Abstract
Rationale
When acutely administered intraperitoneally, the non-psychoactive cannabinoid cannabidiol (CBD), its acidic precursor cannabidiolic acid (CBDA) and a stable methyl ester of CBDA (HU-580) reduce lithium chloride (LiCl)–induced conditioned gaping in male rats (a selective preclinical model of acute nausea) via activation of the serotonin 1A (5-HT1A) receptor.
Objectives
To utilise these compounds to manage nausea in the clinic, we must determine if their effectiveness is maintained when injected subcutaneously (s.c) and when repeatedly administered. First, we compared the effectiveness of each of these compounds to reduce conditioned gaping following repeated (7-day) and acute (1-day) pretreatments and whether these anti-nausea effects were mediated by the 5-HT1A receptor. Next, we assessed whether the effectiveness of these compounds can be maintained when administered prior to each of 4 conditioning trials (once per week). We also evaluated the ability of repeated CBD (7 days) to reduce LiCl-induced vomiting in Suncus murinus. Finally, we examined whether acute CBD was equally effective in male and female rats.
Results
Both acute and repeated (7 day) s.c. administrations of CBD (5 mg/kg), CBDA (1 μg/kg) and HU-580 (1 μg/kg) similarly reduced LiCl-induced conditioned gaping, and these effects were blocked by 5HT1A receptor antagonism. When administered over 4 weekly conditioning trials, the anti-nausea effectiveness of each of these compounds was also maintained. Repeated CBD (5 mg/kg, s.c.) maintained its anti-emetic efficacy in S. murinus. Acute CBD (5 and 20 mg/kg, s.c.) administration reduced LiCl-induced conditioned gaping similarly in male and female rats.
Conclusion
When administered repeatedly (7 days), CBD, CBDA and HU-580 did not lose efficacy in reducing nausea and continued to act via agonism of the 5-HT1A receptor. When administered across 4 weekly conditioning trials, they maintained their effectiveness in reducing LiCl-induced nausea. Repeated CBD also reduced vomiting in shrews. Finally, CBD’s anti-nausea effects were similar in male and female rats. This suggests that these cannabinoids may be useful anti-nausea and anti-emetic treatments for chronic conditions, without the development of tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cancer patients undergoing chemotherapy treatment still experience the side effects of nausea and vomiting, despite utilising the classic anti-emetic regimen (e.g. Chow et al. 2018; Giagnuolo et al. 2019), with up to 50% of patients still experiencing acute nausea (e.g. Araz et al. 2019; Clemmons et al. 2018; Navari et al. 2018; Timaeus et al. 2018). For example, Sepúlveda-Vildósola et al. (2008) reported that treatment with a classic anti-emetic (a serotonin 3 receptor antagonist) resulted in complete acute vomiting control in 72% of patients, but complete acute nausea control in only 38% of patients. Dronabinol and nabilone, synthetic formulations of Δ9-tetrahydrocannabinol (the psychoactive component of cannabis), have been approved for treatment of chemotherapy-induced nausea and vomiting, but these compounds can be associated with more adverse effects such as hallucination and drowsiness (see Ho and MacDougall 2019 for an excellent review). Therefore, more effective anti-nausea and anti-emetic compounds, devoid of psychoactive effects, are needed.
Using a unique rodent model of nausea-induced conditioned gaping disgust responses (Grill and Norgren 1978), we (see Rock and Parker 2016) have demonstrated that the non-psychoactive cannabinoid cannabidiol (CBD, 5 mg/kg, intraperitoneal [i.p.] and subcutaneous [s.c.]; Parker and Mechoulam 2003; Rock et al. 2011, 2012, 2020) and cannabidiolic acid, the acidic precursor of CBD (CBDA, 0.0005–0.01 mg/kg, i.p.; Bolognini et al. 2013; Rock and Parker 2013; Rock et al. 2015, 2020) suppress lithium chloride (LiCl)-induced nausea. Indeed, CBDA was approximately 1000 times more potent than CBD in reducing nausea-induced conditioned gaping. Intuitively, this may point to CBDA as a more favourable treatment for nausea over CBD, but CBDA decarboxylates into CBD upon drying and with heat. This instability of CBDA (Crombie and Crombie 1977) may limit its clinical utility. Recently, a stable analogue of CBDA, its methyl ester (HU-580; 0.1 and 1 μg/kg, i.p.), has also been shown to reduce LiCl-induced conditioned gaping (Pertwee et al. 2018), perhaps making it a more appropriate anti-nausea medication.
The interoceptive insular cortex (IIC) is a critical forebrain region that mediates nausea. Limebeer et al. (2018) demonstrated that administration of LiCl elevated 5-HT selectively in the IIC, while acute systemic pretreatment of CBD (5 mg/kg, i.p.) reduced this LiCl-induced elevation of 5-HT. CBD is likely producing this anti-nausea effect by acting at serotonin 1A (5-HT1A) somatodendritic autoreceptors in the dorsal raphe nucleus (DRN; Rock et al. 2012) to reduce the firing of 5-HT afferent neurons, therefore preventing the LiCl-induced elevation of 5-HT in the IIC. And indeed, when microinfused directly into the DRN, CBD reduced LiCl-induced conditioned gaping and was blocked by administration of a 5-HT1A receptor antagonist (Rock et al. 2012). Similarly, the anti-nausea effects of acutely administered CBDA and HU-580 are also blocked by administration of a 5-HT1A receptor antagonist (Bolognini et al. 2013; Pertwee et al. 2018). These results suggest that IIC 5-HT produces the experience of nausea which may be prevented by CBD (or CBDA or HU-580) to reduce nausea-inducing 5-HT in the IIC.
Using the emetic species Suncus murinus (house musk shrew), our group has also shown that acute pretreatment with CBD (5 and 10 mg/kg, i.p. and/or s.c.; Parker et al. 2004; Rock et al. 2012) or CBDA (0.1 and 0.5 mg/kg, i.p.) reduces LiCl-induced emesis in S. murinus. This anti-emetic effect was blocked by administration of a 5-HT1A receptor antagonist (Rock et al. 2012), suggesting a similar anti-emetic mechanism of action for CBD.
While acute administration (one injection) of CBD, CBDA or HU-580 prior to conditioning has been shown to reduce LiCl-induced conditioned gaping in rats and/or vomiting in shrews, it remains unknown whether repeated administration will similarly reduce nausea (or increase it), due to possible desensitisation of the 5-HT1A receptor in the DRN (or via another mechanism). Indeed, using in vivo single-unit extracellular recordings of DRN 5-HT neurons, De Gregorio et al. (2019) showed that while acute CBD administration reduced the firing rate of DRN 5-HT neurons, repeated CBD (5 mg/kg, s.c.) treatment over 7 days actually increased the firing rate of DRN 5-HT neurons. They suggested that this is likely occurring through desensitisation of 5-HT1A autoreceptors in the DRN.
Clinical data indicates that female cancer patients exhibit more nausea and vomiting from chemotherapy treatment than male patients (e.g. Gralla et al. 1999; Liaw et al. 2001), and female sex continues to be a risk factor for chemotherapy-induced nausea and vomiting, even with the recommended anti-emetic regimen (e.g. Tsuji et al. 2019). Few preclinical studies have focused on directly comparing the effects of CBD in male and female rodents, but those studies that have, report no sex differences for CBD’s effects on intraocular pressure (Miller et al. 2018), hypothermia (Javadi-Paydar et al. 2018), anxiety-like behaviour (Kasten et al. 2019), locomotor activity (Javadi-Paydar et al. 2018; Kasten et al. 2019), conditioned place preference and conditioned taste avoidance (Hempel et al. 2018). To date, the only group to explore sex differences with CBDA methyl ester (HU-580) is that of Zhu et al. (2020), who reported that repeated HU-580 (14-day) treatment produced anti-nociceptive effects in male rats, but these same doses were ineffective in females. Although few sex differences have been reported with CBD in animal models to date, it is not known if CBD is equally effective in reducing nausea when comparing male and female rats.
The experiments described here aim to determine the effectiveness of both acute and repeated s.c. administrations of CBD, CBDA and HU-580 to suppress LiCl-induced nausea and to evaluate whether the effects are 5-HT1A receptor mediated. In addition, we assessed whether these compounds can maintain their effectiveness when administered prior to 4 conditioning trials (once per week). Next, we evaluated the effect of repeated pretreatment with CBD (5 mg/kg, s.c.) daily for 7 days to maintain its potential to reduce LiCl-induced vomiting in S. murinus. Finally, we sought to examine sex differences in the effects of acute s.c. administration of CBD to suppress LiCl-induced nausea. The results of these experiments have important implications for the use of these compounds in the veterinary clinic and in human clinical trials.
Materials and methods
Animals
All procedures complied with the Animals for Research Act of Ontario and the guidelines of the Canadian Council on Animal Care. All procedures were approved by the Institutional Animal Care Committee at the University of Guelph, which is accredited by the Canadian Council on Animal Care. The rat colony room was maintained at an ambient temperature of 21 °C and a 12-h/12-h reverse light–dark schedule (lights off at 07:00 hours). All rat experimental manipulations occurred during the dark phase cycle. Male (N = 145) and female (N = 24) Sprague Dawley rats (Charles River Laboratories, Saint-Constant, QC, Canada) were maintained on ad libitum chow and water. In experiment 1, the rats were 55 days old and ranged from 256 to 356 g on the first day of conditioning. In experiments 2 and 3, the rats were 52 days old and ranged from 235 to 330 g on the first day of conditioning. In experiment 4, the male and female rats were 52 days old, with males ranging from 256 to 302 g and females ranging from 170 to 220 g on the first day of conditioning. They were individually housed in opaque plastic cages (48 cm × 26 cm × 20 cm), containing bed-o-cob bedding from Harlan Laboratories, Inc. (Mississauga, ON, Canada), a brown paper towel and Crink-l’Nest™ from The Andersons, Inc. (Maumee, OH, USA). They were also provided a soft white paper cup (14 cm long and 12 cm in diameter).
The shrews were bred and raised in the University of Guelph colony and ranged from 141 to 261 days old at the time of testing. Male (n = 16) S. murinus (house musk shrews) were individually housed in clear mouse cages in the colony room at an ambient temperature of 21 °C on a 10-h/14-h light–dark schedule (lights off at 19:00 hours). Shrews were tested in their light cycle. They were provided with a soft white paper cup containing Crink-l’Nest™ from The Andersons, Inc. (Maumee, OH, USA), maintained on Medical/Royal Canine Feline Maintenance mixed with Harlan Ferret dry chow and had ad libitum access to water. All shrews were weaned at 25–30 days of age.
Drugs
LiCl (Sigma-Aldrich), prepared in a 0.15 M solution with sterile water, was administered intraperitoneally (i.p.) to rats at a volume of 20 ml/kg (127.2 mg/kg) and to shrews at a volume of 60 ml/kg (381.6 mg/kg). CBD (Toronto Biochemicals), CBDA (provided by Raphael Mechoulam) and its methyl ester (HU-580; provided by Raphael Mechoulam) were dissolved in a glass graduated cylinder in ethanol with Tween 80 added to the solution, and the ethanol was evaporated off with a nitrogen stream, after which saline (SAL) was added (final Tween 80:SAL ratio = 1:9). CBD was mixed at a concentration of 5 mg/ml or 20 mg/ml (experiment 4) and administered subcutaneously (s.c.) at 1 ml/kg (5 and 20 mg/kg). CBDA and HU-580 were mixed at a concentration of 1 μg/ml and administered s.c. at a volume of 1 ml/kg (1 μg/kg). The selective 5-HT1A receptor antagonist WAY100635 (0.1 mg/kg; Sigma-Aldrich) was also mixed as above to a final vehicle (VEH) solution consisting of 1:9 Tween 80:SAL and administered i.p. at a volume of 1 ml/kg. Doses of the antagonist were selected because these doses had no effect on LiCl-induced conditioned gaping on their own (Bolognini et al. 2013; Pertwee et al. 2018; Rock et al. 2012, 2013, 2015, 2017).
Apparatus
The taste reactivity (TR) chambers made of clear Plexiglas (22.5 cm × 26 cm × 20 cm) were placed on a clear glass top table with a mirror positioned under the chamber (at a 45° angle) to allow video recording (Sony video camera, Handycam; Henry’s Camera, Waterloo, ON, Canada) of the ventral surface of the rat to quantify orofacial responses. The videos were later scored using ‘The Observer’ event-recording software (Noldus Information Technology, Inc., Leesburg, VA, USA).
The observation chambers for shrew vomiting in experiment 3 were made of clear Plexiglas (10 cm × 19 cm × 19 cm) and placed on a table with a clear glass top. A mirror (at a 45° angle) beneath the chamber facilitated viewing of the ventral surface of each shrew.
Procedures
Experiment 1: acute and repeated pretreatments with CBD, CBDA or HU-580 in male rats
Rats were implanted with an intraoral cannula under isoflurane anaesthesia as described by Limebeer et al. (2010). Following recovery from surgery (3 days), the rats were repeatedly pretreated for 7 days with s.c. injections of VEH, CBD (5 mg/kg), CBDA (1 μg/kg) or HU-580 (1 μg/kg) once a day. On the 4th day of injections, the rats also received an adaptation trial during which they were placed in the TR chamber and their cannula was attached to a fluid infusion pump (model KDS100; KD Scientific, Holliston, MA, USA). Water was infused into their intraoral cannulae for 2 min at a rate of 1 ml/min.
On the 7th injection day, the rats received a single conditioning trial, with VEH, CBD pretreatment (experiment 1a), CBDA pretreatment (experiment 1b) or HU-580 pretreatment (experiment 1c). For experiment 1a, the rats were randomly assigned to one of the following groups: repeated VEH (n = 8), acute CBD (n = 7) and repeated CBD (n = 7). As well to determine the mechanism of action, an additional three groups were pretreated with the 5-HT1A receptor antagonist WAY100635 (WAY) (0.1 mg/kg, i.p.), 15 min prior to the pretreatment; these are designated as repeated VEH + WAY (n = 7), acute CBD + WAY (n = 7) and repeated CBD + WAY (n = 7). For experiment 1b, the rats were randomly assigned to one of the following groups: repeated VEH (n = 7), acute CBDA (n = 8), repeated CBDA (n = 8), acute CBDA + WAY (n = 8) and repeated CBDA + WAY (n = 8). For experiment 1c, rats were assigned to one of the following groups: repeated VEH (n = 7), acute HU-580 (n = 8), repeated HU-580 (n = 8), acute HU-580 + WAY (n = 8) and repeated HU-580 + WAY (n = 8).
Thirty minutes following the pretreatment injection of VEH, CBD, CBDA or HU-580, the rats were each placed in the TR chamber and intraorally infused with 0.1% saccharin for 2 min at a rate of 1 ml/min. During this time, the orofacial responses were recorded from the mirror beneath the chamber. After the saccharin infusion, each rat was injected i.p. with 20 ml/kg of 0.15 M LiCl and returned to its home cage.
Seventy-two hours later, the rats were tested drug free and the test trial was video recorded. Rats were again placed in the TR chamber and infused with the 0.1% saccharin solution for 2 min (1 ml/min). The videos were scored by an observer blind to the experimental conditions using The Observer for the behaviour of gaping (large openings of the mouth and jaw, with the lower incisors exposed).
To assess whether any of the pretreatments interfered with learning per se, conditioned taste avoidance (CTA) was assessed in a single bottle test. Rats were water restricted at 15:00 hours following their test session. The next morning, a bottle containing 0.1% saccharin solution was placed on the cage at 08:00 hours for 2 h and the amount of saccharin consumed was measured.
Experiment 2: pretreatment with CBD, CBDA or HU-580 prior to multiple conditioning trials in male rats
Following surgery (as in experiment 1), rats were randomly assigned to a pretreatment group: VEH (n = 9), CBD (5 mg/kg, s.c.; n = 9), CBDA (1 μg/kg, s.c.; n = 8) and HU-580 (1 μg/kg, s.c.; n = 9). On each of four conditioning trials (1 week apart), the rats were pretreated with VEH, CBD, CBDA or HU-580 30 min prior to being placed in the TR chamber and conditioned as in experiment 1. Seventy-two hours following the fourth and final conditioning trial, the rats were tested as in experiment 1.
Experiment 3: repeated vs acute pretreatment with CBD on vomiting in S. murinus
Male shrews (n = 4) were repeatedly (designated as repeated CBD group) pretreated for 7 days with CBD (5 mg/kg, s.c.). Each day, shrews were transferred from their home cage to an empty cage in the experimental room that contained four meal worms. After 15 min, they were injected with CBD and returned to their home cage. This repeated group was then compared with shrews not pretreated with CBD prior to the injection of the emetic drug LiCl on the test trial.
On the test day (day 7), the potential of CBD following repeated pretreatment was compared with acute treatment to reduce vomiting produced by the emetic drug LiCl. All shrews (n = 16) were placed in an empty cage containing four meal worms in the experimental room and, 15 min later, were injected with VEH (n = 6) or 5 mg/kg (s.c.) CBD (n = 6, acute group; n = 4, repeated group). After 30 min, they were injected with LiCl (381.6 mg/kg, i. p.) and placed in an observation chamber for 45 min and the frequency of vomiting episodes was counted by an observer blind to the experimental conditions.
Experiment 4: acute pretreatment with CBD in male and female rats
Male and female rats were implanted with an intraoral cannula under isoflurane anaesthesia as described by Limebeer et al. (2010). Following recovery from surgery (3 days), the rats received an adaptation trial (as in experiment 1). On the next day, the rats received a conditioning trial in which they were administered a pretreatment injection of VEH or CBD (5 and 20 mg/kg, s.c.). Thirty minutes later, the rats were individually placed in the TR chamber and conditioned as in experiment 1. The groups were as follows: VEH (males; n = 8), VEH (females; n = 8), CBD 5 mg/kg (males; n = 8), CBD 5 mg/kg (females; n = 8), CBD 20 mg/kg (males; n = 8) and CBD 20 mg/kg (females; n = 8). Seventy-two hours later, a drug-free test was conducted as in experiment 1.
To assess whether any of the pretreatments interfered with learning per se, CTA was assessed in a single bottle test (as in experiment 1).
Data analysis
Data were analysed using SPSS (IBM, version 26). Statistical significance was set as a p value < 0.05. For experiment 1 (a–c), separate single-factor analysis of variance (ANOVA) were conducted on the number of gapes at test for each group, with subsequent Bonferroni post hoc comparisons of significant effects. For experiment 2, a mixed factors ANOVA with the between-group factor of group (4) and the within-group factor of trial (5) was conducted on the number of gapes, and single-factor ANOVAs were conducted for each trial, with subsequent Bonferroni post hoc comparisons of significant group effects. In addition, for experiments 1 and 2, the amount of saccharin consumed (ml) during the 2-h CTA test for each group was entered into separate single-factor ANOVAs, with Bonferroni post hoc comparisons. For experiment 3, a single-factor ANOVA was conducted on the number of vomiting episodes at test for each group, with subsequent Bonferroni post hoc comparisons of significant effects. For experiment 4, a 2 (sex) × 3 (group; VEH, 5 and 20 mg/kg) ANOVA was conducted on the number of gapes at test for each group, with LSD post hoc comparisons. In addition, for experiment 4, the amount (ml) of saccharin consumed during the CTA test was entered into a 2 (sex) × 3 (group; VEH, 5 and 20 mg/kg) ANOVA.
Results
Experiment 1: acute and repeated pretreatments with CBD, CBDA or HU-580 in male rats
Acute administration of CBD, CBDA or HU-580 reduced LiCl-induced conditioned gaping, and these compounds maintained their effectiveness when administered repeatedly for 7 days prior to the conditioning trial. The anti-nausea effects of both acute and repeated administrations of each treatment were prevented by antagonism of the 5-HT1A receptor.
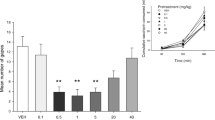
Figure 1 a presents the mean (± SEM) number of gapes displayed by rats pretreated with CBD. The one-way ANOVA revealed a significant group effect (F(5, 37) = 9.3; p < 0.001); Bonferroni post hoc tests revealed that groups acute CBD and repeated CBD displayed significantly fewer gapes than all other groups (p values ≤ 0.01).
The effect of acute or repeated (7 days) administration of VEH, CBD (5 mg/kg, s.c.) (a), CBDA (1 μg/kg, s.c.) (b) or HU-580 (1 μg/kg, s.c.) (c) on LiCl-induced conditioned gaping. Additional groups also evaluated the potential of a 5-HT1A receptor antagonist (WAY) to prevent the anti-nausea effect of each compound. Inserts d–f represent the subsequent CTA test. Each circle represents a single rat’s score within each group. Asterisks indicate a significant difference from the repeated VEH group (**p ≤ 0.01)
Figure 1 b presents the mean (± SEM) number of gapes displayed by rats pretreated with CBDA. The one-way ANOVA revealed a significant group effect (F(4, 34) = 14.1; p < 0.001). Bonferroni post hoc comparisons revealed that groups acute CBDA and repeated CBDA displayed fewer gapes than all other groups (p values < 0.01). Figure 1 c presents the mean (± SEM) number of gapes displayed by rats pretreated with HU-580. The one-way ANOVA revealed a significant group effect (F(4, 34) = 9.5; p < 0.001); Bonferroni post hoc comparisons revealed that groups acute HU-580 and repeated HU-580 displayed fewer gapes than all other groups (p values < 0.01).
None of the treatments modified the strength of the LiCl-induced CTA. As seen in Fig. 1 d–f, none of the groups differed in their saccharin intake. These results indicate that no pretreatment interfered with CTA learning; therefore, administration of these compounds did not interfere with learning per se.
Experiment 2: pretreatment with CBD, CBDA or HU-580 prior to multiple conditioning trials in male rats
Over multiple conditioning trials, CBD, CBDA or HU-580 effectively reduced LiCl-induced conditioned gaping. Figure 2 a presents the mean (± SEM) number of gapes displayed by rats in each group over the four conditioning trials and drug-free test trial. The 4 × 5 mixed factors ANOVA revealed a significant main effect of group (F(3, 31) = 25.6; p < 0.001), a significant main effect of trial (F(4, 124) = 90.2; p < 0.001) and a significant group × trial interaction (F(12, 124) = 9.0; p < 0.001). To analyse the interaction, subsequent single-factor ANOVAS for trials 2, 3 and 4 and test revealed significant effects (p values < 0.001). Bonferroni post hoc comparison tests revealed that on these trials, those rats pretreated with CBD, CBDA or HU-580 gaped significantly less than VEH controls. No other groups differed.
The effect of pretreatment with VEH, CBD (5 mg/kg, s.c.), CBDA (1 μg/kg, s.c.) or HU-580 (1 μg/kg, s.c.) on LiCl-induced conditioned gaping over 4 conditioning trials (C1–C4) and during a drug-free test (a) and the CTA test (b). Each circle represents a single rat’s score within each group. Asterisks indicate a significant difference from VEH (***p < 0.001)
Figure 2 b presents the mean (± SEM) amount of saccharin (ml) consumed. None of the treatments modified the strength of the LiCl-induced CTA. None of the groups differed in their saccharin intake.
Experiment 3: repeated vs acute pretreatment with CBD on vomiting in S. murinus
Both repeated and acute CBD reduced LiCl-induced emesis. Figure 3 presents the mean (± SEM) number of vomiting episodes displayed by shrews pretreated with CBD or VEH. The one-way ANOVA revealed a significant effect of group (F(2, 13) = 19.5; p < 0.001); Bonferroni post hoc tests revealed that acute CBD (p < 0.001) or repeated CBD (p < 0.01)–pretreated shrews vomited less frequently than those pretreated with acute VEH.
Experiment 4: acute pretreatment with CBD in male and female rats
Acute administration of CBD in male and female rats similarly reduced LiCl-induced conditioned gaping, and the strength of LiCl-induced conditioned gaping did not differ in male and female rats. Figure 4 a presents the mean (± SEM) number of gapes displayed by male and female rats pretreated with CBD. The 2 (sex) × 3 (group) ANOVA revealed only a significant effect of group (F(2, 42) = 39.7; p < 0.001). LSD post hoc tests revealed that groups 5 and 20 CBD gaped significantly less than VEH controls (p values < 0.001) and group 5 CBD gaped significantly less than group 20 CBD (p < 0.05), showing a biphasic effect.
The effect of acute administration of VEH or CBD (5, 20 mg/kg, s.c.) on LiCl-induced gaping in male and female rats (a) and the CTA test (b). Each circle represents a single rat’s score within each group. Asterisks indicate a significant difference from VEH (***p < 0.001), and number sign indicates a significant difference from group 20 CBD (#p < 0.05)
Figure 4 b presents the mean (± SEM) amount of saccharin (ml) consumed. The 2 (sex) × 3 (group) ANOVA revealed a significant effect of sex (F(1, 42) = 5.7; p < 0.05), a non-significant effect of group (F(2, 42) = 0.1; p > 0.05) and a non-significant sex × group interaction (F(2, 42) = 0.4; p > 0.05). The females drank significantly less saccharin than males.
Discussion
Here, we demonstrate that CBD, CBDA and HU-580 each maintain their effectiveness to reduce nausea when they are repeatedly administered s.c. and when they are administered s.c. prior to several conditioning trials (approximating several chemotherapy treatments). We also show that s.c. CBD maintains its effectiveness to reduce vomiting when repeatedly administered. Finally, we show that acute s.c. CBD reduces LiCl-induced gaping similarly in male and female rats, with a dose of 5 mg/kg being more effective than 20 mg/kg, as we have previously reported (Rock et al. 2020), with male and female VEH controls showing similar conditioned gaping responses to LiCl. There are many reports of CBD treatment producing dose-dependent, biphasic effects such that low/moderate doses are effective, but higher doses become ineffective. For example, in rodent models of anxiety, low to moderate doses of CBD reduced anxiety but higher doses were ineffective (e.g. Long et al. 2010; Guimarães et al. 1990). In mice, CBD (1 mg/kg) reduced paclitaxel-induced mechanical sensitivity, while higher doses of 1.25, 2.5 and 5 mg/kg were ineffective (King et al. 2017). It is possible that the dose-dependent effects of CBD are due to different mechanisms of action or off-target effects.
These current findings are important for translation to the clinic for either veterinary treatment or, eventually, human clinical trials, as most chemotherapy regimens are not single treatments but are instead given in cycles (ranging from 2 to 6 weeks) with a number of chemotherapy treatment doses administered during that time. Despite repeated administration, the anti-nausea effectiveness of each of these treatments was shown to be 5-HT1A receptor mediated. In addition, we have replicated our previous findings (but now using the s.c. route of administration rather than the i.p. route of administration) that acute treatment with CBD (Parker and Mechoulam 2003; Rock et al. 2011, 2012), CBDA (Bolognini et al. 2013; Rock and Parker 2013; Rock et al. 2015) and HU-580 (Pertwee et al. 2018) reduced LiCl-induced conditioned gaping, by a 5-HT1A receptor–mediated action (Bolognini et al. 2013; Pertwee et al. 2018; Rock and Parker 2013; Rock et al. 2011, 2012, 2015).
Rock et al. (2012) demonstrated that when microinfused directly into the DRN, CBD reduced LiCl-induced conditioned gaping, and this effect was blocked by WAY100635. Activation of these somatodendritic 5-HT1A autoreceptors in the DRN results in a reduction in the firing rate of 5-HT afferents to terminal forebrain regions (Sotelo et al. 1990; Verge et al. 1985), and indeed, in vivo single-unit extracellular recordings of DRN 5-HT neurons show that acute CBD administration reduced the firing rate of DRN 5-HT neurons and this effect was blocked by WAY100635 (De Gregorio et al. 2019). The reduced firing rate of DRN 5-HT neurons by acute CBD administration was also blocked by a transient receptor potential cation channel subfamily V member 1 (TRPV1) antagonist (De Gregorio et al. 2019). This suggests that 5-HT1A receptors and/or TRPV1 may be mediating CBD’s acute effects on DRN 5-HT neuron firing. Furthermore, we (Limebeer et al. 2018) have recently demonstrated that CBD reduces a LiCl-induced elevation of 5-HT selectively in the IIC. Together, these findings suggest that acute administration of CBD, CBDA and HU-580 is likely exerting its anti-nausea effect by activation of somatodendritic 5-HT1A autoreceptors, reducing the firing of 5-HT neurons in the DRN, presumably leading to reduced 5-HT release in forebrain regions such as the IIC.
Interestingly, after repeated exposure (7 days) of CBD (5 mg/kg, s.c.), CBDA (1 μg/kg, s.c.) and HU-580 (1 μg/kg, s.c.), each remained effective in suppressing LiCl-induced gaping (without the development of tolerance) and the effects of each were also reversed by pretreatment with the 5-HT1A receptor antagonist WAY100635. These results may be surprising, given that De Gregorio et al. (2019) reported that after 7 days of treatment, CBD increased the firing rate of DRN 5-HT neurons. Furthermore, they also found that this repeated (7-day) CBD treatment reduced the sensitivity of 5-HT neurons to the suppressive effect of lysergic acid diethylamide, and they suggest this is likely through desensitisation of 5-HT1A autoreceptors in the DRN. However, the effect of a 5-HT1A receptor agonist on the firing rate of the DRN neurons following repeated CBD treatment was not assessed. The DRN projects extensively throughout regions of the midbrain and forebrain, with one projection region being the IIC (e.g. Peyron et al. 1998; Vertes 1991). Although the firing rate of a subset of 5-HT neurons in the DRN was measured by De Gregorio et al. (2019), the projection targets of these particular neurons are unknown. It is possible that the neurons showing increased firing after CBD administration are not those that project to the forebrain regions that mediate nausea (such as the IIC). To fully understand this relationship, these 5-HT DRN neurons would need to be examined by using an anterograde anatomical tracer.
Finally, when administered once a week for 4 weeks (to more closely mimic the potential clinical application of these compounds), CBD (5 mg/kg, s.c.), CBDA (1 μg/kg, s.c.) and HU-580 (1 μg/kg, s.c.) also reduced LiCl-induced conditioned gaping. It should be noted that each compound was similarly effective in its anti-nausea efficacy, despite the difference in doses of these compounds (which were chosen because they are the most effective anti-nausea doses tested to date). That is, CBD (5 mg/kg, s.c.), CBDA (1 μg/kg, s.c.) and HU-580 (1 μg/kg, s.c.) all reduced LiCl-induced conditioned gaping with similar efficacy in each experiment, again providing further evidence for greater anti-nausea potency of CBDA and HU-580 over that of CBD. Together, our findings suggest long-term anti-nausea efficacy of these compounds, without the development of tolerance.
CBD’s neuroprotective, anti-depressant, anxiolytic and anti-allodynic effects are also mediated by 5-HT1A receptors (e.g. Campos et al. 2016; Hind et al. 2016; Jesus et al. 2019; Rock et al. 2017), but few groups have looked at the effect of repeated CBD administration. Campos et al. (2013) have shown that repeated CBD (5 mg/kg, i.p. for 21 days) decreases escape responses in the elevated T-maze, (suggesting a panicolytic effect) which is mediated by activation of 5-HT1A receptors located in the dorsal periaqueductal gray (a brain region mediating panic), but this repeated CBD treatment did not change 5-HT1A receptor messenger RNA (mRNA) expression nor did it modify levels of 5-HT in the dorsal periaqueductal gray. This suggests that repeated CBD reduces panic effects by action at 5-HT1A receptors in the dorsal periaqueductal gray, independent of a change in 5-HT levels or 5-HT1A mRNA expression. In streptozotocin-induced diabetic rats, Jesus et al. (2019) have shown that repeated treatment with CBD (0.3 or 3 mg/kg, i.p., for 14 days) reduced mechanical allodynia (likely through a 5-HT1A receptor–mediated effect) and CBD normalised the lower spinal cord levels of 5-HT found in the diabetic rats. This suggests that CBD may effectively treat diabetic neuropathy through activation of the 5-HT system. Finally, using the olfactory bulbectomy mouse model of depression, Linge et al. (2016) have shown that repeated CBD (50 mg/kg, i.p., for 3 days + 10 mg/kg until day 14) has anti-depressant-like effects (likely through a 5-HT1A receptor–mediated effect) and CBD enhanced serotonin levels in the ventromedial prefrontal cortex, suggesting that CBD is enhancing 5-HT signalling. Taken together, these results suggest an interaction between repeated CBD administration, activation of 5-HT1A receptors (and in some cases, changes in expression of these receptors) and alterations in 5-HT levels in some associated brain regions. Although Limebeer et al. (2018) have shown that acute CBD reduces a LiCl-induced elevation of 5-HT selectively in the IIC, we do not know whether the acute effect is 5-HT1A receptor mediated, nor do we know what would happen to levels of 5-HT in this region with repeated CBD administration.
When repeatedly administered for 7 days, CBD (5 mg/kg, s.c.) maintained its anti-emetic effect, suggesting a lack of tolerance in the shrews. In rats, De Gregorio et al. (2019) showed that repeated CBD (5 mg/kg, s.c.) reduced the firing rate of DRN neurons (a seemingly pro-nausea/vomiting effect). This inconsistency in CBD’s effects may be due to species differences. We also do not know whether the anti-emetic effects of repeated CBD are mediated by the 5-HT1A receptor, as CBD’s acute anti-emetic effects have been shown to be (Rock et al. 2012).
The suppression of conditioned gaping appears to be exclusive to nausea-induced conditioned gaping and not learning per se, because none of these compounds interfered with the strength of learning of the LiCl-paired saccharin in the CTA test (see Parker 2014 for review). This finding is consistent with other CTA tests involving administration of other cannabinoids (e.g. Bolognini et al. 2013; Rock and Parker 2013; Rock et al. 2011, 2013, 2015). In experiment 4, female rats consumed less saccharin than males, regardless of pretreatment group. This reduced consumption for females in comparison to males may simply be a function of the females having a smaller body weight.
Recently, Anderson et al. (2019) demonstrated that i.p. CBDA administration in a Tween-based vehicle (similar to the vehicle used in these studies) improves brain penetration of CBDA (brain–plasma ratio of 1.9 in Tween-based vehicle versus 0.04 in vegetable oil) in mice. Improved brain penetration of CBDA by the use of a Tween-based vehicle may be therapeutically beneficial, because this suggests that more CBDA reaches the brain (as many therapeutic agents for central nervous system diseases are limited by their inability to cross the blood–brain barrier), resulting in the low-dose anti-nausea effect that we see with CBDA when delivered in a Tween-based vehicle. Unfortunately, in this study, we did not measure brain or plasma levels to assess whether repeated treatment might modify this brain penetration.
Taken together, our results suggest that CBD (5 mg/kg, s.c.), CBDA (1 μg/kg, s.c.) or HU-580 (1 μg/kg, s.c.) effectively reduce nausea in male rats when administered acutely, repeatedly (7 days) and once/week for 4 weeks. The anti-nausea effects seem to be mediated by the 5-HT1A receptor. In addition, CBD (5 mg/kg, s.c.) also reduced LiCl-induced vomiting in shrews when administered acutely and repeatedly (7 days). Importantly, tolerance to the anti-nausea and anti-vomiting effects was not observed. Finally, acute CBD (5 and 20 mg/kg, s.c.) reduced nausea in male and female rats in a similar manner; however, we cannot be certain that CBDA and HU-580 are equally effective in combatting nausea in both sexes, especially based on the report by Zhu et al. (2020) that HU-580 is more effective in treating pain in male rats than in female rats. Clinical trials utilising these compounds as adjunct treatments with existing anti-emetic regimens are needed to combat chemotherapy-induced nausea and vomiting.
Abbreviations
- 5-HT1A :
-
Serotonin 1A
- ANOVA :
-
Analysis of variance
- CBD :
-
Cannabidiol
- CBDA :
-
Cannabidiolic acid
- CTA:
-
Conditioned taste avoidance
- DRN :
-
Dorsal raphe nucleus
- IIC :
-
Interceptive insular cortex
- i.p. :
-
Intraperitoneal
- LiCl :
-
Lithium chloride
- SAL :
-
Saline
- s.c. :
-
Subcutaneous
- TR :
-
Taste reactivity
- VEH :
-
Vehicle
- WAY :
-
WAY100635
References
Anderson LL, Low IK, Banister SD, McGregor IS, Arnold JC (2019) Pharmacokinetics of phytocannabinoid acids and anticonvulsant effect of cannabidiolic acid in a mouse model of Dravet syndrome. J Nat Prod 82:3047–3055. https://doi.org/10.1021/acs.jnatprod.9b00600
Araz M, Karaagac M, Korkmaz L, Koral L, Inci F, Beypinar I, Uysal M, Artac M (2019) Comparison of palonosetron and granisetron in triplet antiemetic therapy in nonmetastatic breast cancer patients receiving high emetogenic chemotherapy: a multicenter, prospective, and observational study. Cancer Chemother Pharmacol 83:1091–1097. https://doi.org/10.1007/s00280-019-03831-4
Bolognini D, Rock EM, Cluny NL, Cascio MG, Limebeer CL, Duncan M, Stott CG, Javid FA, Parker LA, Pertwee RG (2013) Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br J Pharmacol 168:1456–1470. https://doi.org/10.1111/bph.12043
Campos AC, de Paula SV, Carvalho MC, Ferreira FR, Vicente MA, Brandão ML, Zuardi AW, Zangrossi H Jr, Guimarães FS (2013) Involvement of serotonin-mediated neurotransmission in the dorsal periaqueductal gray matter on cannabidiol chronic effects in panic-like responses in rats. Psychopharmacology 226:13–24. https://doi.org/10.1007/s00213-012-2878-7
Campos AC, Fogaça MV, Sonego AB, Guimarães FS (2016) Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 112:119–127. https://doi.org/10.1016/j.phrs.2016.01.033
Chow R, Tsao M, Chiu L, Popovic M, Milakovic M, Lam H, DeAngelis C (2018) Efficacy of the combination neurokinin-1 receptor antagonist, palonosetron, and dexamethasone compared to others for the prophylaxis of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med 7:221–233. https://doi.org/10.21037/apm.2018.03.09
Clemmons AB, Orr J, Andrick B, Gandhi A, Sportes C, DeRemer D (2018) Randomized, placebo-controlled, phase III trial of fosaprepitant, ondansetron, dexamethasone (FOND) versus FOND plus olanzapine (FOND-O) for the prevention of chemotherapy-induced nausea and vomiting in patients with hematologic malignancies receiving highly emetogenic chemotherapy and hematopoietic cell transplantation regimens: the FOND-O trial. Biol Blood Marrow Transplant 24:2065–2071. https://doi.org/10.1016/j.bbmt.2018.06.005
Crombie L, Crombie WML (1977) Cannabinoid acids and esters: miniaturized synthesis and chromatographic study. Phytochemistry 16:1413–1420. https://doi.org/10.1016/S0031-9422(00)88794-4
De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, Aboud M, Maione S, Comai S, Gobbi G (2019) Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 160:136–150. https://doi.org/10.1097/j.pain.0000000000001386
Giagnuolo G, Buffardi S, Rossi F, Petruzziello F, Tortora C, Buffardi I, Marra N, Beneduce G, Menna G, Parasole R (2019) Single center experience on efficacy and safety of aprepitant for preventing chemotherapy-induced nausea and vomiting (CINV) in pediatric Hodgkin lymphoma. PLoS One 14:e0215295. https://doi.org/10.1371/journal.pone.0215295
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, Clark-Snow R, Gill DP, Groshen S, Grunberg S, Koeller JM, Morrow GR, Perez EA, Silber JH, Pfister DG (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. J Clin Oncol 17:2971–2994. https://doi.org/10.1200/JCO.1999.17.9.2971
Grill HJ, Norgren R (1978) The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 43:263–279. https://doi.org/10.1016/0006-8993(78)90568-1
Guimarães FS, Chiaretti TM, Graeff FG, Zuardi AW (1990) Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology 100:558–559. https://doi.org/10.1007/BF02244012
Hempel BJ, Clasen MM, Nelson KH, Woloshchuk CJ, Riley AL (2018) An assessment of concurrent cannabidiol and Δ9-tetrahydrocannabinol administration in place aversion and taste avoidance conditioning. Exp Clin Psychopharmacol 26:205–213. https://doi.org/10.1037/pha0000188
Hind WH, England TJ, O’Sullivan SE (2016) Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. Br J Pharmacol 173:815–825. https://doi.org/10.1111/bph.13368
Ho C, MacDougall D (2019) Nabilone for the treatment of nausea and vomiting or anorexia: a review of clinical effectiveness and guidelines. Canadian Agency for Drugs and Technologies in Health, Ottawa
Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, Taffe MA (2018) Effects of Δ9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology 235:2541–2557. https://doi.org/10.1007/s00213-018-4946-0
Jesus CHA, Redivo DDB, Gasparin AT, Sotomaior BB, de Carvalho MC, Genaro K, Zuardi AW, Hallak JEC, Crippa JA, Zanoveli JM, da Cunha JM (2019) Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res 1715:156–164. https://doi.org/10.1016/j.brainres.2019.03.014
Kasten CR, Zhang Y, Boehm SL 2nd (2019) Acute cannabinoids produce robust anxiety-like and locomotor effects in mice, but long-term consequences are age- and sex-dependent. Front Behav Neurosci 13:32. https://doi.org/10.3389/fnbeh.2019.00032
King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ (2017) Single and combined effects of Δ(9) -tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br J Pharmacol 174:2832–2841. https://doi.org/10.1111/bph.13887
Liaw CC, Wang CH, Chang HK, Liau CT, Yeh KY, Huang JS, Lin YC (2001) Gender discrepancy observed between chemotherapy-induced emesis and hiccups. Support Care Cancer 9:435–441. https://doi.org/10.1007/s005200000231
Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, Parker LA (2010) Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol 161:336–349. https://doi.org/10.1111/j.1476-5381.2010.00885.x
Limebeer CL, Rock EM, Sharkey KA, Parker LA (2018) Nausea-induced 5-HT release in the interoceptive insular cortex and regulation by monoacylglycerol lipase (MAGL) inhibition and cannabidiol. eNeuro 5:ENEURO.0256-18.2018. https://doi.org/10.1523/ENEURO.0256-18.2018
Linge R, Jiménez-Sánchez L, Campa L, Pilar-Cuéllar F, Vidal R, Pazos A, Adell A, Díaz Á (2016) Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology 103:16–26. https://doi.org/10.1016/j.neuropharm.2015.12.017
Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T (2010) A behavioural comparison of acute and chronic delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int J Neuropsychopharmacol 13:861–876. https://doi.org/10.1017/s1461145709990605
Miller S, Daily L, Leishman E, Bradshaw H, Straiker A (2018) Δ9-Tetrahydrocannabinol and cannabidiol differentially regulate intraocular pressure. Invest Ophthalmol Vis Sci 59:5904–5911. https://doi.org/10.1167/iovs.18-24838
Navari RM, Rapoport BL, Powers D, Arora S, Clark-Snow R (2018) Rolapitant for the prevention of nausea in patients receiving highly or moderately emetogenic chemotherapy. Cancer Med 7:2943–2950. https://doi.org/10.1002/cam4.1560
Parker LA (2014) Conditioned flavor avoidance and conditioned gaping: rat models of conditioned nausea. Eur J Pharmacol 722:122–133. https://doi.org/10.1016/j.ejphar.2013.09.070
Parker LA, Mechoulam R (2003) Cannabinoid agonists and antagonists modulate lithium-induced conditioned gaping in rats. Integr Physiol Behav 38:133–145. https://doi.org/10.1007/BF02688831
Parker LA, Kwiatkowska M, Burton P, Mechoulam R (2004) Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 171:156–161. https://doi.org/10.1007/s00213-003-1571-2
Pertwee RG, Rock EM, Guenther K, Limebeer CL, Stevenson LA, Haj C, Smoum R, Parker LA, Mechoulam R (2018) Cannabidiolic acid methyl ester, a stable synthetic analogue of cannabidiolic acid, can produce 5-HT1A receptor-mediated suppression of nausea and anxiety in rats. Br J Pharmacol 175:100–112. https://doi.org/10.1111/bph.14073
Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH (1998) Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience 82:443–468. https://doi.org/10.1016/s0306-4522(97)00268-6
Rock EM, Parker LA (2013) Effect of low doses of cannabidiolic acid and ondansetron on LiCl-induced conditioned gaping (a model of nausea-induced behaviour) in rats. Br J Pharmacol 169:685–692. https://doi.org/10.1111/bph.12162
Rock EM, Parker LA (2016) Cannabinoids as potential treatment for chemotherapy-induced nausea and vomiting. Front Pharmacol 7:221. https://doi.org/10.3389/fphar.2016.00221
Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG, Mechoulam R, Parker LA (2011) Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology 215:505–512. https://doi.org/10.1007/s00213-010-2157-4
Rock EM, Bolognini D, Limebeer CL, Cascio MG, Anavi-Goffer S, Fletcher PJ, Mechoulam R, Pertwee RG, Parker LA (2012) Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol 165:2620–2634. https://doi.org/10.1111/j.1476-5381.2011.01621.x
Rock EM, Kopstick RL, Limebeer CL, Parker LA (2013) Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br J Pharmacol 170:641–648. https://doi.org/10.1111/bph.12316
Rock EM, Limebeer CL, Parker LA (2015) Effect of combined doses of Δ(9)-tetrahydrocannabinol (THC) and cannabidiolic acid (CBDA) on acute and anticipatory nausea using rat (Sprague- Dawley) models of conditioned gaping. Psychopharmacol (Berl) 232:4445–4454. https://doi.org/10.1007/s00213-015-4080-1
Rock EM, Limebeer CL, Petrie GN, Williams LA, Mechoulam R, Parker LA (2017) Effect of prior foot shock stress and Δ9-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology 234:2207–2217. https://doi.org/10.1007/s00213-017-4626-5
Rock EM, Sullivan MT, Pravato S, Pratt M, Limebeer CL, Parker LA (2020) Effect of combined doses of Δ9-tetrahydrocannabinol and cannabidiol or tetrahydrocannabinolic acid and cannabidiolic acid on acute nausea in male Sprague-Dawley rats. Psychopharmacology 237:901–914. https://doi.org/10.1007/s00213-019-05428-4
Sepúlveda-Vildósola AC, Betanzos-Cabrera Y, Lastiri GG, Rivera-Márquez H, Villasis-Keever MA, Del Angel VW, Díaz FC, López-Aguilar E (2008) Palonosetron hydrochloride is an effective and safe option to prevent chemotherapy-induced nausea and vomiting in children. Arch Med Res 39:601–606. https://doi.org/10.1016/j.arcmed.2008.04.007
Sotelo C, Cholley B, El Mestikawy S, Gozlan H, Hamon M (1990) Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur J Neurosci 2:1144–1154. https://doi.org/10.1111/j.1460-9568.1990.tb00026.x
Timaeus S, Elder J, Franco K (2018) Evaluation of the use of fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in pediatric patients. J Pediatr Hematol Oncol 40:527–531. https://doi.org/10.1097/MPH.0000000000001213
Tsuji D, Suzuki K, Kawasaki Y, Goto K, Matsui R, Seki N, Hashimoto H, Hama T, Yamanaka T, Yamamoto N, Itoh K (2019) Risk factors associated with chemotherapy-induced nausea and vomiting in the triplet antiemetic regimen including palonosetron or granisetron for cisplatin-based chemotherapy: analysis of a randomized, double-blind controlled trial. Support Care Cancer 27:1139–1147. https://doi.org/10.1007/s00520-018-4403-y
Verge D, Daval G, Patey A, Gozlan H, el Mestikawy S, Hamon M (1985) Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur J Pharmacol 113:463–464. https://doi.org/10.1016/0014-2999(85)90099-8
Vertes RP (1991) A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313:643–668. https://doi.org/10.1002/cne.903130409
Zhu YF, Linher-Melville K, Niazmand MJ, Sharma M, Shahid A, Zhu KL, Parzei N, Sidhu J, Haj C, Mechoulam R, Singh G (2020) An evaluation of the anti-hyperalgesic effects of cannabidiolic acid-methyl ester (CBDA-ME) in a preclinical model of peripheral neuropathic pain. Br J Pharmacol 177:2712–2725. https://doi.org/10.1111/bph.14997
Funding
The research described here was funded by research grants from the Natural Sciences and Engineering Council of Canada (NSERC: 03629) and Canadian Institutes of Health Research (CIHR: 388239).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rock, E.M., Sullivan, M.T., Collins, S.A. et al. Evaluation of repeated or acute treatment with cannabidiol (CBD), cannabidiolic acid (CBDA) or CBDA methyl ester (HU-580) on nausea and/or vomiting in rats and shrews. Psychopharmacology 237, 2621–2631 (2020). https://doi.org/10.1007/s00213-020-05559-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05559-z