Abstract
Rationale
Data from both preclinical and clinical studies have provided proof of concept that modulation of limbic and forebrain glutamate, via mGlu2/3 receptor agonists, might provide therapeutic benefits in many psychiatric disorders including schizophrenia and anxiety.
Objective
The aim of this study was to assess the efficacy of a structurally novel, potent, selective mGlu2/3 receptor agonist with improved bioavailability (LY404039) in animal models predictive of antipsychotic and anxiolytic efficacy.
Materials and methods
LY404039 was assessed in amphetamine- and phencyclidine-induced hyperlocomotion, conditioned avoidance responding, fear-potentiated startle, marble burying, and rotarod behavioral tests. Monoamine release and turnover were assessed using microdialysis and ex vivo tissue levels.
Results
LY404039 attenuated amphetamine- and phencyclidine-induced hyperlocomotion (3–30 and 10 mg/kg, respectively). LY404039 (3–10 mg/kg) inhibited conditioned avoidance responding. LY404039 also reduced fear-potentiated startle in rats (3–30 μg/kg) and marble burying in mice (3–10 mg/kg), indicating anxiolytic-like effects. Importantly, LY404039 did not produce sedative effects or motor impairment as measured by rotarod performance and lack of escape failures in the conditioned avoidance task (at doses up to 30 and 10 mg/kg, respectively). LY404039 (10 mg/kg) also increased dopamine and serotonin release/turnover in the prefrontal cortex.
Conclusions
These results demonstrate the broad preclinical efficacy of LY404039 across multiple animal models of antipsychotic and anxiolytic efficacy. Additionally, this compound modulates mesocortical neurotransmission and provides a novel mechanism for the treatment of psychiatric disorders that may be associated with improved efficacy and reduced incidence of undesirable side effects. As glutamatergic dysfunction has been linked to the etiology of schizophrenia, clinical studies with more potent mGlu2/3 agonists, such as LY404039, may be useful to explore the validity of this hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence suggests that the primary symptoms of psychiatric disorders such as schizophrenia and anxiety may be associated with altered central glutamate transmission (Heresco-Levy 2005; Moghaddam 2002). Noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonists, including phencyclidine (PCP) and ketamine, exacerbate psychotic symptoms in schizophrenic patients and produce working memory deficits and psychotomimetic-like hallucinations in healthy volunteers (Krystal et al. 2005b; Morris et al. 2005). Pathological glutamate release in limbic and cortical regions has also been implicated in stress and anxiety disorders (Bergink et al. 2004; Moghaddam 2002). Medications currently used to treat anxiety and schizophrenia produce therapeutic effects through several different mechanisms including dopamine, serotonin and γ-aminobutyric acid (GABA) and have proven adequate in some patient populations (Heresco-Levy 2005; Swanson et al. 2005). These medications, however, lack efficacy in other patient populations and are associated with adverse side effects including weight gain, motor dysfunction, sedation, tolerance, abuse liability, and dependence (Ascher-Svanum et al. 2005; Gudex 1991; Sachdev 2005). Given that current pharmacotherapies leave marked unmet clinical need, researchers have explored novel mechanisms in the hope of improving treatment outcomes for these patients.
Among those mechanisms receiving considerable attention in recent years is the glutamatergic system. In particular, the group II mGlu receptor subtypes (mGlu2 and mGlu3) stand out as possible drug targets based on their neuroanatomical localization and selective modulatory effects upon glutamatergic tone in these regions. MGlu2 and mGlu3 receptors are highly localized within limbic and forebrain areas associated with anxiety and schizophrenia including the amygdala, hippocampus, and prefrontal cortex (Ohishi et al. 1993a, b; Swanson et al. 2005). MGlu2/3 receptors are negatively coupled to adenylyl cyclase and function as autoreceptors and heteroreceptors to modulate the synaptic release of glutamate and other neurotransmitters (Cartmell and Schoepp 2000; Schoepp 2001). It is presumed that under pathological conditions of excessive glutamatergic tone, characteristic of anxiety states and schizophrenia, activation of mGlu2/3 receptors normalizes neurotransmitter imbalance (Bergink et al. 2004; Moghaddam 2002; Schoepp and Marek 2002).
Preclinical studies indicate that modulation of glutamatergic activity in limbic and forebrain areas may have therapeutic value as a novel mechanism for the treatment of psychotic and anxiety disorders (Heresco-Levy 2005; Schoepp and Marek 2002; Swanson et al. 2005). The mGlu2/3 receptor agonist (1S,2S,5R,6S)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylate monohydrate (LY354740) reversed PCP-evoked hyperlocomotion and working memory deficits while also blocking PCP-induced glutamate release in the prefrontal cortex (Moghaddam and Adams 1998). The amelioration of PCP-induced hyperlocomotion by LY354740, and another mGlu2/3 receptor agonist (−)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY379268), was reversed by the selective mGlu2/3 receptor antagonist 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xant-9-yl) propanoic acid (LY341495, Kingston et al. 1998), indicating that the antipsychotic-like effects were mediated via mGlu2/3 receptors (Cartmell et al. 1999). In contrast, LY354740 has demonstrated less activity in other models predictive of antipsychotic activity, including conditioned avoidance responding and prepulse inhibition of startle (Schreiber et al. 2000; Takamori et al. 2003). LY354740 has also demonstrated efficacy in animal models predictive of anxiolytic efficacy, including fear-potentiated startle, elevated plus-maze, stress-induced hyperthermia (tested as racemate LY314582), and lactate-induced panic (Helton et al. 1998; Linden et al. 2004, 2005b; Shekhar and Keim 2000; Spooren et al. 2002). Collectively, preclinical evidence suggests that mGlu2/3 receptor agonists function to reduce excessive glutamatergic neurotransmission in limbic and forebrain areas and may be useful in the treatment of psychosis as well as anxiety.
Clinical data with LY354740 has produced results consistent with earlier animal studies (Schoepp et al. 2003; Swanson et al. 2005). LY354740 attenuated fear-potentiated startle and reduced subjective measures of anxiety and negative affect in humans without producing sedation (Grillon et al. 2003). Also, treatment with LY354740 decreased the number and severity of CO2-induced anxiety symptoms in panic-prone patients without producing sedative or amnestic effects (Schoepp et al. 2003). Another study reported that LY354740 dose-dependently reversed ketamine-induced working memory deficits in healthy human subjects (Krystal et al. 2005a), indicative of potential therapeutic benefit in treating schizophrenia. However, clinical development of LY354740 was hampered by low oral bioavailability in humans (3–5%) due to poor gastrointestinal absorption and inadequate central exposure levels (Johnson et al. 2002; Bueno et al. 2005). Therefore, emphasis has been placed on the development of potent selective mGlu2/3 receptor agonists with more favorable pharmacokinetic profiles.
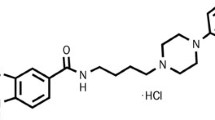
As clinical development of mGlu2/3 receptor agonists will require high potency molecules with sufficient oral bioavailability, the goal of the present study was to characterize the in vivo pharmacology of a new molecule with such characteristics. Thus, the structurally novel mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039, Fig. 1) with high in vitro potency and efficacy and improved rodent bioavailability (Monn et al. 2007; Rorick-Kehn et al. 2007) was evaluated for its ability to influence several in vivo effects that may bear upon the predictive efficacy of this compound against neuropsychiatric disease states. These include amphetamine- and phencyclidine-induced hyperlocomotion, spontaneous locomotor activity, conditioned avoidance responding, fear-potentiated startle, marble burying, motor coordination on the rotarod apparatus, and monoamine release and turnover in the prefrontal cortex. Recently, LY404039 was reported to reduce alcohol-seeking behavior in alcohol-preferring (P) rats (Rodd et al. 2006). These results are consistent with the hypothesis that relapse to drug and alcohol seeking may be associated with excessive glutamatergic tone and that activation of mGlu2/3 receptor agonists may provide a novel approach for the treatment of addiction and craving.
Materials and methods
Animals
All experiments were conducted in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (http://www.nap.edu/readingroom/books/labrats/) and were approved by the Eli Lilly Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing between 200–350 g were tested in the locomotor, fear-potentiated startle, microdialysis/monoamine turnover, and rotarod experiments. The sample sizes for each experiment were as follows: LY404039 effects on spontaneous locomotor activity, n = 10 per group; clozapine effects on spontaneous locomotor activity, n = 6–8/group; LY404039 effects on amphetamine-induced hyperlocomotion, n = 11–12 per group; reversal of LY404039 effects on amphetamine-induced hyperlocomotion, n = 10–12 per group; fear-potentiated startle, n = 8 per group; LY341495 reversal of fear-potentiated startle effects, n = 12 per group; microdialysis experiments, n = 5; ex vivo tissue analysis, n = 8 per group; rotarod performance, n = 8 per group; PCP-induced disruptions of rotarod performance n = 8 per group. For the locomotor activity and fear-potentiated startle experiments, rats were pair-housed and food-fasted for 12 to 18 h before the experiment, with water available ad libitum. The acute fasting procedures were routinely implemented to ensure more consistent drug exposure across the various dose groups and experiments. For the microdialysis experiments, rats were single-housed with standard laboratory chow and water available ad libitum. To allow the implantation of dialysis probes, rats were anesthetized with chloral hydrate/pentobarbital (170 and 36 mg/kg in 30% propylene glycol and 14% ethanol, respectively). After surgery, rats were single-housed to avoid interference with the chronically implanted dialysis probe of the cagemate. For all other experiments, rats were pair-housed with standard laboratory chow and water available ad libitum. All animals were maintained on a 12-h light/dark cycle (lights on at 06:00).
Male Fischer-F344 rats (Harlan, Indianapolis, IN), weighing between 350–400 g, were tested in the conditioned avoidance responding experiment (n = 6). The rats used in this experiment had previous exposure to drug treatments before receiving LY404039. A washout period of 1 week was implemented before the LY404039 experiment. Rats were pair-housed with water and standard laboratory chow available ad libitum.
Male NIH Swiss mice (Harlan, Indianapolis, IN), weighing approximately 28–32 g, were tested in the rotarod and marble-burying experiments (LY404039 or chlordiazepoxide, n = 6; vehicle, n = 12). For these experiments, mice were group-housed (n = 10–12 per cage) with water and laboratory chow available ad libitum.
Drugs
LY404039 and LY341495 were synthesized at Lilly Research Laboratories. Diazepam, chlordiazepoxide hydrochloride, d-amphetamine sulfate, and phencyclidine hydrochloride were obtained from Sigma Aldrich (St. Louis, MO). Clozapine was purchased from Research Biochemicals International (Natick, MA). Clozapine was dissolved in 0.9% NaCl by the drop-wise addition of 8.5% lactic acid. Lilly compounds that were dosed orally were dissolved in sterile water with drop-wise addition of NaOH. Lilly compounds that were dosed parenterally were dissolved in 0.9% NaCl by the drop-wise addition of NaOH. All other compounds were dissolved in 0.9% NaCl. In cases where the salt form of a drug was tested, reported doses reflect the overall weight, rather than the free base weight. For all experiments, drugs were mixed fresh before use. Doses were administered to rats in a volume of 1 ml/kg; doses were administered to mice in a volume of 10 ml/kg. Route of administration is indicated separately for each experiment.
Spontaneous locomotor activity; inhibition of amphetamine-induced hyperlocomotion, reversal by the mGlu2/3 receptor antagonist LY341495
Apparatus and procedures used to assess spontaneous locomotor activity and amphetamine-induced hyperlocomotion were similar to those previously described (Rorick-Kehn et al. 2006). Ambulations, defined as an overall change in the animal’s body position within the chamber, were recorded by a computer for offline analysis.
Spontaneous locomotor activity
To assess the effects of LY404039 and clozapine on spontaneous locomotor activity, animals received an oral gavage of vehicle (sterile water, 1 ml/kg), LY404039 (0.3, 1, 3, or 10 mg/kg), or clozapine (0.3, 1, 3, or 10 mg/kg) and returned to their home cage. After 60 min, rats were placed in the test cage for a 45-min assessment of spontaneous locomotor activity.
Amphetamine-induced hyperlocomotion
Rats were administered a randomly assigned dose of LY404039 (0.3, 1, 3, 10, or 30 mg/kg, p.o.) or sterile water vehicle (1 ml/kg, p.o.) and returned to their home cage for 30 min. Rats were then placed in the test cage for a 30-min habituation period to allow for acclimation to the test cage environment and to measure baseline locomotor activity. After the habituation period, animals received a challenge dose of amphetamine (3 mg/kg, s.c.) or 0.9% NaCl vehicle (1 ml/kg, s.c.) and then observed for an additional 60 min. The effect of the benzodiazepine anxiolytic diazepam on PCP-induced hyperlocomotion was also assessed. For this experiment, animals received diazepam (1, 3, 10, or 30 mg/kg, p.o.) or sterile water vehicle (1 ml/kg, p.o.) and were returned to their home cage for 30 min as described for LY404039. After the 30-min habituation period, rats received a challenge dose of PCP (5 mg/kg, s.c.) or 0.9% NaCl vehicle (1 ml/kg, s.c.) and were observed for 60 min.
Reversal of LY404039 suppression of PCP- and amphetamine-induced hyperlocomotion
Rats were administered LY341495 (1 mg/kg, s.c.) or 0.9% NaCl vehicle (1 ml/kg, s.c.) and were returned to their home cage for 90 min. The parameters used in this experiment were chosen based on PCP time-course analysis, which determined that LY404039 was most potent at reducing PCP-induced behaviors at 1–2 h post-administration (see below). Rats received an injection of LY404039 (10 mg/kg, p.o.) or sterile water vehicle (1 ml/kg, p.o.) and tested for activity levels during a 30-min habituation period. After the 30-min habituation period, a challenge dose of amphetamine (3 mg/kg, s.c.), PCP (5 mg/kg, s.c.), or 0.9% NaCl vehicle (1 ml/kg, s.c.) was delivered, and rats were observed for an additional 60 min.
Onset and duration of action studies
Onset and duration of action studies were carried out using a time-sampling procedure in which the time between dosing (LY404039, 10 mg/kg, p.o.) and experimental testing was systematically varied. The pretreatment times tested were: 1, 2, 3, 4, 8, and 24 h. Animals in the vehicle/vehicle group were distributed evenly across all drug pretreatment groups. All other procedures were as described above.
Conditioned avoidance responding
Rats were trained and tested in standard rat operant shuttle-avoidance chambers (Coulbourn Instruments, Allentown, PA) placed within sound-attenuating chambers. The apparatus consisted of two adjacent stainless steel compartments (25 × 25 × 30 cm) with clear Plexiglas front and rear walls. A stainless steel wall containing a guillotine-style shuttle door (8 × 9 cm) separated the compartments. The floor grid was comprised of 0.5 cm diameter stainless steel rods separated by 1.5 cm (center-to-center). A houselight (5 watts) located approximately 25 cm above the floor grid on each side of the chamber served as the conditional stimulus (CS).
Rats received five training sessions per week. Each session consisted of a 60-s adaptation period followed by 50 trials, with inter-trial intervals of 30 s. The illumination of the CS and opening of the shuttle door signaled trial onset. For each trial, rats were given 10 s (CS period) to perform a shuttle response. After 10 s, a mild footshock (2.5 mA) was initiated through the grid floor for 10 s (shock period). Shuttle responses performed during the 10-s CS period were recorded as avoidance responses, and no shocks were received. Shuttle responses made during the 10-s shock period were recorded as escape responses. If no shuttle responses occurred during the 10-s shock period, an escape failure was recorded, the shuttle door closed, and the shock terminated. For all rats, the session terminated after 50 trials (avoidance + escape) or if 20 escape failures were recorded.
Rats were trained until performance was consistently above 90% avoidance responding (45 avoidance responses out of 50 trials). Drug treatments began once the rats performed greater than 90% avoidance responses on three consecutive days. On the day before each drug day, rats received a sham injection of vehicle 30 min before the session (vehicle day). If rats reached criterion on the vehicle day (greater than 90% avoidance), drug treatments were administered on the following day. On drug days, LY404039 (3 or 10 mg/kg, i.p.) was administered 30 min before the session.
Fear-potentiated startle
Dose-response studies
Rats were trained and tested using previously described procedures (Helton et al. 1998). Briefly, on days 1 and 2, rats received ten conditioning trials in which a 5,000-ms light CS was paired with a co-terminating 1-mA footshock. Forty-eight hours after the second conditioning session (on testing day 3), fear-potentiated startle was assessed. This session consisted of 20 alternating trials of the noise-alone trials (110-dB white noise, 50 ms duration, 70-dB background noise level) or noise preceded by light (light + noise). Startle response amplitudes for each trial type (noise-alone vs light + noise) were averaged for each rat across the entire test session. Data were presented as the difference between noise-alone and light + noise (i.e., fear-potentiation of the startle response). Recorded values represent a maximum change in voltage from baseline and are presented as peak maximum voltage (V max).
For the dose-response assessment of fear-potentiated startle, rats received LY404039 (0.03, 0.3, 3, or 30 μg/kg, p.o.), diazepam (0.6 mg/kg, p.o.), or sterile water vehicle (1 ml/kg, p.o.) 30 min before the session. In a separate group of animals, rats received vehicle (1 ml/kg, p.o.), LY404039 (30 μg/kg, p.o.), LY341495 (1 mg/kg, s.c.), or LY404039 (30 μg/kg, p.o.) + LY341495 (1 mg/kg, s.c.) on testing day 3. For this experiment, LY404039 was administered 30 min before the startle session, and LY341495 was administered 60 min before the startle session.
Onset and duration of action studies
Onset of action and duration of action studies were carried out by varying the time between dosing (LY404039, 30 μg/kg, p.o.) and experimental testing. The pretreatment times tested were: 1, 2, 4, 8, and 24 h. All other procedures were as described above.
Marble burying and rotarod performance in mice
Before the marble-burying experiment, the effects of LY404039 and chlordiazepoxide on motor performance were evaluated using a rotarod apparatus. Mice were acclimated to a dimly lit testing room for 60 min and then dosed with LY404039 (1, 3, or 10 mg/kg, i.p.), chlordiazepoxide (3, 10, or 30 mg/kg, i.p.), or vehicle (physiological saline, 10 ml/kg, i.p.) and returned to their home cage. Thirty minutes later, mice were placed on a rotarod (Ugo Basile, Comerio VA, Italy) operating at a speed of 6 rpm and observed for falling. Mice that fell off the rotarod on two occasions during 2 min were scored as failing. Immediately after the rotarod test, anxiolytic-like effects were evaluated in the marble-burying test. For this test, mice were placed in a plastic chamber (17 × 28 × 12 cm) containing sawdust shavings (5 mm depth; Harlan Sani-Chips, Harlan-Teklad, Indianapolis, IN) and 20 blue marbles (1.5 cm diameter) located on top of the shavings. The number of marbles buried (2/3 covered with sawdust) was recorded after 30 min. All mice were included in the marble-burying experiment regardless of performance in the rotarod test.
Rat rotarod performance
An automated rotarod apparatus (San Diego Instruments, San Diego, CA) tested for motor impairment and ataxia. Ninety minutes before drug administration, rats were trained to stay on the rotarod, rotating at 4 rpm, over four successive trials. Those rats that remained on the rod for consecutive 60-s periods were retested 30 min before drug administration. Rats successful in the retesting session received LY404039 (3, 10, or 30 mg/kg, p.o.) or sterile water vehicle (1 ml/kg, p.o.). Thirty minutes later, rats were again tested on the rotarod for a period of up to 60 s. Data were expressed as the number of seconds the animal remained on the rotarod apparatus.
An additional experiment was conducted to determine the interaction between PCP and LY404039. Experimental procedures were identical to those described above. For this experiment, rats were given injections of PCP (1, 2, 3, 5, or 8 mg/kg, s.c.) or 0.9% NaCl vehicle (1 ml/kg, s.c.) 30 min before administration of LY404039 (3 or 10 mg/kg, p.o.) or sterile water vehicle (1 ml/kg, p.o.).
In vivo neurochemistry
Microdialysis experiments
The microdialysis technique has been previously described (Li et al. 1998). Dialysis probes were implanted into the prefrontal cortex of rat brain and fixed in place with cranioplastic cement (Plastics One, Roanoke, VA). Plastic dialysis probes of loop-type were made using cellulose dialysis membrane (C-D Medical, Miami, FL) with a molecular weight cut-off of 5,000. The exposed tubing of dialysis probe measured 3 mm in length with an outer diameter of 0.6 mm. The coordinates for prefrontal cortex were: A (anterior to bregma), 3.2 mm; L (lateral from the midsagittal suture), 0.8 mm; V (ventral from the dura surface), 4 mm (Paxinos and Watson 1986). Rats with improper probe location and unstable monoamine baseline values were not included in the statistical analysis.
Microdialysis experiments were performed 2 days after surgery to allow the rats to fully recover from the operation. Rats were individually placed in a plastic bowl and connected to a liquid swivel system for freely moving animals (CMA/120, BioAnalytical Systems, West Lafayette, IN). The input tube of the dialysis probe was connected to a syringe pump (Harvard Instruments, Model 22, South Natick, MA) which delivered an unbuffered artificial cerebrospinal fluid containing 150 mM NaCl, 3 mM KCl, 1.7 mM CaCl2, and 0.9 mM MgCl2 (pH 6.0) to the probe at a rate of 2.2 μl/min. The output tubes from the swivels were attached to an electrically actuated switching valve (Valco Instruments, Houston, TX) which enabled the analysis of dialysates from two rats in parallel. Dialysates were collected by an on-line high performance liquid chromatography (HPLC) valve as detailed below, with each experiment lasting between 8 and 9 h. Less than 20% variation of the basal monoamine levels was obtained 2–3 h after the start of experiments. Drugs were given after at least three stable baseline samples were obtained.
Tissue levels of monoamines and monoamine metabolites in the prefrontal cortex
Rats were removed from their home cages and injected s.c. with 10 mg/kg LY404039, 10 mg/kg clozapine, or 0.9% NaCl vehicle and returned to their cages. For the initial experiments, rats were removed after 30 min and killed via decapitation in an adjacent room. For the time-course experiments, rats were removed and decapitated 0.5, 1, 2, 4, or 24 h post-injection. The prefrontal cortex was dissected out and frozen on dry ice. The tissue samples were then weighed individually and stored at −80°C in plastic tubes containing 0.5 ml of 0.01 M HCl until analyzed for DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-HT, and 5-HIAA. Immediately before analysis, samples were thawed at room temperature, and 0.4 ml of 0.01 M HCl (containing isoproterenol as an internal standard) was added. After sonication, 100 μl of 1.5 M perchloric acid was added, and the samples were vortexed and stored at 4°C for 30 min. The samples were then centrifuged for 2 min at 12,000 rpm, and the supernatant was analyzed by HPLC with electrochemical detection.
An HPLC analytical method was used to simultaneously detect the monoamines dopamine (DA) and serotonin (5-HT) and the metabolites DOPAC, 5-HIAA, and HVA in the same dialysates. This method was used to detect levels of monoamines and their metabolites in both the in vivo microdialysis and ex vivo tissue experiments. A BDS-Hypersil 3 μm C18 analytical column (2 × 150 mm from Keystone Scientific, Bellefonte, PA) with a ten-port HPLC valve and a 20-μl sample loop was used in configuration with a small sample clean-up column (BDS-Hypersil 3 μm C18, 2 × 10 mm) which trapped a late-eluting peak contained in the dialysate samples. The mobile phase for both columns was the same and consisted of 75 mM sodium phosphate monobasic, 350 mg/l 1-octanesulfonic acid sodium salt, 0.5 mM ethylenediaminetetraacetic acid, 0.8% tetrahydrofuran (HPLC grade, inhibitor-free), and 8% acetonitrile at pH 3 (adjusted with phosphoric acid). The flow rate for both columns was 0.20 ml/min. The analytical column was maintained at 40°C with a column heater, whereas the sample cleanup column was mounted on the ten-port valve at room temperature. An electrochemical detector (EG&G PARC, Princeton, NJ) with dual glassy carbon electrodes was used (E1 = 500 mV, E2 = −50 mV, range = 0.5 nA on both electrodes). 5-HT and metabolites were detected at E1, and NE and DA were detected at E2.
The present HPLC analytical method detected not only the three monoamines but also the metabolites of DA and 5-HT. As extracellular DOPAC, HVA, and 5-HIAA levels are much higher than DA and 5-HT levels, the DOPAC, HVA, and 5-HIAA peaks were analyzed by utilizing the 10-volt output on the detector (EG&G PARC) which sent a separate channel to the computer that allowed the metabolite peaks to stay on scale. The data of all three channels were collected using an EZChrom chromatography data system (Scientific Software, San Ramon, CA) running on a Compaq computer which calculated peak heights and sample concentrations. The sensitivity for DA, NE, and 5-HT was 0.1 pmol/ml dialysate or 2 fmol per sample (20 μl).
Data analysis
One-way analyses of variance (ANOVAs), followed by Bonferroni post hoc tests for each 5-min bin, were used to analyze the effects of LY404039 and clozapine on spontaneous locomotor activity (GraphPad Prism 4, GraphPad Software, San Diego, CA). One-way ANOVAs, followed by Newman–Keuls post hoc tests, were used to analyze the hyperlocomotion, fear-potentiated startle, and rotarod studies (GraphPad Prism 4, GraphPad Software). Separate repeated-measures ANOVAs, followed by Tukey’s LSD post hoc tests, were used to assess the effects of LY404039 on conditioned avoidance responding and escape failures (SPSS 12.0, SPSS, Chicago, IL). One-way ANOVAs, followed by Dunnett’s post hoc tests, were used to analyze the effects of LY404039 and clozapine on tissue monoamines/metabolites and LY404039 and chlordiazepoxide on marble-burying/rotarod performance in mice (GraphPad Prism 4, GraphPad Software). Repeated-measures ANOVAs were used to assess the effects of LY404039 in the microdialysis experiments (SPSS 12.0, SPSS). For these experiments, post hoc comparisons were carried out using paired t tests, with each time point compared to its respective value at t = 0. For all post hoc tests, alpha was set to α = 0.05.
Results
Spontaneous locomotor activity; inhibition of amphetamine-induced hyperlocomotion; reversal by the mGlu2/3 receptor antagonist LY341495
Spontaneous locomotor activity
Oral administration of the mGlu2/3 receptor agonist LY404039 and the atypical antipsychotic clozapine dose-dependently suppressed spontaneous locomotor activity relative to vehicle controls (Fig. 2). Spontaneous activity was significantly suppressed in rats receiving LY404039 [F(4,32)=10.56, P < 0.0001] and in rats receiving clozapine [F(4,32)=7.60, P < 0.0005]. Post hoc tests indicated that relative to the vehicle group, locomotor activity was significantly lower in rats receiving 10 mg/kg LY404039 during only the first 5-min bin (P < 0.001). Post hoc tests also revealed that 10 mg/kg clozapine produced significantly decreased locomotor activity, compared to vehicle, during the first and second 5-min bins (P < 0.05 and P < 0.01, respectively).
Spontaneous locomotor activity after oral administration of the mGlu2/3 receptor agonist LY404039 (a) or the atypical antipsychotic clozapine (b). Values represent the mean (±SEM) number of ambulations observed during the 45-min period. *p < 0.05 vs vehicle; **p < 0.01 vs vehicle; ***p < 0.001 vs vehicle
Amphetamine-induced hyperlocomotion
As expected, amphetamine (3 mg/kg, s.c.) produced a robust hyperlocomotor response. LY404039 dose-dependently inhibited amphetamine-induced hyperlocomotion within a range of 3–30 mg/kg (Fig. 3a), suggesting antipsychotic-like effects, F(6,74)=18.57, P < 0.001.
Inhibition of amphetamine (AMPH)-induced (3 mg/kg, s.c.) hyperlocomotion by the oral administration of the mGlu2/3 receptor agonist LY404039 (a). Values represent the mean (±SEM) number of ambulations observed during the 60-min observation. #p < 0.05, ##p < 0.01, ###p < 0.001 vs veh/veh control; *p < 0.05, **p < 0.01, ***p < 0.001 vs veh/AMPH. (b) Reversal of LY404039 (10 mg/kg, p.o.) inhibition of phencyclidine (PCP; 5 mg/kg, s.c.)- and AMPH-induced (3 mg/kg, s.c.) hyperlocomotion by the selective mGlu2/3 receptor antagonist LY341495 (1 mg/kg, s.c.). *p < 0.05, **p < 0.01 vs corresponding veh/veh group; #p < 0.05, ##p < 0.01 vs LY404039/vehicle group; $$p < 0.01 vs veh/LY341495 group. (c and d) Effect of LY404039 on amphetamine (3 mg/kg, s.c.)- and PCP (5 mg/kg, s.c.)-evoked hyperlocomotion using a time-sampling method. e Diazepam does not reverse PCP (5 mg/kg, s.c.)-induced hyperlocomotion
Reversal of LY404039 effects on stimulant-induced hyperlocomotion by LY341495
The ability of LY404039 to inhibit PCP-induced hyperlocomotion was reversed by pretreatment with the selective mGlu2/3 receptor antagonist LY341495 (Fig. 3b), F(3,47)=9.92, P < 0.001 (see Monn et al. 2007 for dose-response effects of LY404039 on PCP-induced hyperlocomotion). LY404039 suppression of amphetamine-induced hyperlocomotion was also reversed by pretreatment with LY341495 [Fig. 3b; F(3,41)=6.09, P < 0.002], demonstrating the effects were likely mediated by group II mGlu receptors.
Onset and duration of action studies
Onset and duration of action studies indicated that LY404039 (10 mg/kg, p.o.) attenuated amphetamine (3 mg/kg, s.c.; Fig. 3c)- and PCP (5 mg/kg, s.c.; Fig. 3d)-induced hyperlocomotion by ~90% at 1–2 h [amphetamine: F(8,53)=10.97, P < 0.0001 and PCP: F(7,47)=6.89, P < 0.0001], and the effect was maintained for 4 h. The benzodiazepine anxiolytic diazepam (1–30 mg/kg, p.o.) failed to attenuate PCP (5 mg/kg, s.c.)-induced hyperlocomotion at any dose tested (Fig. 3e).
Conditioned avoidance responding
As illustrated in Fig. 4, LY404039 inhibited conditioned avoidance responding in a dose-related manner, indicative of antipsychotic-like efficacy, F(2,10)=4.15, P < 0.05. Post hoc tests indicated that 3 mg/kg LY404039 significantly reduced avoidance responses (P < 0.05), whereas the 10 mg/kg dose did not reach significance (P = 0.07), likely due to low power and high variability. The 48% reduction in avoidance responding induced by LY404039 in this experiment is similar to previous observations after subcutaneous administration of various atypical antipsychotics (10 mg/kg clozapine produced a 41.25% reduction, 0.1 mg/kg risperidone produced a 42.0% reduction, and 0.05 mg/kg haloperidol produced a 37.1% reduction in avoidance responding). Escape failures after 10 mg/kg clozapine, 0.1 mg/kg risperidone, and 0.05 mg/kg haloperidol were 0, 2.0, and 6.29%, respectively. Importantly, LY404039 did not significantly increase the incidence of escape failures, indicating that LY404039 does not produce sedation or motor side effects characteristic of neuroleptic medications such as haloperidol.
LY404039 selectively disrupted conditioned avoidance responding in Fischer-F344 rats without affecting escape responses, indicating antipsychotic effects. Values represent the mean (±SEM) percentage of avoidance responses and escape failures. *p < 0.05 vs vehicle control, #p = 0.07 vs vehicle control
Fear-potentiated startle
Consistent with previous reports (Helton et al. 1998), administration of diazepam (0.6 mg/kg, p.o.) suppressed the fear-potentiated startle response. As shown in Fig. 5a, oral administration of LY404039 also produced a dose-related attenuation of fear-potentiated startle responding [F(5,42)=5.59, P < 0.001), with significant effects observed at 3 and 30 μg/kg. Oral administration of 30 μg/kg LY404039 demonstrated efficacy during the first hour after dosing, and the effect lasted 4 h post-dose [F(6,49)=6.30, P < 0.0001; Fig. 5b]. LY404039-induced attenuation of fear-potentiated startle was reversed by pretreatment with the selective mGlu2/3 receptor antagonist LY341495 (Fig. 5c), indicating that the effects of LY404039 were mediated by group II metabotropic glutamate receptors, F(3,44)=4.48, P < 0.01. Overall, LY404039 produced very mild and transient effects on baseline startle responding as reported in Table 1. Results indicated a modest effect on baseline startle during the dose-response experiment, F(5,42)=2.74, P < 0.05, with 0.3 μg/kg LY404039 producing a significant increase in startle responding relative to the vehicle group (Table 1). ANOVA also revealed a significant effect on startle responding during the time-course experiment, F(6,49) = 5.30, P < 0.001. Post hoc tests indicated that baseline startle responding was lower in rats receiving diazepam (P < 0.05) and in experimental rats at 8 and 24 h after LY404039 administration (P < 0.001 and 0.05, respectively, Table 1). No significant effects on baseline startle were observed during the LY341495 reversal experiment (P > 0.05). Importantly, general sedative effects of LY404039 were not observed.
Oral administration of LY404039 or diazepam (0.6 mg/kg, positive control) attenuated fear-potentiated startle responding (a). (b) The LY404039-induced attenuation of fear-potentiated startle was reversed by the selective mGlu2/3 receptor antagonist LY341495 (1 mg/kg, s.c.). Values represent the mean (±SEM) startle amplitude (V max) expressed as the difference between noise-alone and light + noise trials. *p < 0.05 vs vehicle group; **p < 0.01 vs vehicle group; #p < 0.05 vs LY404039 group. (c) Effect of LY404039 (30 µg/kg) on fear-potentiated startle responding using a time-sampling method
Marble burying and rotarod performance in mice
The effects of LY404039 and chlordiazepoxide on motor performance were evaluated using a rotarod apparatus. Immediately after the rotarod test, anxiolytic-like effects were evaluated in the marble-burying test. Rotarod failures and the number of marbles buried by mice receiving LY404039 or chlordiazepoxide are reported in Table 2. LY404039 dose-dependently reduced the number of marbles buried during the 30-min session, indicating anxiolytic-like effects, F(3,26)=14.15, P < 0.0001. Post hoc tests indicated significant reductions in marbles buried at 3 and 10 mg/kg LY404039. Importantly, LY404039 demonstrated no significant motor impairment at any dose evaluated in the rotarod test (P > 0.05). Likewise, chlordiazepoxide dose-dependently reduced the number of marbles buried, F(3,26)=12.87, P < 0.01; 30 mg/kg chlordiazepoxide, resulting in fewer marbles buried than mice receiving vehicle. However, chlordiazepoxide (30 mg/kg) also significantly increased the percentage of mice falling off the rotarod, indicating motor impairment (P < 0.05; Table 2).
Rat rotarod performance
LY404039 did not affect rotarod performance of rats at doses up to 30 mg/kg, indicating a lack of motor side effects (Table 3), P > 0.05. As shown in Table 3, LY404039 did not reverse or worsen PCP-induced motor ataxia on the rotarod apparatus, P > 0.05.
In vivo neurochemistry
Microdialysis experiments
Atypical antipsychotics, including clozapine and olanzapine, induce increases in cortical dopamine release and turnover. These increases have been hypothetically linked to improvement of negative symptoms in schizophrenia (Li et al. 1998; Goldman-Rakic 1999). Microdialysis in freely moving rats indicated that LY404039 (10 mg/kg, s.c.) produced increases in the release of dopamine and its metabolites DOPAC and HVA in the prefrontal cortex [Fig. 6a and b, dopamine: F(10,30)=13.05, P < 0.001; DOPAC: F(10,30)=29.14, P < 0.001; HVA: F(10,30)=26.99, P < 0.001]. While LY404039 did not significantly alter the release of 5-HT (Fig. 6a, P > 0.05), the serotonin metabolite 5-HIAA was significantly altered after 10 mg/kg LY404039, F(10,30)=2.62, P = 0.02 (Fig. 6b).
Effect of LY404039 on monoamine release and turnover in rat prefrontal cortex. Dialysates obtained from the prefrontal cortex indicated that LY404039 (10 mg/kg, s.c.) evoked an increase in the release and turnover of DA in the prefrontal cortex (a and b), while also slightly and transiently increasing 5-HT turnover (b). Values represent the mean (±SEM) percent of the respective pre-injection baseline values. *p < 0.05, ***p < 0.005 vs pre-injection DA baseline; @p < 0.05, @@@p < 0.005 vs pre-injection DOPAC baseline; #p < 0.05, ##p < 0.01, ###p < 0.005 vs pre-injection HVA baseline; $ p < 0.05 vs pre-injection 5-HIAA baseline
Tissue levels of monoamines and monoamine metabolites in the prefrontal cortex
Within the prefrontal cortex, a significant increase in the DOPAC/DA ratio was observed, F(3,28)=10.84, P < 0.0001. Post hoc tests indicated that this increase was significant after injection of either LY404039 (10 mg/kg, s.c.) or clozapine (10 mg/kg, s.c.; Fig. 7a). Increased dopamine turnover in the prefrontal cortex was observed within the first 30 min and lasted for 2 h, F(5,42)=21.42, P < 0.0001 (Fig. 7b). LY404039 also increased serotonin turnover, F(3,28)=5.93, P < 0.003, as indicated by the increased 5-HIAA/5-HT ratio relative to the saline control group (Fig. 7a). This increase in serotonin turnover was observed within 30 min and persisted at the 4-h time point, but had returned to baseline by 24 h post-administration, F(5,42)=20.76, P < 0.0001 (Fig. 7c). Clozapine did not significantly affect serotonin turnover in the prefrontal cortex (Fig. 7a).
Effect of LY404039 on monoamine turnover in rat prefrontal cortical tissue. LY404039 (10 mg/kg, s.c.) increased the turnover of both DA and 5-HT in the prefrontal cortex, while clozapine (10 mg/kg, s.c.) only increased DA turnover (a). Values represent the mean (±SEM) metabolite/neurotransmitter ratio. The LY404039-evoked increases in DA turnover in the PFC were observed within the first 30 min and persisted for 2 h (b). The increased 5-HT turnover evoked by LY404039 was also observed within the first 30 min, and the effect persisted at the 4-h time point (c). *p < 0.05, **p < 0.01 vs respective control group
Discussion
Due to the poor oral bioavailability of previous generation mGlu2/3 receptor agonists, we discovered LY404039, a novel agent with improved potency and bioavailability (Monn et al. 2007) that represents a potentially viable clinical investigational tool. LY404039 is structurally unique, potent, and highly selective for group II mGlu receptors, while also showing no appreciable affinity at group I or group III mGlu receptors, ionotropic (NMDA, AMPA, kainate) glutamate receptors, or glutamate transporters (Rorick-Kehn et al. 2007). To determine if these improvements translated into enhancements of in vivo pharmacology relevant to neuropsychiatric disorders, we investigated pertinent in vivo effects of this molecule. In pharmacological models predictive of antipsychotic efficacy, LY404039 exhibited a profile similar to that of clozapine. Orally administered LY404039 inhibited spontaneous locomotor activity and dose-dependently attenuated amphetamine- and PCP-induced hyperlocomotion. The reduction of non-habituated locomotor activity by both LY404039 and clozapine indicates a propensity to modulate behavioral states, including pathological states, rather than a mild sedative effect. Indeed, compounds that induce sedation at doses close to the therapeutic levels, such as diazepam, do not reverse the psychotomimetic-like effects of PCP (Sams-Dodd 1998; see also Fig. 3). Suppression of both PCP- and amphetamine-induced hyperlocomotion by LY404039 was reversed by the mGlu2/3 receptor antagonist LY341495, indicating that the effects were mediated by mGlu2/3 receptors. In support of mGlu mediation of PCP reversal, the attenuation of PCP-induced hyperlocomotion in wild-type mice was absent in mice lacking mGlu2 receptors (Spooren et al. 2000). Interestingly, the mGlu2/3 antagonist LY341495 was without effect on behavior when administered alone, but potentiated the behavioral effects of PCP when administered without the agonist LY404039. This effect is consistent with blockade of mGlu2 and mGlu3 autoreceptors and heteroreceptors and suggests that LY341495 exacerbates the glutamate release associated with PCP administration. The potentiation by LY341495 was selective in that it was not observed after amphetamine administration, consistent with an indirect but functional interaction between mGlu2/3 receptors and dopaminergic neurocircuitry. These findings provide further support for the involvement of glutamatergic mechanisms in producing PCP-induced hyperlocomotion in rats and psychotomimetic-like effects in humans and also exacerbating psychotic symptoms in schizophrenic patients (Morris et al. 2005). Contrary to previous results with LY354740 (Takamori et al. 2003), we demonstrated here that LY404039 inhibited conditioned avoidance responding, indicating antipsychotic-like effects. While Takamori and colleagues demonstrated that the mGlu2/3 receptor agonists MGS0008 and MGS0028 produced escape failures at efficacious doses, we demonstrated here that LY404039 did not produce escape failures in this task, indicating reduced propensity for inducing motor side effects. Importantly, LY404039 also failed to produce sedative effects or motor impairment in the rotarod task in both rats and mice, providing further evidence that the novel mechanism of action of mGlu2/3 receptor agonists may confer them with a reduced propensity to produce adverse side effects typically observed with currently available anxiolytic and antipsychotic medications (Schoepp and Marek 2002; Swanson et al. 2005).
In animal models predictive of anxiolytic efficacy, LY404039 attenuated fear-potentiated startle in rats and reduced marble burying in mice. The fear-potentiated startle paradigm appeared to be exquisitely sensitive to LY404039, as evidenced by the microgram doses required for anxiolytic-like effects. This is consistent with previous results showing that both an mGlu2/3 agonist and an mGlu2 receptor potentiator were significantly more effective in the fear-potentiated startle paradigm, relative to other behavioral experiments (Helton et al. 1998; Johnson et al. 2005). One explanation for the increased sensitivity may be the fasted state of the animals during the assessment of fear-potentiated startle. Perhaps the acute fasting induced a mild anxiety-like state in the animals, which may then have increased their sensitivity to the anxiolytic effects of mGlu2/3 receptor agonists. An alternative explanation for the apparent increased potency of mGlu2/3 receptor agonists in this model may be related to receptor reserve, such that the fear-potentiated startle paradigm may require lower receptor occupancy levels relative to other paradigms. Classical receptor theory predicts that the action of an agonist is associated with a broad range of potencies depending on the amount of receptor reserve, which can vary widely in different brain regions. Consistent with this, Swanson et al. (2005) demonstrated that LY354740 was more potent at inhibiting excitatory postsynaptic potentials in amygdala synapses, relative to corticohippocampal or prefrontal cortical synapses. Thus, only a smaller proportion of receptors may need to be occupied in the amygdala to achieve maximal anxiolytic-like response in the fear-potentiated startle model.
LY404039 increased the release of dopamine and its metabolites, DOPAC and HVA, in the prefrontal cortex and increased dopamine turnover, as measured by microdialysis and ex vivo tissue levels. These results are similar to previous results for clozapine and another mGlu2/3 receptor agonist LY379268 (Cartmell et al. 2000a, b). Such increases in cortical dopamine have been linked to the improvement of negative symptoms of schizophrenia (Goldman-Rakic 1999; Li et al. 1998). Given the parenteral route of administration in the microdialysis and ex vivo tissue studies, the dose at which LY404039 increased dopamine turnover and release (10 mg/kg, s.c.) was slightly higher than the doses that were active in the behavioral experiments (3–10 mg/kg, p.o.). Similar to LY379268 but unlike clozapine, the current results demonstrated that LY404039 also increased serotonin turnover in the prefrontal cortex (Cartmell et al. 2000a, b). That LY404039 modulates serotonergic, in addition to dopaminergic, activity in vivo may represent a significant advantage over other antipsychotic medications in demonstrating potential therapeutic benefits for patients with co-morbid anxiety and depressive disorders (Cartmell et al. 2000a; Rickels and Rynn 2002; Schoepp and Marek 2002). Given the novel profile of neurochemical effects, it is tempting to speculate that LY404039 might also improve negative symptoms in schizophrenic patients via modulation of neural substrates involved in affective regulation.
Medications currently used to treat anxiety and schizophrenia have proven satisfactory in some populations, but often fail to adequately treat all symptoms of these disorders. For example, none of the current treatments effectively manages the negative and cognitive symptoms of schizophrenia (Heresco-Levy 2005), and only 60–70% of patients with generalized anxiety disorder report a significant improvement in symptoms after 8 weeks of anxiolytic therapy (Rickels and Rynn 2002). Moreover, these medications are associated with adverse side effects, including weight gain and motor side effects for antipsychotics, and sedation, tolerance, abuse liability, and dependence for anxiolytics (Ascher-Svanum et al. 2005; Gudex 1991; Heresco-Levy 2005; Sachdev 2005). Consequently, considerable attention has been focused on discovering novel medications for these disorders that may be devoid of such undesirable side effects, while also demonstrating improved therapeutic benefits. While currently available antipsychotic medications produce antipsychotic effects in animal models, they all demonstrate some level of antagonism at dopamine receptors (Wadenberg et al. 2001) and also produce motor side effects at higher doses in both the rotarod and conditioned avoidance tasks in rats, effects that may be related to dopamine antagonism (Steinpreis et al. 1999; Wadenberg et al. 2001). In contrast to current medications, we recently demonstrated that LY404039 has no affinity for dopamine D1 or D2 receptors or other non-glutamatergic receptors implicated in the mechanism of action of current antipsychotics and anxiolytics (Rorick-Kehn et al. 2007). In this paper, we demonstrated that LY404039 did not produce sedative effects or motor impairment, as measured by rotarod performance and lack of escape failures in the conditioned avoidance task. Moreover, LY404039 did not reverse or worsen the motor-impairing effects of PCP on the rotarod test of motor coordination. Thus, our data add to a growing literature suggesting that modulation of mGlu receptors may have therapeutic utility as a novel treatment for neuropsychiatric disorders, while also demonstrating a lower propensity for inducing undesirable side effects.
In the last two decades, our understanding of mGlu receptor pharmacology and the role of these receptors in various psychiatric disorders has advanced at a rapid pace, aided by the cloning and pharmacological characterization of the eight different mGlu receptor subtypes, the development of mGlu receptor knockout mice, and the discovery of potent and selective mGlu receptor agonists and antagonists. Since its discovery a decade ago (Monn et al. 1997), LY354740 has exhibited broad anxiolytic-like efficacy in animal models, including fear-potentiated startle, elevated plus maze, lactate-induced panic, and stress-induced hyperthermia (Helton et al. 1998; Linden et al. 2004, 2005b; Shekhar and Keim 2000; Spooren et al. 2002), as well as tempering withdrawal symptoms from nicotine or morphine (Helton et al. 1997; Vandergriff and Rasmussen 1999). In contrast to the extensive preclinical and clinical literature supporting the anxiolytic-like effects of mGlu2/3 receptor agonists, a recent report demonstrated that the mGlu2/3 receptor antagonists LY341495 and MGS0039 produced anxiolytic-like effects in the stress-induced hyperthermia model in mice (Iijima et al. 2007). However, whether the anxiolytic-like effects observed in that study were mediated by group II mGlu receptor antagonism is unclear. In addition to its affinity for mGlu2 and mGlu3 receptors, LY341495 also shows sub-micromolar affinity for mGlu8 receptors (Kingston et al. 1998), and the affinity of MGS0039 for mGlu8 receptors has not been reported (Nakazato et al. 2004). Moreover, the results of Iijima et al. directly contradict evidence that stress increases glutamate release in cortical and subcortical regions, particularly the prefrontal cortex, and that the stress-induced increase in glutamate transmission also mediates the well-known increase in mesocortical dopaminergic transmission in response to stress (Moghaddam 2002). Indeed, LY341495 has been demonstrated to increase anxiety-like behaviors in the elevated plus-maze and induce c-fos expression in the amygdala and other brain areas (Linden et al. 2005a), consistent with an exacerbation of glutamate release by the mGlu2/3 antagonist. LY354740 has also previously demonstrated antipsychotic efficacy in several measures of schizophrenia, including suppression of serotonin-evoked EPSPs in the prefrontal cortex (Marek et al. 2000), as well as reversal of behavioral hyperactivity and stereotypy and the increased prefrontal cortical activity induced by MK-801 and PCP (Cartmell et al. 1999; Moghaddam and Adams 1998; Schoepp and Marek 2002).
In support of the preclinical results, LY354740 produced encouraging results in clinical trials. LY354740 attenuated fear-potentiated startle and reduced subjective anxiety ratings under stressful conditions in healthy volunteers (Grillon et al. 2003), reduced the number and severity of CO2-inhalation-induced anxiety symptoms in panic-prone patients (Schoepp et al. 2003), and reversed ketamine-induced working memory deficits in healthy volunteers (Krystal et al. 2005a). More recently, LY354740 and its prodrug LY544344 failed to prevent panic attacks in smaller clinical trials (Bergink and Westenberg 2005; Kellner et al. 2005). However, in one experiment, LY544344 did significantly reduce anxiety and panic symptoms in the subgroup of patients who showed an endocrine response to CCK-4, which was used to induce panic symptoms in that trial (Kellner et al. 2005). The lack of positive findings in recent experiments may have resulted from low statistical power in those trials or, alternatively, inadequate central exposure. Indeed, the clinical development of LY354740 has been hampered by poor oral bioavailability in animals and humans (Rorick-Kehn et al. 2006). Importantly, in all clinical trials to date, LY354740 was well tolerated and, unlike benzodiazepine anxiolytics, cessation of treatment did not induce withdrawal symptoms. Results of these clinical trials suggest that LY354740 may represent a novel approach for the safe and effective treatment of anxiety and psychosis with reduced incidence of unwanted side effects.
LY404039 resulted from an effort to discover selective, potent, orally active mGlu2/3 receptor agonists for the treatment of psychiatric disorders. In this paper, we demonstrated that oral administration of LY404039 produced antipsychotic- and anxiolytic-like effects in several animal models and increased monoamine turnover and release in the prefrontal cortex at lower oral doses than those previously reported for LY354740. Specifically, while LY354740 failed to reverse PCP-induced hyperlocomotion at oral doses up to 100 mg/kg (Rorick-Kehn et al. 2006), LY404039 was effective at doses as low as 1 mg/kg when administered orally (Monn et al. 2007). Using another model of schizophrenia, we report here that LY404039 effectively reversed amphetamine-induced hyperlocomotion at an oral dose of 3 mg/kg. In previous experiments, parenteral routes were required to observe anxiolytic effects with LY354740 (Rorick-Kehn et al. 2006), whereas the current results demonstrate oral anxiolytic-like effects at a dose of 3 μg/kg in the fear-potentiated startle paradigm, demonstrating markedly improved oral potency in vivo. The increased bioavailability observed in rats (63%) relative to LY354740 (~10%; Monn et al. 2007; Johnson et al. 2002) suggests that LY404039 may be an attractive candidate for clinical development in the treatment of neuropsychiatric disorders.
Although not addressed in the current experiments, the relative contribution of mGlu2 versus mGlu3 receptors is an issue that should be examined in future studies, particularly as more selective ligands are discovered. For example, a recent report demonstrated that an mGlu2 receptor potentiator produced behavioral effects similar to those produced by mGlu2/3 receptor agonists in animal models predictive of antipsychotic efficacy, suggesting that mGlu2 receptors may be primarily responsible for the behavioral effects (Galici et al. 2005). Further support is provided by the demonstration that the racemate of LY354740 reversed PCP-induced hyperlocomotion in wild-type, but not mGlu2 receptor knock-out mice (Spooren et al. 2000). Whether the activation of mGlu3 receptors further contributes to the in vivo efficacy of group II mGlu agonists requires further exploration.
Pathological glutamatergic and dopaminergic neurotransmission in limbic and cortical areas is hypothesized to underlie the production of both positive and negative symptoms in schizophrenic patients (Goldman-Rakic 1999; Heresco-Levy 2005). Stress and anxiety disorders are also associated with altered glutamatergic activity in limbic and cortical regions (Bergink et al. 2004; Moghaddam 2002). Many clinically effective antipsychotics are believed to alleviate the positive symptoms of schizophrenia by reducing mesolimbic dopamine release and concomitantly increasing dopamine activity in mesocortical pathways (Goldman-Rakic 1999; Heresco-Levy 2005). However, recent experiments support the contention that the most effective atypical antipsychotics do not work solely through the dopaminergic system, but rather interact through a broad class of neurotransmitter systems (Heresco-Levy 2005; Krystal et al. 2005b). Anxiolytics produce their effects by increasing inhibitory activity in the brain, but a converse approach is to decrease excessive central excitatory activity through modulatory metabotropic glutamatergic mechanisms (Swanson et al. 2005). Described herein is a demonstration of the broad preclinical efficacy of the structurally novel, potent selective mGlu2/3 receptor agonist LY404039. The results also indicate that LY404039 modulates mesocortical glutamatergic and dopaminergic neurotransmission and, in doing so, may provide a novel mechanism for the treatment of psychiatric disorders that is associated with improved efficacy and reduced incidence of undesirable side effects. As glutamatergic dysfunction has been linked to the etiology of schizophrenia, clinical studies with more potent mGlu2/3 agonists, such as LY404039, may be useful to explore the validity of this hypothesis in the clinic.
References
Ascher-Svanum H, Stensland MD, Kinon BJ, Tollefson GD (2005) Weight gain as a prognostic indicator of therapeutic improvement during acute treatment of schizophrenia with placebo or active antipsychotic. J Psychopharmacol 19:110–117
Bergink V, Westenberg HG (2005) Metabotropic glutamate II receptor agonists in panic disorder: a double blind clinical trial with LY354740. Int Clin Psychopharmacol 20:291–293
Bergink V, van Megen HJ, Westenberg HG (2004) Glutamate and anxiety. Eur Neuropsychopharmacol 14:175–183
Bueno AB, Collado I, de Dios A, Dominguez C, Martin JA, Martin LM, Martinez-Grau MA, Montero C, Pedregal C, Catlow J, Coffey DS, Clay MP, Dantzig AH, Lindstrom T, Monn JA, Jiang H, Schoepp DD, Stratford RE, Tabas LB, Tizzano JP, Wright RA, Herin MF (2005) Dipeptides as effective prodrugs of the unnatural amino acid (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid (LY354740), a selective group II metabotropic glutamate receptor agonist. J Med Chem 48:5305–5320
Cartmell J, Monn JA, Schoepp DD (1999) The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 291:161–170
Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75:889–907
Cartmell J, Perry KW, Salhoff CR, Monn JA, Schoepp DD (2000a) The potent, selective mGlu2/3 receptor agonist LY379268 increases extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindole-3-acetic acid in the medial prefrontal cortex of the freely moving rat. J Neurochem 75:1147–1154
Cartmell J, Salhoff CR, Perry KW, Monn JA, Schoepp DD (2000b) Dopamine and 5-HT turnover are increased by the mGlu2/3 receptor agonist LY379268 in rat medial prefrontal cortex, nucleus accumbens and striatum. Brain Res 887:378–384
Galici R, Echemendia NG, Rodriguez AL, Conn PJ (2005) A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther 315:1181–1187
Goldman-Rakic PS (1999) The relevance of the dopamine-D1 receptor in the cognitive symptoms of schizophrenia. Neuropsychopharmacology 21:S170–S180
Grillon C, Cordova J, Levine LR, Morgan CA 3rd (2003) Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology (Berl) 168:446–454
Gudex C (1991) Adverse effects of benzodiazepines. Soc Sci Med 33:587–596
Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ (1997) LY354740: a metabotropic glutamate receptor agonist which ameliorates symptoms of nicotine withdrawal in rats. Neuropharmacology 36:1511–1516
Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ (1998) Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther 284:651–660
Heresco-Levy U (2005) Glutamatergic neurotransmission modulators as emerging new drugs for schizophrenia. Expert Opin Emerg Drugs 10:827–844
Iijima M, Shimazaki T, Ito A, Chaki S (2007) Effects of metabotropic glutamate 2/3 receptor antagonists in the stress-induced hyperthermia test in singly housed mice. Psychopharmacology (Berl) 190:233–239
Johnson JT, Mattiuz EL, Chay SH, Herman JL, Wheeler WJ, Kassahun K, Swanson SP, Phillips DL (2002) The disposition, metabolism, and pharmacokinetics of a selective metabotropic glutamate receptor agonist in rats and dogs. Drug Metab Dispos 30:27–33
Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD (2005) Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology (Berl) 179:271–283
Kellner M, Muhtz C, Stark K, Yassouridis A, Arlt J, Wiedemann K (2005) Effects of a metabotropic glutamate(2/3) receptor agonist (LY544344/LY354740) on panic anxiety induced by cholecystokinin tetrapeptide in healthy humans: preliminary results. Psychopharmacology (Berl) 179:310–315
Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD (1998) LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology 37:1–12
Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A (2005a) Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 179:303–309
Krystal JH, Perry Jr EB, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D’Souza DC (2005b) Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 62:985–994
Li XM, Perry KW, Wong DT, Bymaster FP (1998) Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl) 136:153–161
Linden A-M, Greene SJ, Bergeron M, Schoepp DD (2004) Anxiolytic activity of the mGlu2/3 receptor agonist LY354740 on the elevated plus maze is associated with the suppression of stress-induced c-Fos in the hippocampus and increases in c-Fos induction in several other stress-sensitive brain regions. Neuropsychopharmacology 29:502–513
Linden AM, Bergeron M, Schoepp DD (2005a) Comparison of c-Fos induction in the brain by the mGlu2/3 receptor antagonist LY341495 and agonist LY354740: evidence for widespread endogenous tone at brain mGlu2/3 receptors in vivo. Neuropharmacology 49(Suppl 1):120–134
Linden AM, Shannon H, Baez M, Yu JL, Koester A, Schoepp DD (2005b) Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology (Berl) 179:284–291
Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK (2000) Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther 292:76–87
Moghaddam B (2002) Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry 51:775–787
Moghaddam B, Adams BW (1998) Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281:1349–1352
Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD (1997) Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem 40:528–537
Monn JA, Massey SM, Valli MJ, Henry SS, Stephenson GA, Bures M, Herin M, Catlow J, Giera D, Wright RA, Johnson BG, Andis SL, Kingston A, Schoepp DD (2007) Synthesis and metabotropic glutamate receptor activity of S-oxidized variants of (−)-4-amino-2-thiabicyclo-[3.1.0]hexane-4,6-dicarboxylate: identification of potent, selective, and orally bioavailable agonists for mGlu2/3 receptors. J Med Chem 50:233–240
Morris BJ, Cochran SM, Pratt JA (2005) PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol 5:101–106
Nakazato A, Sakagami K, Yasuhara A, Ohta H, Yoshikawa R, Itoh M, Nakamura M, Chaki S (2004) Synthesis, in vitro pharmacology, structure-activity relationships, and pharmacokinetics of 3-alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as potent and selective group II metabotropic glutamate receptor antagonists. J Med Chem 47:4570–4587
Ohishi H, Shigemoto R, Nakanishi S, Mizuno N (1993a) Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience 53:1009–1018
Ohishi H, Shigemoto R, Nakanishi S, Mizuno N (1993b) Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol 335:252–266
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic, New York
Rickels K, Rynn M (2002) Pharmacotherapy of generalized anxiety disorder. J Clin Psychiatry 63(Suppl 14):9–16
Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ (2006) The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res 171:207–215
Rorick-Kehn LM, Perkins EJ, Knitowski KM, Hart JC, Johnson BG, Schoepp DD, McKinzie DL (2006) Improved bioavailability of the mGlu2/3 receptor agonist LY354740 using a prodrug strategy: in vivo pharmacology of LY544344. J Pharmacol Exp Ther 316:905–913
Rorick-Kehn LM, Johnson BG, Burkey JL, Wright RA, Calligaro DO, Marek GJ, Nisenbaum ES, Catlow JT, Kingston AE, Giera DD, Herin MF, Monn JA, McKinzie DL, Schoepp DD (2007) Pharmacological and pharmacokinetic properties of a structurally-novel, potent, selective mGlu2/3 receptor agonist: in vitro characterization of LY404039. J Pharmacol Exp Ther (in press) DOI 10.1124/jpet.106.110809
Sachdev PS (2005) Neuroleptic-induced movement disorders: an overview. Psychiatr Clin North Am 28:255–274
Sams-Dodd F (1998) Effects of diazepam, citalopram, methadone and naloxone on PCP-induced stereotyped behaviour and social isolation in the rat social interaction test. Neurosci Biobehav Rev 23:287–293
Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299:12–20
Schoepp DD, Marek GJ (2002) Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr Drug Targets CNS Neurol Disord 1:215–225
Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ (2003) LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress 6:189–197
Schreiber R, Lowe D, Voerste A, De Vry J (2000) LY354740 affects startle responding but not sensorimotor gating or discriminative effects of phencyclidine. Eur J Pharmacol 388:R3–R4
Shekhar A, Keim SR (2000) LY354740, a potent group II metabotropic glutamate receptor agonist prevents lactate-induced panic-like response in panic-prone rats. Neuropharmacology 39:1139–1146
Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R (2000) Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur J Pharmacol 397:R1–R2
Spooren WP, Schoeffter P, Gasparini F, Kuhn R, Gentsch C (2002) Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608). Eur J Pharmacol 435:161–170
Steinpreis RE, Anders KA, Branda EM, Kruschel CK (1999) The effects of atypical antipsychotics and phencyclidine (PCP) on rotorod performance. Pharmacol Biochem Behav 63:387–394
Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD (2005) Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov 4:131–144
Takamori K, Hirota S, Chaki S, Tanaka M (2003) Antipsychotic action of selective group II metabotropic glutamate receptor agonist MGS0008 and MGS0028 on conditioned avoidance responses in the rat. Life Sci 73:1721–1728
Vandergriff J, Rasmussen K (1999) The selective mGlu2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropharmacology 38:217–222
Wadenberg ML, Soliman A, VanderSpek SC, Kapur S (2001) Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology 25:633–641
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rorick-Kehn, L.M., Johnson, B.G., Knitowski, K.M. et al. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacology 193, 121–136 (2007). https://doi.org/10.1007/s00213-007-0758-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0758-3