Abstract
Rationale
LY354740, a structural analogue of glutamate that shows specificity at the mGluR2/3 receptor, has anxiolytic effects in animal models.
Objective
This study investigated the anxiolytic effects of LY354740 in humans using the fear-potentiated startle reflex methodology.
Methods
Subjects were given either placebo (n=16), 20 mg LY354740 (n=15), or 200 mg LY354740 (n=13). The fear-potentiated startle tests examined startle potentiation to shock anticipation and to darkness.
Results
Consistent with previous results, startle was increased by threat of shock and by darkness. LY354740 did not affect baseline startle. Correspondingly, subjects did not report LY354740 to be sedative. LY354740 significantly reduced the increase in startle magnitude during shock anticipation, but not during darkness. Subjective reports of state anxiety and negative affectivity during the fear-potentiated startle tests were also reduced in a dose-dependent manner by LY354740.
Conclusions
These results suggest that LY354740 has an anxiolytic profile in humans without being sedative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in new anxiolytics stems from the fact that current anxiolytics, in particular the benzodiazepines, have undesirable side effects such as sedation and dependency (Dantzer 1977; Woods et al. 1992). Here, we report that the metabotropic receptor agonist LY354740 has an anxiolytic profile in the fear-potentiated startle paradigm in humans, without being sedative.
l-Glutamate (glutamate) is ubiquitous in the central nervous system (CNS) and plays a major role as an excitatory neurotransmitter in most CNS processes. Glutamate-stimulated, excitatory synaptic transmission is mediated via ionotropic receptors (iGlu: e.g. NMDA, AMPA, kainate receptors), as well as modulated via metabotropic receptors (mGlu: e.g. G–protein coupled). Metabotropic receptors can modulate the pre- and postsynaptic release of glutamate. Several types of mGlu receptors have been cloned. These can be separated structurally into three groups that share similar pharmacology and second messenger coupling. The present study is concerned with LY354740, a structural analogue of glutamate that shows specificity at the group II mGluR2/3. LY354740 reduces glutamatergic transmission in brain regions involved in anxiety and stress, such as the hippocampus, the amygdala, the prefrontal cortex, and the locus coeruleus (Schoepp 1994).
There is growing evidence that LY354740 has anxiolytic effects. Pre-clinical investigations in rats indicate that oral administration of LY354740 at doses that do not produce sedation block fear-potentiated startle (Helton et al. 1998). Intra-amygdala injection of LY354740 also blocks fear-potentiated startle. In addition, systemic injection of LY354740 prevents lactate-induced panic-like responses in panic-prone rats (Shekar and Keim 2000). Finally, LY354740 reduces CO2-provoked anxiety symptoms in patients with panic disorder (Levine et al. 2002). Importantly, LY354740 was not found to be sedative in these studies, suggesting that its anxiolytic effects are not secondary to sedation. If confirmed in further studies in humans, this later characteristic of LY354740 would be a potential advantage over benzodiazepines for which sedation is an important undesirable side effect.
The aim of the present study was to examine the anxiolytic and sedative effects of LY354740 in humans using the fear-potentiated startle reflex paradigm. The startle reflex presents several advantages to investigate both anxiolysis and sedation. The startle reflex is highly sensitive to aversive states in rodents and in humans (Lang et al. 1990; Davis 1992), an effect attributed to structures believed to be involved in fear and anxiety (e.g. the amygdala and bed nucleus of the stria terminalis) (Davis 1998). Importantly, startle is a cross-species reflex. This characteristic makes startle an ideal tool to develop very similar experiments in humans and animals. Finally, LY354740 has been found to be anxiolytic in the fear-potentiated startle reflex paradigm in rats (Helton et al. 1998; Tizzano et al. 2002).
The sensitivity of startle to sedation has been demonstrated in the large majority of human studies. Drugs known for their sedative effects, such as alcohol and the benzodiazepines, produce a dramatic reduction in baseline startle (Grillon et al. 1994, 2000; Kumari et al. 1996; Bitsios et al. 1999; Rodriguez-Fornells et al. 1999; Riba et al. 2001). There is only one exception to this finding. Patrick et al. (1996) reported that diazepam did not affect baseline startle.
"Fear-potentiated startle" refers to the increase in the amplitude of the startle reflex during anticipation of an aversive stimulus (Davis and Astrachan 1978). In the fear-potentiated startle paradigm, an explicit neutral stimulus (conditioned stimulus; e.g. a light) is repeatedly paired with an aversive stimulus (unconditioned stimulus; e.g. a shock). Subsequent to this conditioning procedure, the amplitude of the startle reflex is greater when the reflex is elicited in the presence of the conditioned stimulus compared to when it is absent. The degree of potentiation, which is expressed as a difference or a proportional change from baseline (Grillon and Baas 2002; Walker and Davis 2002b), is called fear-potentiated startle. Fear-potentiated startle constitutes an operational definition of fear (Davis 1992). Animal studies indicate that drugs known to reduce anxiety in humans (e.g., lorazepam) reduce or block fear-potentiated startle (Davis 1979), whereas drugs that increase human anxiety (e.g., yohimbine) increase fear-potentiated startle (Davis et al. 1979). In addition to fear-potentiated startle to phasic discrete cues (e.g. conditioned stimulus), startle can also be potentiated in a more sustained manner by aversive contexts. For example, bright lights facilitate startle. Drugs that reduce anxiety in humans also reduce this "light-enhanced startle" (de Jongh et al. 2002; Walker and Davis 1997b, 2002a).
Animal studies suggest that different brain systems mediate fear-potentiated startle to explicit threat cues and to contextual stimuli (e.g. bright lights) (Davis 1998). Lesions of the central nucleus of the amygdala block explicit cued fear-potentiated startle, but do not affect the facilitation of startle by bright lights (Walker and Davis 1997a). By contrast, inactivation of the bed nucleus of the stria terminalis (BNST) blocks the facilitation of startle by bright lights, but not by explicit threat cues (e.g. conditioned stimulus) (Walker and Davis 1997a). Thus, fear-potentiated startle in rats is mediated by a basolateral amygdala-central nucleus of the amygdala connection and the light-enhanced startle by a basolateral amygdala-BNST connection (Walker and Davis 1997a). The identification of divergent projection mediating these two types of aversive responses is significant to psychopharmacological studies because it suggests that different neurobiological mechanisms, perhaps sensitive to different psychopharmacological agents, underlie various forms of aversive states.
Fear-potentiated startle can also be obtained in humans using the eyeblink reflex, the most persistent component of the startle reflex pattern (Landis and Hunt 1939). Startle potentiation to an explicit threat signal can be obtained using either aversive conditioning or verbal threat procedures (Grillon et al. 1991; Grillon and Davis 1997). In a verbal threat procedure, subjects are told that unpleasant shocks may be delivered only in the presence of a specific cue (e.g. a threat signal). Verbal threat procedures produce a highly robust and reliable startle potentiation (Grillon et al. 1991). There have been discrepancies concerning the effects of benzodiazepines on fear-potentiated startle to threat in humans. Bitsios et al. (1999) and Riba et al. (2001) reported that benzodiazepines reduced fear-potentiated startle. However, Baas et al. (2002) recently reported the results of four separate studies showing that benzodiazepines did not affect fear-potentiated startle. Discrepancies between studies may reflect differences in the procedures and/or method of data analysis. Regarding data analysis, the fear-potentiated startle to a threat stimulus is expressed as a change from baseline startle amplitude to startle amplitude in the threat condition. This change can either be calculated as a difference or as a proportional change. Walker and Davis (2000) recently recommended the use of proportional change scores rather than difference change scores when the drug being investigated affects baseline startle. Because benzodiazepines are sedative and reduce overall startle reactivity, the analysis of fear-potentiated startle should take into account the non-specific effect of benzodiazepines on baseline startle (Grillon and Baas 2002; Walker and Davis 2002b). Failure to do so may lead to potentially erroneous results. Finally, it is possible that different types of aversive states are differently affected by benzodiazepines. To the best of our knowledge, only one study has examined the effect of benzodiazepines using a model other that the threat of shock. Patrick et al. (1996) found that facilitation of startle by unpleasant slides was blocked by diazepam.
Startle can also be facilitated by darkness in humans, increasing by about 15–20% when elicited in a dark room, compared to an illuminated room (Grillon et al. 1997a). This startle-facilitation by darkness effect in humans may mirror the light-enhanced startle in the rat. Grillon et al. (1997b) have suggested that these two effects have similar evolutionary bases. Rodents are nocturnal animals and are vulnerable to predators in bright spaces, whereas humans are diurnal and are more vulnerable in the dark.
The present study examined the potential anxiolytic effects of LY354740 on these two models of anxiety in humans, that is, fear-potentiated startle to threat and the facilitation of startle in the dark. A third situation that leads to enhanced startle was investigated, that which is associated with placing the shock electrodes (Grillon and Ameli 1998). Based on animal studies, we postulated that LY354740 would reduce fear-potentiated startle to the anticipation of shocks. We did not have a specific hypothesis concerning the effect of LY354740 on the facilitation of startle by darkness or by placement of the shock electrodes. On the one hand, animal studies showed that drugs that affect fear-potentiated startle have also been shown to affect light-enhanced startle (e.g. Walker and Davis 2002a). On the other, the neurobiology of fear-potentiated startle and light-enhanced startle is different. To the best of our knowledge, the effect of anxiolytics on the potentiation of startle by the shock electrodes has not been specifically investigated.
Materials and methods
Subjects
Subjects were healthy male and female volunteers, mainly recruited via posted signs in local universities and colleges. They were paid $160 for completion of the study. Volunteers had to have a body mass index between 19 and 28 kg/m2 to be included in the study. Subjects were excluded from participation if they met criteria for any psychiatric diagnosis using structured diagnostic criteria (DSM-IV SCID), suffered from any clinically significant abnormality of the 12-lead EKG, showed any clinically significant abnormality on chemistry, hematology tests, or medical examination, or displayed small startle responses (see below). Subjects were also excluded if they had any history of alcoholism or substance abuse/dependence, or if they screened positive for an illicit drug on urine toxicology. Additional exclusion criteria included a history of severe allergies or multiple adverse drug reactions, any clinically significant active disease, any medically significant disease history, positive hepatitis B virus surface antigen and/or hepatitis C virus antibody test, or a positive human immunodeficiency virus antibody test.
Eighty-four subjects were screened but only 47 were eligible for participation in the study. These subjects were randomized to one of three groups: placebo, 20 mg LY354740 (mGlu20), or 200 mg LY354740 (mGlu200). The two doses were chosen because they were identical to doses that were going to be used in future phase II anxiety studies. The initial protocol was designed to include 16 subjects per group. The study was considerably delayed because of administrative issues when one of the Principle Investigators (C.G.) took a new position at NIMH. The study was finally stopped before its full completion when the date of validity of the LY354740 expired.
However, the study had to be discontinued before its full completion. Consequently, only the 47 eligible subjects could be assigned to the three different treatments. Five subjects in the mGlu20 group, six subjects in the mGlu200 group, and no subjects in the placebo group experienced nausea following drug administration. Nausea did not prevent participation in the fear-potentiated startle experiment (see also Results). In addition, three subjects in the mGlu200 group dropped out due to emesis. These subjects did not complete the study. As a result, there were 16 subjects (ten females) in the placebo group, 15 subjects (seven females) in the mGlu20 group, and 13 subjects (seven females) in the mGlu200 group. Mean ages (23.2, 24.8, and 26.2 years in the placebo, mGlu/20, and mGlu200 groups, respectively) did not significantly differ among groups [F(2,41)=1.3]. Spielberger trait anxiety scores (Spielberger 1983) also did not significantly differ among groups [F(2,41)=1.3]. Mean trait anxiety scores were 31.3, 31.3, and 27.3 in the placebo, mGlu20, and mGlu200 groups, respectively.
Procedure
The study was approved by the Yale University Institutional Review Board and was conducted ethically in accordance with the Helsinki Declaration of 1964 (revised 1984). It consisted of two sessions, an initial screening session (session 1) and a testing session (session 2), which took place between 3 and 10 days following session 1. The screening session consisted of a medical and a psychiatric examination, an assessment of baseline startle, and a memory test (see below). Subjects with low startle responses (mean of less than 50 μV over the nine startle responses) or with a noisy recording during the baseline startle assessment were not asked to participate in the drug challenge.
On the challenge day, the overall testing consisted of an assessment of baseline startle before (baseline startle test 1) and after drug (baseline startle test 2) ingestion, followed by a test of threat of an electric shock and darkness (fear-potentiated startle test). The fear-potentiated startle test was designed such that the effect of placing the shock electrodes could be assessed (see below). The drug treatments were given 180 min before baseline startle test 2 and subsequent fear-potentiated startle, which corresponds to the time of Cmax (mGlu20=9.86 ng/ml; mGlu200=98 ng/ml) of LY354740.
Subjects were first given the state anxiety form, the PANAS, and the measure of sedation. Two electrodes were placed under subjects' left eye to record the eyeblink reflex. Subjects' baseline startle (test 1) was then assessed with nine acoustic startle stimuli. The time between startle stimuli varied from 20 to 30 s. The drug treatments were given orally immediately after the baseline startle test. One hour after drug ingestion, the subjects were given a light breakfast. A memory test was conducted 3 h after drug ingestion. This was immediately followed by a second baseline startle assessment (test 2: nine startle stimuli). The shock electrodes were then placed on the subjects' left forearm, and their left hand was placed on a vibrator. Activation of the vibrator produced a weak vibration, used to signal the onset and the end of the threat conditions (i.e. the vibrator was turned on during the duration of the threat signal). Pilot investigations showed that the vibrator did not potentiate or inhibit startle. Instructions concerning the fear-potentiated startle experiments were then given to the subjects. Subjects were told that they would receive between one and three shocks during the entire experiment. The shock (which they did not experience prior to the experiment) was described as being rather unpleasant but not painful. Subjects were informed that the shock could only be given during threat conditions, signaled by the vibrator being activated, but not during safe conditions, when the vibrator was turned off. They were also told that the light in the room would be turned off several times for about 1 min during the experiment. Subjects were informed that there were several breaks during testing. They were not informed of the exact duration of the experiment.
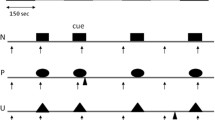
The fear-potentiated startle test started with four startle stimuli followed by three alternating light and dark phases (the offset of one phase was immediately followed by the onset of the other) with each phase lasting 2 min. Each dark/light phase was divided into a 1-min duration threat (vibrator on) and a 1-min duration safe (vibrator off) conditions. Thus, threat and safe conditions alternated six times (six threat and six safe conditions) [e.g. light (safe, threat), dark (safe, threat), light (safe, threat), dark (safe, threat)].
Each subject participated in two fear-potentiated startle tests with a 5-min break between tests, at which time they were asked to fill out subjective assessments. Four sequences of the different conditions were created starting with one of the following four combinations: 1) light (safe, threat), 2) light (threat, safe), 3) dark (safe, threat), or 4) dark (threat, safe). Subjects were randomly assigned to either sequences 1 and 4, 2 and 3, 4 and 1, or 3 and 2.
A single shock was given. It was given in the last threat condition, after the last startle stimuli, if the experiment terminated with a threat condition. If the experiment terminated with a safe condition, an additional threat condition was presented during which the shock was administered. The results during the additional condition were not included in the analysis. (The Yale Investigation Review Board requested the administration of the shock.)
Acoustic startle stimuli were delivered in the threat and in the safe conditions. The acoustic startle stimulus used to screen subjects, to assess baseline startle, and to examine fear-potentiated startle was a 40-ms duration white noise with an intensity of 100 dB(A) delivered binaurally through headphones. The time interval between startle probes varied from 20 to 30 s. Two startle stimuli were delivered during each of the safe and threat conditions.
The eyeblink EMG activity was filtered (1–500 Hz; a 60–Hz notch filter was in place), digitized at 1000 Hz for 250 ms from the onset of the acoustic stimulus, and rectified. Peak amplitudes were identified in the 21- to 100-ms time frame following stimulus onset relative to a baseline value (mean activity in 20-ms post-startle stimulus). Trials for which baseline activity were three times the mean baseline activity were rejected. One subject in each treatment group and two subjects in the placebo group had one trial rejected.
Subjective assessments and memory test
The following questionnaires were used: 1) a measure of positive and negative affect (PANAS) (Watson et al. 1988) and 2) the State and Trait Anxiety Inventory (STAI-state and STAI-trait) (Spielberger 1983). These questionnaires were given to the subjects during the second session 1) upon their arrival in the laboratory prior to drug ingestion, 2) after drug ingestion, just prior to placement of the shock electrodes on subjects' forearm, and 3) just after each fear-potentiated startle test to assess ambient feelings (contextual fear) at different times during the experiment. In addition, a subjective assessment of the sedative effect of LY354740 was conducted using visual analog scales (VAS) (Charney et al. 1984) prior to drug ingestion, and after drug ingestion, prior to placement shock electrodes. The following items were rated: talkative, happy, drowsy, nervous, sad, calm, depressed, anxious, energetic, fearful, mellow, high, angry, irritable, tired, and hungry. Subjects were asked to rate each item on 10-cm horizontal lines. The score ranged from 0 (not at all) to 10 (extremely). Finally, after each fear-potentiated startle test, subjects were asked to retrospectively rate their subjective level of anxiety (from not at all anxious to extremely anxious) using analog scales for each of the four conditions (i.e. light on/safe, light on/threat, light off/safe, light off/threat).
The memory test that was given during the screening day and during the testing day consisted of reading a list of 20 words (different on the first and second occasion) and asking the subjects to recall immediately as many words as possible.
Data reduction and statistical analyses
The startle magnitude data were averaged over each condition within each fear-potentiated startle test. Statistical analyses were conducted with analyses of variance (ANOVAs) and covariance (ANCOVAs) with repeated measures. Orthogonal polynomial contrasts were generated to test linear effects (placebo, mGlu20, mGlu200). Preliminary analyses indicated that there was no difference between males and females. Hence, gender was not included as a factor in the analysis. Because the targeted number of subjects per group was not reached, the overall ANOVAs comparing the three groups, were followed by specific focused contrasts comparing the placebo group to the two LY354740 groups (placebo versus mGlu20/mGlu200). Note that Cohen's d effect size is provided for relevant comparisons.
Results
Startle/eyeblink
Figure 1 shows baseline startle amplitude: 1) before drug ingestion, 2) 180 min after drug ingestion (just before placement of the shock electrodes), and 3) just after placement of the shock electrodes (mean of the four startle stimuli delivered at the beginning of the first fear-potentiated startle test). LY354740 did not affect baseline startle significantly. This result was confirmed with a two-way ANOVA with Treatment (placebo, mGlu20, mGlu200) and Time (before, after ingestion) as the two factors. The Treatment [F(2,41)=1.31], and Time [F(1,41)=0.14], main effect, as well as the Treatment×Time interaction [F(2,41)=0.45], were not significant. There was no significant effect with the factor Group.
Figure 1 shows that placing the shock electrodes on subjects' forearm increased startle, a result consistent with prior findings (Grillon and Ameli 1998). We investigated whether this effect was affected by LY354740 by comparing startle amplitude recorded just prior to and just after placement of the shock electrodes. The results were analyzed with a two-way ANOVA with Treatment (Placebo, mGlu20, mGlu200) and Shock electrodes (without, with) as the two factors. There was a Shock Electrodes main effect [F(1,41)=5.1, P<0.03]. Although the increase in startle magnitude was greater in the placebo, compared to the LY354740 group, Treatment×Shock electrode interaction was not significant [F(2,41)=0.60].
Table 1 presents the results of the fear-potentiated startle experiment and Fig. 2 shows the main findings. The data were analyzed with an ANOVA with repeated measures using Treatment (Placebo, mGlu20, mGlu200), Test (first, second), Darkness (light, dark), and Condition (safe, threat) as factors. The main Treatment effect was not significant [F(2,41)=1.47]. As expected, startle was greatly potentiated by the threat of shock [F(1,41)=60.2, P<0.001, and to a lesser extent by darkness [F(1,41)=8.8, P<0.01; Cohen's d=0.46]. Startle magnitude habituated from the first to the second test [F(1,41)=73.9, P<0.001]. Neither the Treatment×Condition interaction nor the linear trend for Treatment×Condition were significance [F(2,41)=2.25 and F(1,41)=2.7, respectively]. However, the specific contrast placebo versus mGlu20/mGlu200 reached significance [F(1,41)=4.4, P<0.04, d=0.63]. Thus, consistent with the animal data (Helton et al. 1998), the degree of startle potentiation in the threat condition was reduced in the LY354740 groups. Post-hoc pairwise comparisons revealed that startle was potentiated in the threat condition, compared to the safe condition in each group [placebo: F(1,41)=41.5, P<00009, d=1.0; mGlu20: F(1,41)=12.3, d=0.55, P<0001; mGlu200: F(1,41)=13.0, P<0001, d=0.56], suggesting that LY354740 reduced but did not block fear-potentiated startle to shock anticipation.
An analysis was conducted using the presence or absence of nausea in the subjects as a covariate. For the fear-potentiated startle to the threat signal, the level of significance fell just outside the significance criteria [F(1,40)=3.75, P<0.06, d=0.61]. The lack of significance is likely due to small sample size because the effect size was large. In fact, the fear-potentiated startle effect was slightly greater in the subjects with nausea (mean=61.3, SE=16.3) compared to the subjects without nausea (mean=57.8, SE=12.2), indicating that nausea did not reduce fear-potentiated startle.
Subjective reports
Analysis of the VAS ratings for the different adjectives assessing sedative effects was conducted with a two-way ANOVA with Treatment (Placebo, mGlu20, mGlu200) and Time (before, after ingestion) as the two factors. There was no significant main effect or interaction effect for any of the adjectives (results not shown).
Subjective ratings of mood and anxiety were taken at baseline (before and after drug ingestion) and just after each fear-potentiated startle test. Results for STAI-state anxiety and PANAS are presented in Fig. 3. For any given measure, there was no significant difference between scores at baseline before and after drug ingestion. Similarly, there was no significant difference following the first and the second fear-potentiated startle tests. Consequently, the data for each questionnaire were averaged separately within the baseline condition and within the fear-potentiated startle condition. The results were then analyzed using ANOVA with repeated measures using Treatment (Placebo, mGlu20, mGlu200) and Condition (baseline, fear-potentiated startle test) as the two factors. Participation in the fear-potentiated startle tests increased state anxiety [F(1,41)=44.3, P<0.00001], and negative affect [F(1,41)=12.9, P<0.0009], compared to baseline levels, and decreased positive affect [F(1,41)=82.8, P<0.003]. However, the increase in state anxiety and in negative affectivity was smaller in the LY354740 groups, compared to the placebo group resulting in significant Treatment×Time interactions for state anxiety [F(2,41)=3.9, P<0.03] [contrast Placebo versus mGlu20/mGlu200: F(1,41)=7.2, P<0.01, d=0.8], and a trend for negative affect [F(2,41)=2.97, P<0.06] [contrast Placebo versus mGlu20/mGlu200: F(1,41)=5.6, P<0.02, d=0.73]. Importantly, there were significant linear effects for state anxiety [F(1,41)=7.3, P<0.01] and for negative affect [F(1,41)=5.3, P<0.02], indicating that LY354740 reduced subjective reports of anxious mood in a dose-dependent fashion.
State anxiety (STAI) and negative affect (PANAS) scores before (baseline=average of scores upon subjects' arrival in the laboratory and after drug ingestion, just prior to placement of the shock electrodes) and after each fear-potentiated startle test (average over the two tests). See text for details. Columns show mean values and error bars show SEM
Retrospective ratings of anxiety during the fear-potentiated startle tests were analyzed with Treatment (Placebo, mGlu20, mGlu200), Test (first, second), Darkness (light, dark), and Condition (safe, threat) ANOVA (data not shown). Subjects were more anxious in the threat than in the safe condition [F(2,41)=123.2, P<0.00009], and in the dark than in the light condition [F(2,41)=15.8, P<0.0003]. The only significant difference was a Treatment main effect [F(2,41)=6.0, P<0.005], indicating the overall level of anxiety during the test was lower in the LY354047 groups, compared to the placebo group.
Memory performance scores (number of word remembered out of a list of 20 words) during the screening day and after drug ingestion were: placebo/screening=8.7 (0.5); Placebo/after drug=8.6 (0.5); mGlu20/screening=8.2 (0.5); mGlu20/after drug=8.5 (0.5); mGlu200/screening=8.3 (0.5); mGlu200/after drug=9.4 (0.6). These scores were investigated with a Treatment (Placebo, mGlu20, mGlu200)×Test (first, second) ANOVA. None of the effects or interactions was significant, suggesting that short-term memory was not affected by LY354740.
Discussion
The main result of this study was that the metabotropic receptor agonist LY354740 was anxiolytic in the fear-potentiated startle paradigm without producing sedation. More specifically, the startle data suggest that LY354740 reduced fear induced by the threat of a shock. The subjective data suggest that LY354740 reduced the overall anxiety level associated with participation in an experiment where unpleasant shocks were administered.
The study was designed to examine three types of startle potentiation: 1) fear-potentiated startle during threat of a shock; 2) startle facilitation in the dark; and 3) enhancement of startle caused by placing the shock electrodes. Consistent with previous results, the anticipation of shock (Grillon et al. 1991), darkness (Grillon et al. 1997a), and the placement of the shock electrodes (Grillon and Ameli 1998) increased significantly the magnitude of the eyeblink reflex component of the startle response. Startle potentiation in aversive situations in animals have been taken as an index of fear and anxiety based on brain lesion and psychopharmacological studies (Davis 1992). Along with this objective physiological measure of aversive states, we obtained subjective ratings of anxiety that confirmed that both shock anticipation in the threat condition and darkness were anxiogenic.
As postulated based on pre-clinical data (Helton et al. 1998; Walker et al. 2002), LY354740 reduced fear-potentiated startle during shock anticipation. This result was suggested by the significantly smaller threat-induced potentiation of startle in the LY354740 group, compared to the placebo group. Given the relatively large effect size of the placebo versus mGlu20/mGlu200 comparison, it is likely that the lack of a significant Treatment (placebo, mGlu20, mGlu200)×Condition (safe, threat) interaction was due to the small sample size. Of note, using very similar threat of shock paradigms, our group has been unable to obtain differences in fear-potentiated startle between patient groups (e.g. patients with panic disorder versus healthy controls; Grillon et al. 1994) or drug treatment (Baas et al. 2002). In particular, we were unable to find in four separate experiments that benzodiazepine reduced fear-potentiated startle (Baas et al. 2002). These results suggest that fear-potentiated startle to threat is a very robust effect. Its partial disruption by LY354740 is a significant finding.
The absence of a dose-response effect for potentiated startle is surprising given the 10-fold increase in LY354740 in the mGlu200 compared to the mGlu20 group. One possibility is that LY354740 has a non-monotonic effect at the doses given in the present study. This is unlikely for at least two reasons. First, animal studies report a linear dose-response effect of LY354740 on fear-potentiated startle (e.g. Walker et al. 2002). Second, the present study found a significant dose-response effect for the subjective measures (state anxiety and negative affectivity). Hence, the lack of differential reduction in fear-potentiated startle in the mGlu20 and mGlu200 groups cannot be attributed to a lack of differential anxiolytic effect of LY344740. More likely, it reflects a lack of differential sensitivity of fear-potentiated startle to threat to the differential anxiolytic effects of the two doses of LY344740. One possibility is that the relation between startle potentiation and the intensity of fear elicited by shock anticipation is not linear. For example, a high level of startle potentiation could be reached for moderate levels of fear with little increase with high levels of fear. Under these circumstances, a small reduction in fear-potentiated startle could reflect a substantial reduction in the intensity of fear. An alternative is that the eyeblink response reached ceiling levels in the threat condition, preventing accurate assessment of any reduction in fear. This latter explanation does not seem to be supported by the data. The Table shows that although startle habituated from the first to the second fear-potentiated startle test, the degree of startle potentiation remained approximately the same across the two tests. If startle reached a ceiling level in the threat condition of the first test, one would have expected a greater increase in potentiated startle in the second test. Future studies should examine the relation between subjective fear intensity and levels of fear-potentiated startle.
The reduction of fear-potentiated startle by LY354047 cannot be explained by a non-specific sedative effect of the drug on baseline startle, which would prevent accurate assessment of fear-potentiated startle (Grillon and Baas 2002; Walker and Davis 2002b). LY354740 did not significantly affect baseline startle and the two LY354740 groups and the placebo group had comparable baseline startle. Any difference in fear-potentiated startle among these groups cannot be attributed to difference in baseline startle.
A similar effect of LY354740 on fear-potentiated startle was reported in rats. Helton et al. (1998) showed that LY354740 reduced fear-potentiated startle in a dose-dependent fashion. A likely target for the effect of LY354740 is the amygdala. This structure has been shown repeatedly to play a central role in the acquisition and in the expression of conditioned fear. A functioning amygdala is also necessary for the acquisition and expression of fear-potentiated startle in rodents (Hitchcock and Davis 1986). In addition, group II metabotropic receptors are found in high concentration in the amygdala, and blockade of glutamatergic synaptic transmission within the amygdala prevents anxiogenic responses (Kim et al. 1993). Similarly, LY354740 depresses excitatory glutamate transmission at the level of the basolateral/central nucleus of the amygdala, both structures that are necessary for fear-potentiated startle. Thus one possible explanation for the anxiolytic and non-sedative effect of LY354740 is that it targets activity in a specific fear pathway. By contrast, because benzodiazepines may target gamma-aminobutyric acid A (GABA-A) receptor throughout the brain and spinal cord, their effect on fear-potentiated startle may be less specific and they induce sedation, including a robust reduction in baseline startle (Bitsios et al. 1999; Riba et al. 2001; Baas et al; 2002).
In traditional conditioning experiments, reduced fear to a signal for shock could be due to impaired learning or to reduced fear. Because subjects were instructed of the association between the shock and the threat signal, it is unlikely that deficient learning played a role in the findings. It could be argued that the LY354740 subjects forgot the instruction. The fact that subjects 1) showed both physiological and subjective fear of the threat signal and that 2) LY354740 did not affect memory performance for the word list argues against this possibility. Hence, it is very likely that LY354740 affected the expression of fear. Consistent with this interpretation, results in rodents show that following acquisition of conditioned fear, pre-test infusion of the AMPA receptor antagonist NBQX into the basolateral amygdala blocks the expression of fear-potentiated startle (Walker and Davis 1997a). Furthermore, oral injection of LY354740 in rats disrupts the expression, but not the acquisition, of fear-potentiated startle (Tizzano et al. 2002). Evidence for the role of the amygdala in this process comes from a study showing that after acquisition, pre-test intra-amygdala injection of LY35470 interferes with the expression of fear-potentiated startle (Walker et al. 2002). A recent study suggests that, in addition to glutamatergic receptors, GABAergic pathways can mediate the anxiolytic effects of LY354740. This follows from the finding that the benzodiazepine antagonist, flumazenil, blocks the anxiolytic effects of LY354740 in rats on the elevated plus maze (Ferris et al. 2001). However, Tizzano et al. (2002) showed that GABA receptors in rats are not involved in the anxiolytic effects of LY354740 on fear-potentiated startle because flumazenil does not antagonize the effect of LY354740 in this model. Taken together, these results suggest that LY354740 is anxiolytic and that its anxiolytic effects result from reduced glutamatergic transmission, at least to some extent, within the amygdala.
LY354740 did not reduce the facilitation of startle in the dark, suggesting that it did not have anxiolytic properties in this paradigm. One possibility to explain this result is that the facilitation of startle in the dark is not due to an increase in anxiety. Several recent reports indicate that startle can be increased by non-specific arousal in humans (e.g. Lipp 2002). If the facilitation of startle in the dark were due to a non-specific increase in arousal, it would not be inconsistent to find that this effect is not affected by a drug such as LY354740, that has anxiolytic but no sedative properties. In contrast, an arousal interpretation of the facilitation of startle in the dark would be consistent with the finding that the benzodiazepine diazepam, which has sedative effects, reduced the facilitation of startle in the dark in humans (Baas et al. 2002).
Another possibility is that darkness is anxiogenic in humans and that LY354740 is not anxiolytic in this model. Results supporting darkness as a model for anxiety include 1) psychopharmacological and neurobiological studies of the effect of bright lights in rodents (Walker and Davis 1997b, 2002a; de Jongh et al. 2002), 2) the finding that diazepam blocks the facilitation of startle in the dark in humans (Baas et al. 2002), and 3) studies in Vietnam veterans with PTSD showing increased facilitation of startle in the dark in this population (Grillon et al. 1998).
Finally, it is possible that the facilitation of startle in the dark is due to anxiety, but that we were unable to detect an anxiolytic effect of LY354740 because the signal may, as suggested by one of the reviewers, be too small and noisy to detect such an effect. Testing the effect of LY354740 in subjects that may be overly sensitive to darkness (e.g. Vietnam veterans with PTSD: Grillon et al. 1998) may help address this issue.
Experimental contexts where electric shocks are administrated are anxiogenic, raising the baseline level of startle reactivity in humans (Grillon and Morgan 1996; Grillon and Ameli 1998). In the present experiment, the anxiogenic nature of the context was suggested by two measures: 1) the enhanced startle after the placement of the shock electrodes and 2) the various anxiety and mood questionnaires (STAI-state, PANAS) given at baseline and just after each fear-potentiated startle test. Rating of anxiety and negative affectivity were significantly higher when measured just after each fear-potentiated startle experiment, compared to baseline. LY354740 had a dose-dependent anxiolytic effect on these measures (Fig. 3), reducing the level of anxiety during the experiment. Similarly, the enhancement of startle after placing the shock electrodes was smaller in the LY354740 group compared to the placebo group, but this effect did not reach significance. Thus, there is evidence that LY354740 affected not only fear to an explicit threat cue, but also the more sustained level of anxious apprehension of being in a stressful environment.
Subjects were also asked to retrospectively rate their level of anxiety during the safe and the threat conditions after each fear-potentiated startle experiment. Although subjects felt more anxious during the threat than during the safe condition, this effect was not reduced by LY354740. However, here again, the LY354740 group showed an overall reduction in subjective anxiety, compared to the placebo group. The lack of differential effect of LY354740 on anxiety ratings to the safe and threat signals contrast with the startle data. This finding is not surprising given that the ratings were retrospective and that subjects were asked to differentiate between four separate conditions (safe/light, safe/dark, threat/light, threat/dark). Retrospective ratings are biased toward providing a global evaluation that may not be sensitive to real-time rating (Kahneman 1999). Future studies wishing to address this issue should provide an online measure of subjective anxiety. However, such a measure could interfere with anxiety (e.g. external inhibition). This is one of the reasons that psychophysiological approaches are advantageous. In particular, the startle reflex provides a specific assessment of ongoing changes in affective states.
In conclusion, LY354740 reduced the expression of fear-potentiated startle to a threat signal without affecting baseline startle. It also reduced in a dose-dependent manner subjective feelings of anxiety and negative affectivity. These results are consistent with reports indicating that LY354740 is anxiolytic in several animal models, including fear-potentiated startle, but has no sedative effect, even at doses that are 100–1000 times higher than anxiolytic doses (Helton et al. 1998). In this respect, the utility of LY354740 as a potential anxiolytic is not hampered by sedation, one of the major side effects of benzodiazepines. In chronic treatment, it is also well tolerated without evidence of sedation, amnesic, or withdrawal effects (Levine et al. 2002). Results of ongoing clinical trials will tell us whether the anxiolytic effects of LY354740 in human and animal models extend to the treatment of anxiety disorders.
References
Baas JM, Grillon C, Bocker KB, et al. (2002) Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology 161:233–247
Bitsios P, Philpott A, Langley RW, Bradshaw CM, Szabadi E (1999) Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. J Psychopharmacol 13:226–234
Charney DS, Heninger GH, Breier A (1984) Noradrenergic function in panic anxiety: effects of yohimbine in healthy subjects and patients with agoraphobia and panic disorder. Arch Gen Psychiatry 41:751–763
Dantzer R (1977) Behavioral effects of benzodiazepines: a review. Biobehav Rev 1:71–86
Davis M (1979) Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology 62:1–7
Davis M (1992) The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15:353–375
Davis M (1998) Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry 44:1239–1247
Davis M, Astrachan DI (1978) Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used during training. J Exp Psychol [Anim Behav Proc] 4:95–103
Davis M, Redmond DE Jr, Baraban JM (1979) Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology 65:111–118
de Jongh R, Groenink L, van der Gugten J, Olivier B (2002) The light-enhanced startle paradigm as a putative animal model of anxiety: effects of chlordiazepoxide, flesonoxan and fluvoxamine. Psychopharmacology 159:176–180
Ferris P, Seward E, Dawson GR (2001) Interactions between LY354740, a group II metabotropic agonist and the GABA(A)-benzodiazepine receptor complex in the rat elevated plus-maze. J Psychopharmacol 15:76–82
Grillon C, Ameli R (1998) Effects of threat of shock, shock electrode placement, and darkness on startle. Int J Psychophysiol 28:223–231
Grillon C, Baas JM (2002) Comments on the use of the startle reflex in psychopharmacological challenges: impact of baseline startle on measurement of fear-potentiated startle. Psychopharmacology 164:236–238
Grillon C, Davis M (1997) Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired vs. unpaired training. Psychophysiology 34:451–458
Grillon C, Morgan CA (1996) Fear-potentiated startle in Vietnam veterans with PTSD. Biol Psychiatry 39:555
Grillon C, Ameli R, Woods SW, Merikangas K, Davis M (1991) Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28:588–595
Grillon C, Sinha R, O'Malley SS (1994) Effects of ethanol on the acoustic startle reflex in humans. Psychopharmacology 114:167–171
Grillon C, Pellowski M, Merikangas KR, Davis M (1997a) Darkness facilitates the acoustic startle in humans. Biol Psychiatry 42:453–460
Grillon C, Ameli R, Goddard A, Woods S, Davis M (1997b) Baseline and fear-potentiated startle in panic disorder patients. Biol Psychiatry 35:431–439
Grillon C, Morgan CA III, Davis M, Southwick SM (1998) Effect of darkness on acoustic startle in Vietnam veterans with PTSD. Am J Psychiatry 155:812–8217
Grillon C, Sinha R, Ameli R, O'Malley SS (2000) Effects of ethanol on baseline startle and prepulse inhibition in young men at risk for alcoholism and/or anxiety. J Stud Alcohol 61:46–54
Helton DR, Tizzano JP, Monn JA, Schoepp DD, Kallman MJ (1998) Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J Pharmacol Exp Ther 284:651–660
Hitchcock JM, Davis M (1986) Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle Kehne paradigm. Behav Neurosci 100:11–22
Kahneman D (1999) Objective hapiness. In: Kahneman D, Diener E, Schwarz N (eds) Well-being: the foundations of hedonic psychology. Russell Sage Foundation, New York, N.Y., pp 3–25
Kim M, Campeau S, Falls WA, Davis M (1993) Intra-amygdala infusions of the non-NMDA receptor antagonist CNQX blocks the expression of fear-potentiated startle. Behav Neural Biol 59:5–8
Klowsowicz BA, O'Donnell A, Volicer L (1979) Effects of ethanol on acoustic startle response and cerebellar cyclic GMP level: interaction with pilocarpine and atropine. Curr Alcohol 5:173–180
Kumari V, Cotter P, Corr PJ, Gray JA, Checkley SA (1993) Effect of clonidine on the human acoustic startle reflex. Psychopharmacology 123:353–360
Landis C, Hunt WA (1939) The startle pattern. Farrar and Rinehart, New York
Lang PJ, Bradley MM, Cuthbert BN (1990) Emotion, attention, and the startle reflex. Psychol Rev 97:1–19
Levine LR, Gaydos B, Sheehan D, et al. (2001) The mGlu2/3 receptor agonist, LY354740, reduces panic anxiety induced by CO2 challenge in patients diagnosed with panic disorder. Neuropharmacology 43:294
Lipp OV (2002) Anticipation of a non-aversive reaction time task facilitates the blink startle reflex. Biol Psychol 59:147–62
Patrick C, Berthot B, Moore J (1996) Diazepam blocks fear potentiated startle in humans. J Abnorm Psychol 105:89–96
Riba J, Rodriguez-Fornells A, Urbano G, Morte A, Antonijoan R, Barbanoj MJ (2001) Differential effects of alprazolam on the baseline and fear-potentiated startle reflex in humans: a dose-response study. Psychopharmacology 157:358–367
Rodriguez-Fornells A, Riba J, Gironell A, Kulisevsky J, Barbanoj MJ (1999) Effects of alprazolam on the acoustic startle response in humans. Psychopharmacology 143:280–285
Schoepp DD (1994) Novel functions for subtypes of metabotropic glutamate receptors. Neurochem Int 24:439–449
Shekhar A, Keim SR (2000) LY354740, a potent group II metabotropic glutamate agonist, prevents lactate induced panic response in panic-prone rats. Neuropharmacology 39:1139–1146
Spielberger CD (1983) Manual for the state-trait anxiety inventory. Consulting Psychologist Press, Palo Alto, Calif.
Tizzano JP, Griffey KI, Schoepp DD (2002) The anxiolytic action of mGlu2/3 receptor agonist, LY354740, in the fear-potentiated startle model in rats is mechanistically distinct from diazepam. Pharmacol Biochem Behav 73:367–374
Walker D, Davis M (1997a) Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci 17:9375–9383
Walker DL, Davis M (1997b) Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol Psychiatry 42:461–471
Walker DL, Davis M (2000) Quantifying fear-potentiated startle: a comparison of absolute versus percent increase scoring methods. Soc Neurosci Abstr 26:482
Walker DL, Davis M (2002a) Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology 159:304–310
Walker DL, Davis M (2002b) Quantifying fear potentiated startle using absolute versus percent increase scoring methods: implication for the neurocircuitry of fear and anxiety. Psychopharmacology 164:318–328
Walker DL, Rattiner L, Davis M (2002) Group II metabotropic glutamate receptor within the amygdala regulate anxiety in rats as assessed with fear-potentiated startle. Behav Neurosci 116:1075–1083
Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Person Soc Psychol 54:1063–1070
Woods JH, Katz JL, Winger L (1992) Benzodiazepines: use, abuse, and consequences. Pharmacol Rev 44:151–347
Acknowledgements
The authors acknowledge Lilly Research Laboratories for their financial assistance and for providing LY354740. The work was conducted at Yale University School of Medicine, Department of Psychiatry, New Haven, Conn., USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grillon, C., Cordova, J., Levine, L.R. et al. Anxiolytic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology 168, 446–454 (2003). https://doi.org/10.1007/s00213-003-1444-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1444-8