24
Fride E, Mechoulam R (1996) Developmental aspects of anandamide: ontogeny of response and prenatal exposure. Psychoneuroendocrinology 21:157–172
Fried PA (1980) Marihuana use by pregnant women: neurobehavioral effects in neonates. Drug Alcohol Depend 6:415–424
Fried PA (1989a) Cigarettes and marijuana: are there measurable long-term neurobehavioral teratogenic effects? Neurotoxicology 10:577–583
Fried PA (1989b) Postnatal consequences of maternal marijuana use in humans. Ann N Y Acad Sci 562:123–132
Fried PA (2002a) Adolescents prenatally exposed to marijuana: examination of facets of complex behaviors and comparisons with the influence of in utero cigarettes. J Clin Pharmacol 42:97S–102S
Fried PA (2002b) Conceptual issues in behavioral teratology and their application in determining long-term sequelae of prenatal marihuana exposure. J Child Psychol Psychiatry 43:81–102
Fried PA, Smith AM (2001) A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol 23:1–11
Fried PA, Watkinson B, Gray R (2003) Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol 25:427–436
Goldschmidt L, Day NL, Richardson GA (2000) Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol 22:325–336
Goldschmidt L, Richardson GA, Cornelius MD, Day NL (2004) Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol Teratol 26:521–532
Golub MS, Sassenrath EN, Chapman LF (1981) Mother–infant interaction in rhesus monkeys treated clinically with delta-9-tetrahydrocannabinol. Child Dev 52:389–392
Gray KA, Day NL, Leech S, Richardson GA (2005) Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol 27:439–448
Handley SL, Mithani S (1984) Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol 327:1–5
Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci 28:83–92
Hermann H, Marsicano G, Lutz B (2002) Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109:451–460
Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T (1989) Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sci 44:697–701
Insel TR (2003) Is social attachment an addictive disorder? Physiol Behav 79:351–357
Insel TR, Hill JL, Mayor RB (1986) Rat pup ultrasonic isolation calls: possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav 24:1263–1267
Jakubovic A, Hattori T, McGeer PL (1977) Radioactivity in suckled rats after giving 14C-tetrahydrocannabinol to the mother. Eur J Pharmacol 22:221–223
Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF (2001) Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci 21:9506–9518
Lamprea MR, Cardenas FP, Silveira R, Morato S, Walsh TJ (2000) Dissociation of memory and anxiety in a repeated elevated plus maze paradigm: forebrain cholinergic mechanisms. Behav Brain Res 117:97–105
Laviola G, Renna G, Bignami G, Cuomo V (1988) Ontogenetic and pharmacological dissociation of various components of locomotor activity and habituation in the rat. Int J Dev Neurosci 6:431–438
Leech S, Larkby CA, Day R, Day NL (2006) Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry 45:223–230
Lester BM (1987) Developmental outcome prediction from acoustic cry analysis in term and preterm infants. Pediatrics 80:529–534
MacLean P (1990) The triune brain in evolution: role in paleocerebral functions. Plenum, New York
Maier SE, Miller JA, Blackwell JM, West JR (1999) Fetal alcohol exposure and temporal vulnerability: regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exper Res 23:726–734
Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O (2002) Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 159:379–387
McGregor IS, Dastur FN, McLellan RA, Brown RE (1996) Cannabinoid modulation of rat pup ultrasonic vocalizations. Eur J Pharmacol 313:43–49
Mereu G, Fa M, Ferraro L, Cagiano R, Antonelli T, Tattoli M, Ghiglieri V, Tanganelli S, Gessa GL, Cuomo V (2003) Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A 100:4915–4920
Michelsson K, Sirviö P, Wasz-Höckert O (1977) Sound spectrographic cry analysis of infants with bacterial meningitis. Dev Med Child Neurol 3:309–315
Millan MJ (2003) The neurobiology and control of anxious states. Prog Neurobiol 70:83–244
Molina-Holgado F, Molina-Holgado E, Leret ML, Gonzalez MI, Reader TA (1993) Distribution of indoleamines and [3H]paroxetine binding in rat brain regions following acute or perinatal delta 9-tetrahydrocannabinol treatments. Neurochem Res 18:1183–1191
Molina-Holgado F, Amaro A, Gonzalez MI, Alvarez FJ, Leret ML (1996) Effect of maternal delta 9-tetrahydrocannabinol on developing serotonergic system. Eur J Pharmacol 316:39–42
Moreno M, Trigo JM, Escuredo L, Rodriguez de Fonseca F, Navarro M (2003) Perinatal exposure to delta 9-tetrahydrocannabinol increases presynaptic dopamine D2 receptor sensitivity: a behavioral study in rats. Pharmacol Biochem Behav 75:565–575
Navarro M, Rubio P, de Fonseca FR (1995) Behavioural consequences of maternal exposure to natural cannabinoids in rats. Psychopharmacology (Berl) 122:1–14
Noirot E (1972) Ultrasounds and maternal behavior in small rodents. Dev Psychobiol 5:371–387
Onaivi ES, Green MR, Martin BR (1990) Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther 253:1002–1009
O’Shea M, McGregor IS, Mallet PE (2006) Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar long-lasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol 20(5):611–621
Paria BC, Dey SK (2000) Ligand-receptor signaling with endocannabinoids in preimplantation embryo development and implantation. Chem Phys Lipids 108:211–220
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24:525–529
Pellow S, Chopin P, File SE, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Rasmussen DD, Mitton DR, Green J, Puchalski S (2001) Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res 25:999–1005
Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ (1993) Presence of cannabinoid binding sites in the brain from early postnatal ages. Neuroreport 4:135–138
Rodriguez de Fonseca F, Rubio P, Menzaghi F, Merlo-Pich E, Rivier J, Koob GF, Navarro M (1996) Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther 276:56–64
Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F (1997) Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276:2050–2054
Rubio P, Rodriguez de Fonseca F, Munoz RM, Ariznavarreta C, Martin-Calderon JL, Navarro M (1995) Long-term behavioral effects of perinatal exposure to delta 9-tetrahydrocannabinol in rats: possible role of pituitary-adrenal axis. Life Sci 56:2169–2176
Schlicker E, Kathmann M (2001) Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol Sci 22:565–572
Sloan LB, Gay JW, Snyder SW, Bales WR (1992) Substance abuse during pregnancy in a rural population. Obstet Gynecol 79:245–248
Tattoli M, Cagiano R, Gaetani S, Ghiglieri V, Giustino A, Mereu G, Trabace L, Cuomo V (2001) Neurofunctional effects of developmental alcohol exposure in alcohol-preferring and alcohol-nonpreferring rats. Neuropsychopharmacology 24:691–705
Tournier M, Sorbara F, Gindre C, Swendsen JD, Verdoux H (2003) Cannabis use and anxiety in daily life: a naturalistic investigation in a non-clinical population. Psychiatry Res 118:1–8
Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411
Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM (1999) Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav Brain Res 106:133–142
Vanderschuren LJ, Niesink RJ, Van Ree JM (1997) The neurobiology of social play behavior in rats. Neurosci Biobehav Rev 21:309–326
Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H (2002) Comparison of the subjective effects of delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 161:331–339
Witkin JM, Tzavara ET, Nomikos GG (2005) A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol 16:315–331
Acknowledgments
We thank Dr. L.J.M.J. Vanderschuren for critical reading of the manuscript and valuable suggestions and Daniela Valeri, Angela Saraceno, and Alessandra Sordi for technical help. This study was supported by grants PRIN 2005 (to M.R.C.), and FIRB 2006 (to V.C.) from Ministero dell’Università e della Ricerca Scientifica-Italy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trezza and Campolongo contributed equally to the present study.
Rights and permissions
About this article
Cite this article
Trezza, V., Campolongo, P., Cassano, T. et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology 198, 529–537 (2008). https://doi.org/10.1007/s00213-008-1162-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1162-3