Abstract
Rationale
Nicotinic agonists may improve attention and memory in humans and may ameliorate some cognitive deficits associated with neuropsychiatric disorders such as schizophrenia.
Materials and methods
We investigated the effects of a single dose of nicotine on episodic memory performance in 10 adults with schizophrenia and 12 healthy controls. Participants were nonsmokers in order to avoid confounding effects of nicotine withdrawal and reinstatement on memory. At each of two study visits, participants performed a test of episodic memory before and 4 h after application of a 14-mg transdermal nicotine (or identical placebo) patch in counterbalanced order.
Results
Compared with placebo, nicotine treatment was associated with more rapid and accurate recognition of novel items. There was a trend for a treatment by diagnosis interaction, such that the effect of nicotine to reduce false alarms was stronger in the schizophrenia than the control group. There was no effect of nicotine on accuracy or reaction time for identification of previously viewed items.
Conclusions
These data suggest that nicotine improves novelty detection in non-smokers, an effect that may be more pronounced in non-smokers with schizophrenia. Because memory deficits are associated with functional impairment in schizophrenia and because impaired novelty detection has been linked to the positive symptoms of schizophrenia, study of the effects of chronic nicotinic agonist treatment on novelty detection may be warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive deficits, including impaired verbal memory, are a significant component of the schizophrenia syndrome and are a strong predictor of functional outcome for those with this disorder (Green 1996). Nicotinic acetylcholine receptor (nAChR) agonists are among several classes of cognitive-enhancing agents under investigation for treatment of cognitive deficits associated with schizophrenia (Levin and Rezvani 2002). Nicotine improves memory in animals (Arendash et al. 1995; Ciamei et al. 2001; Levin and Simon 1998) and improves attention, learning, and memory in healthy adults (Ernst et al. 2001; Lawrence et al. 2002; Levin et al. 1998; Mancuso et al. 1999; McClernon et al. 2003; Rusted et al. 1998; Rusted et al. 2005; Levin et al. 2006). In addition, nicotine has demonstrated benefits in these domains in patients with Alzheimer’s disease (Wilson et al. 1995), age-associated memory impairment (White and Levin 2004), attention deficit hyperactivity disorder (Poltavski and Petros 2006), and depression (McClernon et al. 2006).
Several lines of research suggest that nicotine may be therapeutic in patients with schizophrenia. First, the smoking rate among those with schizophrenia is several-fold higher than in the general population (de Leon et al. 1995; de Leon and Diaz 2005; Hughes 1986), with estimated rates as high as 72–90%, and smokers with schizophrenia smoke each cigarette more intensively and extract more nicotine per cigarette than smokers without psychiatric illness (Olincy et al. 1997; Strand and Nyback 2005; Tidey et al. 2005; Williams et al. 2005). There is evidence for disordered nicotinic neurotransmission in schizophrenia relative to controls, including reduced expression of α4β2 and α7 nAChRs in post-mortem brain tissue (Durany et al. 2000; Freedman et al. 1995) and reduced upregulation of high affinity neuronal nAChR expression in response to smoking (Breese et al. 2000). Third, exogenous nicotine ameliorates some aspects of cognitive and neurophysiologic dysfunction in schizophrenia, including deficits in auditory sensory gating (Adler et al. 1993; Adler et al. 1992), eye tracking (Sherr et al. 2002), working memory (Jacobsen et al. 2004), and attention (Barr et al. 2007; Depatie et al. 2002; Smith et al. 2006). In addition, nicotine deprivation and nAChR blockade impair visuospatial working memory in smokers with schizophrenia but not controls (George et al. 2002; Sacco et al. 2005), although this finding has not been consistently demonstrated (Evins et al. 2005).
These findings support the hypothesis that nAChR agonists may enhance abnormally low nicotinic cholinergic activity in schizophrenia. Perhaps critically, stimulation of presynaptic α4β2 and α7 nAChRs on glutamatergic and dopaminergic neurons increases activity of these neurons in relevant brain regions, including the hippocampus and prefrontal cortex (Janhunen and Ahtee 2007; Kiba and Jayaraman 1994; Lambe et al. 2003; Mansvelder and McGehee 2000; Nomikos et al. 2000; Sziraki et al. 1998), which may have relevance for improving cognitive performance in patients (Laruelle et al. 2003; Newcomer et al. 1999; Olney and Farber 1995). Evidence for a modulatory effect of nicotine on N-methyl-d-aspartate (NMDA) glutamate function at the molecular level has been illustrated via differences in gene expression related to NMDA post-synaptic density and other gene groups in the postmortem hippocampi of smokers and nonsmokers. Most notably, the significant interaction between smoking status and the diagnosis of schizophrenia suggests that nicotine results in differential expression of genes related to hippocampal NMDA glutamate receptor complex function in patients with schizophrenia (Mexal et al. 2005).
There have been few investigations of the effect of nicotine on memory performance in schizophrenia despite strong evidence for impaired verbal memory in this disorder (Cirillo and Seidman 2003) and the important role of the cholinergic system in memory processes (Hasselmo and Giocomo 2006). We therefore conducted a study of the effects of a single dose of transdermal nicotine on recognition memory performance in non-smoking adults with schizophrenia and matched non-psychiatrically ill controls using a double-blind, placebo-controlled, crossover design. This was an ancillary study conducted in a subset of participants in a larger study of the effect of nicotine on cognitive performance in non-smokers (Barr et al. 2007). Non-smokers were studied to avoid the potentially confounding effects of nicotine withdrawal and reinstatement on memory function. Our hypothesis was that nicotine would improve memory accuracy as assessed by increased hit rate, improved source memory accuracy, reduced false alarm rate, and reduced hit reaction time in both groups, with greater improvement expected with nicotine treatment in those with schizophrenia. Secondary analyses were planned for the commonly used signal detection variables of discriminability and response bias.
Materials and methods
The Institutional Review Boards of the Massachusetts General Hospital and the Commonwealth of Massachusetts Department of Mental Health approved the study. All participants provided written informed consent prior to participation.
Participants
Non-smoking adults with a Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association 1994) diagnosis of schizophrenia or schizoaffective disorder, depressed type, were recruited from an urban community mental health clinic. Psychiatric diagnosis was made by a research psychiatrist (AEE, RSB) based on clinical interview and chart review. All patients were on a stable antipsychotic treatment regimen for ≥4 weeks prior to enrollment and remained on these medications during the study. Non-smoking adults without psychiatric illness were recruited from the local community and completed a screening interview using the Structured Clinical Interview for DSM-IV non-patient edition to rule out lifetime history of any Axis I psychiatric disorders and a supplemental questionnaire to rule out schizophrenia spectrum disorders in first-degree relatives.
English was the first-learned language for all participants. Exclusion criteria for both groups included a lifetime diagnosis of cognitive impairment secondary to head injury, dementia, or general medical illness, current diagnosis of substance abuse or dependence (except for caffeine), use of investigational medications in the past month, current diagnosis of major depressive disorder, current unstable medical illness, known allergy to any constituents of the nicotine patch, tobacco smoking within the last 3 months, or a Wide Range Achievement Test-3 (WRAT-3 Blue Reading Subset, Jastak Associates, Wilmington, DE, USA) raw score of <35 (range = 0–57). During the preliminary visit, substance use was assessed with a semi-quantitative salivary assay (Accutest Saliva Test™, JANT Pharmacal, Encino, CA, USA; ALCO Screen, CHEMATICS, North Webster, IN, USA), and non-smoking status was confirmed by self-report, by a salivary cotinine <10 ng/ml (Nicalert™, JANT Pharmacal), and by an expired air carbon monoxide (CO) <9 ppm (Micro Smokerlyzer III, Bedfont Scientific, Kent, UK).
Study design
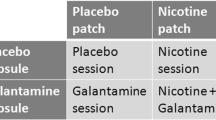
Each participant completed two study visits in which they received either nicotine or placebo by patch in counterbalanced order. Each visit contained two experimental sessions, one in the morning (pre-dose) and one in the afternoon (post-dose). At the beginning of each visit day, participants were evaluated to reconfirm non-smoking status by self-report, expired air CO (<9 ppm), and salivary cotinine (<30 ng/ml). Saliva was analyzed for ethanol, tetrahydrocannabinol (THC), cocaine, phencyclidine, opiates, amphetamine, and methamphetamine. Participants then performed the episodic memory task as part of a neurocognitive battery assessing attention [Continuous Performance Test Identical Pairs (CPT-IP), three-card Stroop, and letter number sequencing], processing speed and cognitive interference (three-card Stroop), working memory (letter number sequencing), and lateralized psychomotor speed (Grooved Pegboard; Barr et al. 2007). After participants performed baseline cognitive testing, the randomly assigned treatment patch was administered—either two 7-mg transdermal nicotine patches (Nicoderm CQ Alza, Mountain View, CA, USA) or two identical placebo patches (1-800-Patches, Salt Lake City, UT, USA). Participants repeated the episodic memory task 4 h after patch application, at the time of expected peak serum nicotine levels (Benowitz 1995; Gupta et al. 1993). Patches were removed upon completion of the episodic memory task (approximately 4.5 h after patch placement), and blood was drawn for serum nicotine levels. For male and postmenopausal female participants (nine controls, ten patients), the mean time between visits was 9.3 days (range = 6–15 days). Due to known effects of estrogen on cognitive performance in women with schizophrenia (Hoff et al. 2001), cycling female participants (none with schizophrenia, three controls) returned for the second visit at the same phase in their cycle as they had been on the first visit (mean inter-visit period = 42.3 days; range = 28–66 days).
Procedure
The episodic memory paradigm consisted of a previously described source monitoring task (Weiss et al. 2007), modified here to consist of three interleaved encoding and testing sessions. During each encoding session, participants simultaneously saw and heard 26 English words (13 spoken by a man, 13 spoken by a woman) and were asked to identify the gender of the voice using a keypad button. Words were presented visually on a screen approximately 18 in. from each participant and aurally via Dell Inspiron 8600 laptop speakers at a clearly audible sound level using Presentation version 0.80 (Neurobehavioral Systems, Albany, CA, USA). Immediately following each encoding block, a test containing the 26 previously studied items and 26 new items was presented visually, and participants were asked to indicate whether the word had been spoken by the man, the woman, or was new by pressing one of three labeled keys. Thus the paradigm tests both simple recognition memory (distinguishing previously presented from novel items) as well as source memory (remembering the gender of the voice that spoke the previously presented items).
Statistical analysis
Simple recognition memory was assessed by hit rate (rate of correct identification of old items as old) and false alarm rate (rate of incorrect identification of new words as old). Source memory accuracy was calculated as the percentage of correctly identified old items attributed to their correct source.
Mean hit reaction time was calculated for correct identification of old items (regardless of source accuracy) and new items. Accuracy and response time values were collapsed across the three test runs at each of the four memory assessments (Wilding 1999). Additional composite signal-detection parameters for discriminability (d′) and response bias (C) were calculated in standard fashion based on the hit rate and false alarm rate values (Macmillan and Creelman 1991; Snodgrass and Corwin 1988).
Baseline (visit 1 pre-dose) between-group differences in accuracy and reaction time were compared using an unpaired Student’s t test. To assess the effect of treatment (nicotine vs. placebo), difference scores for outcome measures were calculated for each visit as (post-dose − pre-dose score). These difference scores were then entered into separate repeated-measures linear mixed models with treatment (nicotine vs. placebo), diagnosis (schizophrenia vs. control), and a group-by-treatment interaction term as fixed effects and participant as a random effect. Age and gender were included as covariates in all models and removed if not significant. As there were four primary outcomes of interest, the statistical significance was set at a two-sided alpha of 0.0125. Analyses were conducted on SPSS version 11 for Mac OSX or SAS version 9.1 for Windows.
Results
Of the 38 participants who were screened, 29 met eligibility requirements and were enrolled. One participant with schizophrenia and three controls were withdrawn from the study prior to randomization due to either change in interest in participating in the study or being lost to follow-up. The remaining 25 subjects were randomized for order of receiving nicotine and placebo patches. One participant in each group was unable to complete the memory task due to nausea, and one additional participant with schizophrenia had unusable data due to technical error. Analyses therefore included the 10 participants with schizophrenia and 12 controls who completed the post-dose episodic memory task. Demographic characteristics of these participants are presented in Table 1
. The groups differed in age, intelligence quotient (IQ; WRAT-3 raw scores converted into age-adjusted standard scores), and prior smoking history. Eight participants with schizophrenia were on a second-generation antipsychotic medication, and two participants were taking a combination of first- and second-generation antipsychotic medications. Half of the participants with schizophrenia were taking clozapine. A list of the patients’ medications is presented in Table 2
. Ten participants (four subjects with schizophrenia, six control subjects) agreed to have blood drawn for serum nicotine at the end of each testing sessions. Mean (±SD) serum nicotine levels were 0.43 (±0.87) ng/ml after the placebo patch condition and 6.14 (±2.44) ng/ml after the nicotine patch condition (t = −7.10, p < 0.0001). Serum nicotine levels did not differ between the schizophrenia group and control group in either the placebo patch condition (t = 0.98, p = 0.36) or the nicotine patch condition (t = 0.74, p = 0.48).
Baseline memory performance
At baseline, participants with schizophrenia demonstrated poorer recognition memory performance compared with controls, controlling for age, with a lower hit rate (67.2 ± 14.8% vs. 83.2 ± 10.8%, t = 2.93, p < 0.01), a higher false alarm rate (15.1 ± 16.6 vs. 2.5 ± 4.0, t = −2.4, p < 0.05), and lower source memory accuracy (68.6 ± 14.9% vs. 86.0 ± 10.4%, t = 3.21, p < 0.01). Patients with schizophrenia were slower when making correct responses compared with controls [hit reaction time for old items (1,798 ± 294 m vs. 1,308 ± 299 ms, t = 3.86, p < 0.01), new items (1,562 ± 342 ms vs. 946 ± 80 ms, t = 5.57, p < 0.001)]. Those with schizophrenia also had poorer discriminability than controls at baseline (1.7 ± 0.7 vs. 3.1 ± 0.7, t = 5.15, p < 0.001), but there was no between-group difference in response bias (0.37 ± 0.47 vs. 0.51 ± 0.36, t = 0.77, p = 0.45).
Nicotine effect on identification of old and new items
As shown in Table 3
, there was no effect of treatment on hit rate. There was an effect of treatment on false alarms such that there was a reduction in false alarms in the nicotine treatment condition relative to placebo [F(1,17) = 8.47, p < 0.01, Cohen’s d effect size = 0.71). There was a trend level treatment by diagnosis interaction that reflects a greater effect of nicotine on reduction in false alarms in the schizophrenia group as compared with the control group [medication by diagnosis interaction: F(1,17) = 3.13; p = 0.095, Cohen’s d effect size = 0.43; Fig. 1a].
Source memory accuracy
There was no effect of nicotine on source memory accuracy: F(1,18) = 0.42, p = 0.53.
Reaction time
As shown in Fig. 1b, hit reaction time was faster during correct identification of new items in the nicotine condition [main effect of study medication, F(1,18) = 9.11; p = 0.007, Cohen’s d effect size = 0.71]. There was no treatment effect on the reaction time for correct identification of old items [F(1,18) = 2.06; p = 0.17].
Discriminability and response bias
When the responses to new and old items were considered together, there was no effect of treatment on discriminability. There was a main effect of treatment on response bias, such that participants on nicotine showed a decreased tendency to respond “old” to a test item, regardless of the stimulus type [main effect for nicotine, F(1,18) = 10.06; p = 0.005], an effect that did not differ by diagnosis.
Control analyses
Inclusion of age and gender as a covariate did not change the outcome of any of the above analyses. There was no effect of order of treatment administration for any of the four primary outcome measures, indicating no significant carry-over effect of nicotine administration on any of the four primary outcome measures examined.
Discussion
The main finding of this study was that nicotine significantly and selectively improved accuracy and speeded reaction times for identification of novel items on an episodic memory task. Improvement in false alarms on this task indicates improvement in recognition of novel items as novel, as measured by reduction in incidence of reporting having previously viewed items that were in fact novel. There was some indication that this improvement in novelty detection with nicotine may be greater in those with schizophrenia than in normal control participants. Improvements in novelty detection may be of clinical significance, as irregularities in novel item recognition have been associated with positive symptoms in schizophrenia (Brebion et al. 1998; Ishigaki and Tanno 1999).
As in our study, prior studies have not found effects of nicotine on overall verbal memory performance in schizophrenia (Harris et al. 2004; Smith et al. 2006). These studies did not specifically assess treatment effect on recognition of new vs. previously viewed words, and as with the present study, measures of recognition memory that assess overall memory performance (e.g., discriminability) may not detect an effect of nicotine if these effects are specific to novelty detection. In one prior study that investigated the effect of nicotine on novelty detection during a delayed recognition memory task, nicotine (1 mg via nasal spray) reduced false alarm rates in 2-h-withdrawn smokers with schizophrenia, an effect not observed in non-smokers with schizophrenia or healthy controls (Myers et al. 2004). As in the present study, nicotine did not improve hit rate. These results differ from those of the current study in which nicotine was associated with improved novelty detection in those with and without schizophrenia. This dissimilarity may be due to differences in the recognition memory paradigms utilized in the experiments. Myers et al. (2004) employed a self-paced test of visuospatial design recognition, as opposed to a paced test that utilized written and spoken words employed in the present study.
The type of novelty effect demonstrated in the present paradigm is known as stimulus novelty, a phenomenon that has been well studied in humans, non-human primates, and rodents (Kumaran and Maguire 2007; Ranganath and Rainer 2003). The neural basis of stimulus novelty involves a suppression of neural firing with stimulus repetition, an effect observed within 90 ms of stimulus presentation (Brown and Bashir 2002). Although these repetition-suppression effects are seen across many regions of the cortex, they appear to be most robust within the cortical aspects of the medial temporal lobe, especially within the perirhinal cortex. This region receives a high degree of cholinergic projections and contains a uniquely high density of nicotinic receptors (Perry et al. 1993); thus cholinergic mechanisms are thought to be at the core of these novelty-detection capabilities (Hasselmo and Giocomo 2006).
The repetition-suppression mechanism underlying stimulus novelty detection is similar to two other electrophysiological sensory gating effects: prepulse inhibition (PPI) of the acoustic startle response and P50 auditory-evoked potential suppression. The potential link between stimulus novelty detection, PPI, and P50 suppression is of particular interest as these latter two effects are abnormal in patients with schizophrenia and their first-degree relatives, are considered leading candidates for endophenotypes of the illness (Turetsky et al. 2007), and are ameliorated by nicotine (Adler et al. 1993; Adler et al. 1992; Duncan et al. 2001; Kumari et al. 2001; Postma et al. 2006). While novelty detection in the episodic memory test differs in processing time from that of sensory gating studies, nicotine improves differentiation of target from on-target stimuli in all of these tasks. The effects of nicotine on stimulus novelty detection may therefore be related to these well-described sensory gating findings. Further research is necessary to determine the relationship between stimulus novelty detection and these sensory gating findings.
The specific mechanism by which nicotine may improve performance on these tasks is incompletely understood. Most work has focused on nicotine’s stimulation of α-7 nAChRs on γ-aminobutyric acid (GABA) interneurons of the hippocampus (Albuquerque et al. 1998). Such stimulation increases GABA activity, which is thought to inhibit a hyperactive hippocampal response to repeated stimuli in schizophrenia, thereby enhancing response inhibition to irrelevant stimuli (Hajos et al. 2005; Ji and Dani 2000).
Alterations in early electrophysiological responses, e.g., improved repetition suppression, may lead to improved downstream decision-making, including greater capacity for inhibitory control of inappropriate responses. In the larger study, of which this study was a part, Barr et al. (2007) found that nicotine reduced errors of commission on the CPT-IP test of attention and improved performance on the Stroop Task in non-smokers with schizophrenia and non-psychiatric controls, effects that were more pronounced in those with schizophrenia than controls. The authors postulate that these effects are the result of a nicotine-mediated improvement in inhibitory control. Our finding that nicotine shifted participants’ response bias in the conservative direction may be an indication that inhibitory mechanisms are involved.
While we are proposing that enhanced pre-attentional mechanisms are responsible for the effects of nicotine seen in this study, it remains possible that nicotine’s effects on attention and working memory also play a role. Nicotine is thought to improve attention and working memory by increasing release of dopamine in the frontal lobe. Memory encoding has been shown to be dependent on attention and working memory, and it is impaired when carried out under conditions of divided attention (Craik et al. 1996; Naveh-Benjamin et al. 2000). By improving attention and working memory, and thereby memory encoding, nicotine may enhance a participant’s ability to differentiate words that have not been previously viewed from those that have been.
Individuals with schizophrenia have difficulty filtering relevant from irrelevant material as well as determining internally generated from externally generated information (Brebion et al. 1997; Keefe et al. 1999). These deficits have been associated with positive symptomatology in schizophrenia (Brebion et al. 2000; Brebion et al. 1998; Franck et al. 2000), such that hallucinations were associated with an increased false alarm rate on a recognition memory task. If nicotine has an effect of reducing false alarm rates and/or improving filtering of irrelevant from relevant events, it is conceivable that it could play some role in improving positive symptoms. A recent study demonstrating reduced positive symptoms in smokers relative to non-smokers with schizophrenia provides some preliminary evidence for this reasoning (Zhang et al. 2007).
The results of the present study are limited by several factors. The small sample size may have limited our power to detect an effect of nicotine on memory, potentially leading to type II errors, although the crossover design increased the power to detect a treatment effect. That said, the effect size of treatment on false alarm rate and on reaction time to novel items was moderate to large, while the effect size of the diagnosis by treatment interaction on false alarm rate was moderate. Second, the non-smoking status of our participants may limit the generalizability of the findings to non-smokers with schizophrenia, as the pathophysiology of schizophrenia has been postulated to differ between smokers and non-smokers (Kelly and McCreadie 1999), although the present study did include former smokers. Third, the effects of clozapine and other antipsychotic medications may modulate the effects of nicotine. Clozapine has been shown to attenuate the effects of nicotine on attention and memory in an animal model of schizophrenia (Rezvani et al. 2007), suggesting that clozapine treatment could reduce the effects of nicotine in the patient group. We were unable to detect a nicotine treatment by clozapine treatment interaction on false alarm rate scores or reaction times to new items, but we were severely limited in our power to detect such effect. This issue should be prospectively explored in a larger sample. Finally, it is possible that the cognitive tests administered before the episodic memory test may have impacted subjects’ performance on the episodic memory test. While this is a potential confounding variable in all neurocognitive batteries, such effect was minimized by the placebo-controlled, crossover design of the study and because other neurocognitive tests administered were not verbally intensive, and there was no delay or intervening task between encoding and retrieval in the episodic memory test.
Overall, our findings support the general hypothesis that nicotine improves recognition of novel events in non-smokers, particularly in those with schizophrenia, with little or no modulation of overall episodic memory ability. Further study utilizing neuroimaging or electrophysiological approaches may be warranted to investigate the neural correlates of this effect.
References
Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R (1992) Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biol Psychiatry 32:607–616
Adler LE, Hoffer LD, Wiser A, Freedman R (1993) Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 150:1856–1861
Albuquerque EX, Pereira EF, Braga MF, Alkondon M (1998) Contribution of nicotinic receptors to the function of synapses in the central nervous system: the action of choline as a selective agonist of alpha 7 receptors. J Physiol Paris 92:309–316
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV). American Psychiatric Association, Washington, DC, USA
Arendash GW, Sanberg PR, Sengstock GJ (1995) Nicotine enhances the learning and memory of aged rats. Pharmacol Biochem Behav 52:517–523
Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE (2008) The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology 33:480–490
Benowitz NL (1995) Clinical pharmacology of transdermal nicotine. Eur J Pharm Biopharm 41:168–174
Brebion G, Smith MJ, Gorman JM, Amador X (1997) Discrimination accuracy and decision biases in different types of reality monitoring in schizophrenia. J Nerv Ment Dis 185:247–253
Brebion G, Smith MJ, Amador X, Malaspina D, Gorman JM (1998) Word recognition, discrimination accuracy, and decision bias in schizophrenia: association with positive symptomatology and depressive symptomatology. J Nerv Ment Dis 186:604–609
Brebion G, Amador X, Smith M, Malaspina D, Sharif Z, Gorman JM (2000) Depression, psychomotor retardation, negative symptoms, and memory in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol 13:177–183
Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S (2000) Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology 23:351–364
Brown MW, Bashir ZI (2002) Evidence concerning how neurons of the perirhinal cortex may effect familiarity discrimination. Philos Trans R Soc Lond B Biol Sci 357:1083–1095
Ciamei A, Aversano M, Cestari V, Castellano C (2001) Effects of MK-801 and nicotine combinations on memory consolidation in CD1 mice. Psychopharmacology (Berl) 154:126–130
Cirillo MA, Seidman LJ (2003) Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev 13:43–77
Craik FI, Govoni R, Naveh-Benjamin M, Anderson ND (1996) The effects of divided attention on encoding and retrieval processes in human memory. J Exp Psychol Gen 125:159–180
de Leon J, Diaz FJ (2005) A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 76:135–157
de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (1995) Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry 152:453–455
Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S (2002) Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology 27:1056–1070
Duncan E, Madonick S, Chakravorty S, Parwani A, Szilagyi S, Efferen T, Gonzenbach S, Angrist B, Rotrosen J (2001) Effects of smoking on acoustic startle and prepulse inhibition in humans. Psychopharmacology (Berl) 156:266–272
Durany N, Zochling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P (2000) Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson’s syndrome. Neurosci Lett 287:109–112
Ernst M, Heishman SJ, Spurgeon L, London ED (2001) Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology 25:313–319
Evins AE, Deckersbach T, Cather C, Freudenreich O, Culhane MA, Henderson DC, Green MF, Schoenfeld DA, Rigotti NA, Goff DC (2005) Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. J Clin Psychiatry 66:1184–1190
Franck N, Rouby P, Daprati E, Dalery J, Marie-Cardine M, Georgieff N (2000) Confusion between silent and overt reading in schizophrenia. Schizophr Res 41:357–364
Freedman R, Hall M, Adler LE, Leonard S (1995) Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry 38:22–33
George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE (2002) Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology 26:75–85
Green MF (1996) What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153:321–330
Gupta SK, Okerholm RA, Coen P, Prather RD, Gorsline J (1993) Single- and multiple-dose pharmacokinetics of Nicoderm (Nicotine Transdermal System). J Clin Pharmacol 33:168–174
Hajos M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, Groppi VE (2005) The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 [N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther 312:1213–1222
Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R (2004) Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology 29:1378–1385
Hasselmo ME, Giocomo LM (2006) Cholinergic modulation of cortical function. J Mol Neurosci 30:133–135
Hoff AL, Kremen WS, Wieneke MH, Lauriello J, Blankfeld HM, Faustman WO, Csernansky JG, Nordahl TE (2001) Association of estrogen levels with neuropsychological performance in women with schizophrenia. Am J Psychiatry 158:1134–1139
Hughes J (1986) Prevalence of smoking among psychiatric outpatients. Am J Psychiatry 143:993–997
Ishigaki T, Tanno Y (1999) The signal detection ability of patients with auditory hallucination: analysis using the continuous performance test. Psychiatry Clin Neurosci 53:471–476
Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH (2004) Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry 55:850–858
Janhunen S, Ahtee L (2007) Differential nicotinic regulation of the nigrostriatal and mesolimbic dopaminergic pathways: implications for drug development. Neurosci Biobehav Rev 31:287–314
Ji D, Dani JA (2000) Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol 83:2682–2690
Keefe RS, Arnold MC, Bayen UJ, Harvey PD (1999) Source monitoring deficits in patients with schizophrenia; a multinomial modelling analysis. Psychol Med 29:903–914
Kelly C, McCreadie RG (1999) Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry 156:1751–1757
Kiba H, Jayaraman A (1994) Nicotine induced c-fos expression in the striatum is mediated mostly by dopamine D1 receptor and is dependent on NMDA stimulation. Brain Res Mol Brain Res 23:1–13
Kumaran D, Maguire EA (2007) Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci 27:8517–8524
Kumari V, Soni W, Sharma T (2001) Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Hum Psychopharmacol 16:321–326
Lambe EK, Picciotto MR, Aghajanian GK (2003) Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology 28:216–225
Laruelle M, Kegeles LS, Abi-Dargham A (2003) Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci 1003:138–158
Lawrence NS, Ross TJ, Stein EA (2002) Cognitive mechanisms of nicotine on visual attention. Neuron 36:539–548
Levin ED, Simon BB (1998) Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 138:217–230
Levin ED, Rezvani AH (2002) Nicotinic treatment for cognitive dysfunction. Curr Drug Target CNS Neurol Disord 1:423–431
Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE (1998) Transdermal nicotine effects on attention. Psychopharmacology (Berl) 140:135–141
Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184:523–539
Macmillan N, Creelman C (1991) Detection theory: a user’s guide. Cambridge University Press, Cambridge
Mancuso G, Andres P, Ansseau M, Tirelli E (1999) Effects of nicotine administered via a transdermal delivery system on vigilance: a repeated measure study. Psychopharmacology (Berl) 142:18–23
Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27:349–357
McClernon FJ, Gilbert DG, Radtke R (2003) Effects of transdermal nicotine on lateralized identification and memory interference. Hum Psychopharmacol 18:339–343
McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED (2006) Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 189:125–133
Mexal S, Frank M, Berger R, Adams CE, Ross RG, Freedman R, Leonard S (2005) Differential modulation of gene expression in the NMDA postsynaptic density of schizophrenic and control smokers. Brain Res Mol Brain Res 139:317–332
Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, Thaker GK (2004) Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology (Berl) 174:334–340
Naveh-Benjamin M, Craik FI, Gavrilescu D, Anderson ND (2000) Asymmetry between encoding and retrieval processes: evidence from divided attention and a calibration analysis. Mem Cognit 28:965–976
Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW (1999) Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 20:106–118
Nomikos GG, Schilstrom B, Hildebrand BE, Panagis G, Grenhoff J, Svensson TH (2000) Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav Brain Res 113:97–103
Olincy A, Young DA, Freedman R (1997) Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 42:1–5
Olney JW, Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52:998–1007
Perry EK, Court JA, Johnson M, Smith CJ, James V, Cheng AV, Kerwin JM, Morris CM, Piggott MA, Edwardson JA et al (1993) Autoradiographic comparison of cholinergic and other transmitter receptors in the normal human hippocampus. Hippocampus 3:307–315
Poltavski DV, Petros T (2006) Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol Behav 87:614–624
Postma P, Gray JA, Sharma T, Geyer M, Mehrotra R, Das M, Zachariah E, Hines M, Williams SC, Kumari V (2006) A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology (Berl) 184:589–599
Ranganath C, Rainer G (2003) Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci 4:193–202
Rezvani AH, Kholdebarin E, Dawson E, Levin ED (2007) Nicotine and clozapine effects on attentional performance impaired by the NMDA antagonist dizocilpine in female rats. Int J Neuropsychopharmacol:1–8
Rusted JM, Graupner L, Tennant A, Warburton DM (1998) Effortful processing is a requirement for nicotine-induced improvements in memory. Psychopharmacology (Berl) 138:362–368
Rusted JM, Trawley S, Heath J, Kettle G, Walker H (2005) Nicotine improves memory for delayed intentions. Psychopharmacology (Berl) 182:355–365
Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP (2005) Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry 62:649–659
Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK (2002) The effects of nicotine on specific eye tracking measures in schizophrenia. Biol Psychiatry 52:721–728
Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, Khan A (2006) Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology 31:637–643
Snodgrass JG, Corwin J (1988) Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117:34–50
Strand JE, Nyback H (2005) Tobacco use in schizophrenia: a study of cotinine concentrations in the saliva of patients and controls. Eur Psychiatry 20:50–54
Sziraki I, Sershen H, Benuck M, Hashim A, Lajtha A (1998) Receptor systems participating in nicotine-specific effects. Neurochem Int 33:445–457
Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM (2005) Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend 80:259–265
Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR (2007) Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull 33:69–94
Weiss A, Goff D, Duff M, Roffman J, Schacter D (2008) Distinguishing familiarity-based from source-based memory in patients with schizophrenia. Schizophr Res 99(1–3):208–217
White HK, Levin ED (2004) Chronic transdermal nicotine patch treatment effects on cognitive performance in age-associated memory impairment. Psychopharmacology (Berl) 171:465–671
Wilding EL (1999) Separating retrieval strategies from retrieval success: an event-related potential study of source memory. Neuropsychologia 37:441–454
Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL (2005) Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res 79:323–335
Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, McFalls E, Kovera C, McCarten JR (1995) Nicotine patches in Alzheimer’s disease: pilot study on learning, memory, and safety. Pharmacol Biochem Behav 51:509–514
Zhang X, Tan Y, Zhou D, Haile C, Wu G, Cao L (2007) Nicotine dependence, symptoms and oxidative stress in male patients with schizophrenia. Neuropsychopharmacology 32(9):2020–2024
Acknowledgements
This work was funded by a grant from the Stanley Medical Research Institute (Dr. Evins). Dr. Evins was also supported by a career development award from NIDA for the study of nicotine in schizophrenia, NIDA 1K23DA00510-01. Dr. Weiss was supported by a career development award from NIMH for the study of episodic memory in schizophrenia, NIMH K23 MH06019. Ms. Jubelt was supported by a Harvard-Pasteur Doris Duke Clinical Research Fellowship. The authors wish to acknowledge Rana Mufti, Michael A. Dyer, and Margaret Duff for their technical assistance in data collection and management in the completion of this research.
Disclosure/Conflicts of Interest
Dr. Evins reports a collaborative agreement with GSK in conjunction with a Cooperative Drug Discovery Group for Nicotine Dependence funded by NIDA U01 DA19378-01. GSK is the maker of Nicoderm Patch, the brand of patch used in this trial.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Evins and Weiss contributed equally to the project.
Rights and permissions
About this article
Cite this article
Jubelt, L.E., Barr, R.S., Goff, D.C. et al. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology 199, 89–98 (2008). https://doi.org/10.1007/s00213-008-1133-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1133-8