Abstract
The monoamine stabilizer (3S)-3-[3-(methenesulfonyl)phenyl]-1-propylpiperidine hidrochloride [(−)-OSU6162] is a promising compound for the treatment of neurological and psychiatric disorders, such as schizophrenia. Here, we tested the hypothesis that (−)-OSU6162 prevents hyperlocomotion and sensorimotor deficits in prepulse inhibition of the startle response (PPI) induced by psychomimetic drugs. Male Swiss mice received injections of (−)-OSU6162 (1, 3, 10, or 30 mg/kg), and their motor responses were investigated in the open field and in the catalepsy tests, which predicts liability to induce sedation and extrapyramidal side effects, respectively. Next, in independent experiments, this compound was evaluated for its efficacy to prevent hyperlocomotion induced by cocaine (10 mg/kg; dopamine transporter inhibitor) or ketamine (60 mg/kg; glutamate NMDA channel blocker) in the open field. Finally, we tested if (−)-OSU6162 prevents PPI disruption induced by MK-801 (0.5 mg/kg; glutamate NMDA channel blocker). (−)-OSU6162 induced neither locomotion impairment nor catalepsy. This compound prevented cocaine-induced hyperlocomotion at the doses of 10 and 30 mg/kg and ketamine-induced hyperlocomotion at the doses of 1 and 3 mg/kg. In the sensorimotor test, (−)-OSU6162 failed to reverse MK-801-induced PPI deficits. The dopamine stabilizer (−)-OSU6162 prevents the hyperactivity induced by dopaminergic and anti-glutamatergic drugs at doses that preserve motor functions, although it failed in the PPI test. Its therapeutic potential for specific symptoms of schizophrenia warrants further investigation in both preclinical and clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a severe, multifactorial, and chronic psychiatric disorder, whose complex symptomatology has led to the concept of symptom dimensions, divided in positive (psychosis, hyperactivity), negative (alogy, affective flattening, social withdrawal), and cognitive symptoms (Kapur et al. 2005; van Os and Kapur 2009). Current treatment options focus mainly on pharmacological approaches with antipsychotic drugs. These are divided in first-generation antipsychotics (FGAs, “typical” antipsychotics), such as chlorpromazine and haloperidol, and second-generation antipsychotics (SGAs; “atypycal” antipsychotic), which include the prototype clozapine, as well as olanzapine, risperidone, quetiapine, among others. Although affinity and selectivity vary among compounds, virtually all FGAs and SGAs act as antagonists at the dopamine D2 receptor (Gardner et al. 2005; Kapur 2004; Millan et al. 2015). More recently, the concept of third-generation antipsychotic drugs (TGAs) has emerged. TGAs, such as aripiprazole and brexpiprazole, are thought to exert their effects through a more subtle mechanism of action, consisting of partial agonism at dopamine receptors (thus termed “dopaminergic modulators”), in addition to other targets (Ohlsen and Pilowsky 2005; Tamminga and Carlsson 2002).
Antipsychotic therapy is limited by various side effects resulting from D2 receptor antagonism and interference with other neurochemical processes. FGAs use results in various motor side effects, ranging from Parkinsonism to tardive dyskinesia (Haddad et al. 2012). Although SGAs are less prone to cause motor impairment, they are not necessarily always more efficacious and may lead to autonomic nervous system misbalancing and metabolic malfunctioning, such as weigh gain, impaired glucose tolerance, and dyslipidemia (Hartling et al. 2012; Leucht et al. 2009; Lieberman et al. 2005). Finally, the concept of TGAs is still controversial, with few representative compounds so far (Mailman and Murthy 2010; Millan et al. 2015). Moreover, a significant number of patients are refractory to pharmacotherapy, suffering from treatment-resistant schizophrenia (Demjaha et al. 2017; Howes et al. 2017).
One possible reason for treatment failure is the complex neurochemical misbalance that causes schizophrenia (Demjaha et al. 2017; Howes et al. 2017). The main biological hypothesis of schizophrenia still focuses on the exacerbation of dopamine neurotransmission projecting from the ventral tegmental area to the nucleus accumbens (Kapur et al. 2005). Another attempt to explain the various symptoms of this disorder points to a hypofunction of N-methyl-D-aspartate (NMDA) glutamate receptor (Laruelle 2014; Laruelle et al. 2003; Moghaddam and Javitt 2012). Thus, there have been attempts to develop new pharmacotherapies beyond dopamine antagonists.
In this context, the compound (3S)-3-[3-(Methenesulfonyl)phenyl]-1-propylpiperidine hidrochloride [(−)-OSU6162] is a promising agent for the treatment of some neurological and psychiatric disorders. In animal models predictive of antipsychotic activity, (−)-OSU-6162 prevents the behavioral effects of dopaminergic and glutamatergic drugs at doses that do not impair motor responses (Natesan et al. 2006; Rung et al. 2005). In the catalepsy test, which predicts liability to induce parkinsonism and other extrapyramidal side effects (Gobira et al. 2013; Sanberg et al. 1988), (−)-OSU6162 has a favorable profile even at high levels of striatal D2 receptor occupancy (Natesan et al. 2006). This compound has also the unusual property to reverse behavioral responses resulting from both hypo- and hyperdopaminergic activity in experimental animals, at doses ranging from 5 to 120 mg/kg in rats (Rung et al. 2008). Possible explanations for its mechanisms of action are preferential antagonism at D2 auto-receptor over post-synaptic receptor (Sonesson et al. 1994); partial agonism at D2 receptor (Burstein et al. 2011; Kara et al. 2010; Lin et al. 2006; Seeman and Guan 2007); allosteric and orthosteric effects at the dopamine D2 receptor, with stimulating or inhibitory effects depending on the prevailing dopaminergc tonus (Rung et al. 2008). (−)-OSU6162 may act as a “dopamine stabilizer”, reverting the consequence of excessive dopamine neurotransmission by antagonizing post-synaptic D2 receptor and reverting low-dopaminergic states due to its higher affinity for presynaptic D2 auto-receptor (Rung et al. 2008). Apart from dopamine, (−)-OSU6162 also displays partial agonism on 5-HT2A serotonin receptor (Burstein et al. 2011; Carlsson et al. 2011) as well as nanomolar affinity for the human σ-1 receptor (Sahlholm et al. 2013).
In the present study, we tested the hypothesis that (−)-OSU6162 exerts antipsychotic-like effects in locomotor and sensorimotor models, based on both dopaminergic and glutamatergic theories of schizophrenia. We hypothesized that (−)-OSU6162 prevents the hyperlocomotion induced by cocaine, a dopamine transporter blocker, and ketamine, an NMDA channel blocker, in an open field. In the sensorimotor test, we investigated if this dopamine stabilizer prevents the deficit in prepulse inhibition of the startle reflex (PPI) induced by MK-801, another NMDA-channel blocker. We also tested if (−)-OSU6162 impairs spontaneous locomotion and induces catalepsy in mice.
Material and methods
Animals
Male Swiss mice (25–35 g, 6–8 weeks of age) were obtained from the Animal Care Facilities (CEBIO) of the Institute of Biological Sciences (ICB)–Federal University of Minas Gerais, Brazil. The animals were maintained in a room with controlled temperature and a light/dark cycle (25 ± 1 °C, lights on at 07:00 a.m., off at 7:00 p.m.). Mice were housed in groups, with free access to food and water. The experiments were performed between 08:00 a.m. and 4:00 p.m. All procedures used in this study followed the ethical principles of animal experimentation adopted by the Ethic Committee on Animal Use of Federal University of Minas Gerais (CEUA–UFMG), and institutionally approved under protocol number 227/2013. Each animal was used only once. All efforts were made to minimize the number of animals used and their suffering.
Drugs
(3S)-3-[3-(Methenesulfonyl)phenyl]-1-propylpiperidine hidrochloride [(−)-OSU6162] (Tocris Bioscience), cocaine (Merck), ketamine (Syntec), haloperidol (Teuto), and clozapine (Sigma) were dissolved in saline. Solutions were prepared immediately before use and administered via intraperitoneal route (i.p.) in a volume of 10 mL/kg. We performed dose-response curves for (−)-OSU6162, cocaine, and ketamine, based on previous literature (Leite et al. 2008; Moreira and Guimaraes 2005; Natesan et al. 2006; Rung et al. 2008). Doses of haloperidol, positive control for the catalepsy test, and clozapine, positive control for the PPI test, were also selected based on previous studies (Issy et al. 2009; Moreira and Guimaraes 2005).
Behavioral protocols
Evaluation of the locomotor activity was carried in a square arena (40 × 40 cm) located in an isolated room. The animals were filmed, and the total distance moved was analyzed automatically by the Any-maze® software (Stoelting, Wood Dale, IL, USA). For the evaluation of a possible alteration in the basal locomotion induced by (−)-OSU6162, after the habituation measurement for 10 min in arena, the animals received an injection of saline or (−)-OSU6162 (1, 3, 10, and 30 mg/kg) and were placed in the arena 15 min later. The distance moved in the arena was measured during 20 min (Viana et al. 2013).
Next, (−)-OSU6162 was evaluated in the catalepsy test. In this test, the capacity of the animal to remove itself from an unusual and uncomfortable posture is measured (Gobira et al. 2013; Sanberg et al. 1988). For this measurement, saline, haloperidol (0.5 mg/kg), or (−)-OSU6162 (1, 3, 10, and 30 mg/kg) were injected, and 30 min later, the mice forepaws were placed gently over a horizontal bar (diameter: 0.5 cm) elevated 4.5 cm from the floor. The time in seconds during which the animal maintained this position until the removal of one of its forepaws was registered for a maximum of 300 s. Three immediate attempts to return the animal in cataleptic position were allowed within the first 10 s. The doses and times of injections were selected in accordance with previous literature (Moreira and Guimaraes 2005).
Dose-response curves of the effect of cocaine and ketamine at the open-field were also performed. As described previously for the experiments with (−)-OSU6162, after the habituation measurement for 10 min in arena, the animals received an injection of saline or cocaine (2.5, 5, and 10 mg/kg) or ketamine (30 and 60 mg/kg) and were placed in the arena 15 min later. The distance moved in the arena was measured for 20 min. To evaluate the effect of (−)-OSU6162 on the hyperlocomotion induced by the psychomimetic drugs, after the habituation for 10 min in arena, animals received an injection of vehicle or (−)-OSU6162 (1, 3, 10, and 30 mg/kg). Following 15 min later, animals received an injection of ketamine (60 mg/kg) or cocaine (10 mg/kg). Then, 30 min after the first injection, the animals were placed in the arena for 20 min. The protocols for the time of injections were selected according to the previous studies (Leite et al. 2008; Moreira and Guimaraes 2005).
Prepulse inhibition of the startle reflex (PPI)
The experiments measuring the PPI were conducted in a sound-isolated chamber. A continuous acoustic signal produced a background white noise level of 65 dB. The pulse was a noise of 105 dB with 20 ms of duration. The prepulse intensities were 80, 85, and 90 dB in 7000 Hz frequency and duration of 10 ms (Issy et al. 2009). The mice were subjected to a pretest session, to select those animals with a PPI response superior to 0%. In the experiment, the animals were first submitted to 5 min of acclimatization. In this period, they received 65-dB background noise and were presented to a series of 10 stimuli (pulse alone). After this period, they received pulse alone P (105 dB), prepulse alone PP (80, 85, or 90 dB), prepulse + pulse with 100 ms interval between prepulse and pulse, and null, in which no stimulus was presented. Mean acoustic startle response to pulse-alone (P) and each prepulse + pulse (PP + P) trial was calculated for each subject. The level of PPI was determined using the formula %PPI = 100 − [100 × (PP + P/P)] (Issy et al. 2009). Using the same protocol, mice were exposed to a test session receiving (−)-OSU6162 (3, 10, 30 mg/kg) or clozapine (5 mg/kg) followed in 30 min by MK-801 (0.5 mg/kg) treatment. We used MK-801 instead of ketamine in this test based on previous experiments showing the efficacy of this compound to disrupt PPI in mice (Issy et al. 2009).
Statistical analysis
Data distribution was analyzed by the Smirnov-Kolmolgorov test. Data from the latency in the catalepsy test did not fit in a normal distribution and were compared by the non-parametric tests Kruskal-Wallis followed by Mann-Whitney. The distance traveled in the open field was subjected to analysis of variance (ANOVA) followed by the Bonferroni’s test. In the PPI test, drug effects across different prepulse intensities were analyzed by two-way ANOVA (treatment and prepulse as experimental factors) followed by the Bonferroni test. The significance level was set at p < 0.05. Data from the catalepsy test are presented as median and interquartile range, whereas the distance moved and the %PPI are presented as mean and standard error of the mean (SEM).
Results
We first investigated the effects of (−)-OSU6162 in behavioral tests predictive of motor side effects in mice. None of the doses of this compound reduced spontaneous locomotion in the open field [F(3,16) = 0.7233, ns; ANOVA; Fig. 1], indicating a low propensity to induce sedative effects. To test the liability of (−)-OSU6162 to induce extrapyramidal side effect (parkinsonims), we compared this compound with the first-generation antipsychotic, haloperidol. As expected, haloperidol increased the time spent in an atypical posture at the doses of 0.5 and 1 mg/kg (H = 27.15, p < 0.0001; Kruskal-Wallis followed by Dunn test; Fig. 2a). In another experiment, haloperidol, but not (−)-OSU6162, induced catalepsy (H = 23.76, p = 0.0002; Kruskal-Wallis followed by Dunn test; Fig. 2b).
Effects of haloperidol (Hal) and (−)-OSU6162 on the catalepsy test in mice. a Haloperidol induced catalepsy at the doses of 0.5 and 1 mg/kg (n = 8, 9, 9, 7). b Haloperidol (0.5 mg/kg), but not (−)-OSU6162, induced catalepsy (n = 6/group). **p < 0.01, ***p < 0.001, ****p < 0.0001 compared with respective vehicle (Veh) groups; Kruskal-Wallis followed by Dunn test. Data are presented as median and intequartile range
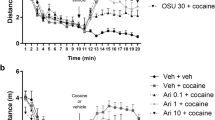
Next, we tested (−)-OSU6162 in dopaminergic and anti-gluatamatergic locomotor models. Cocaine, a dopamine uptake blocker, increases the distance moved in the open field at the dose of 10 mg/kg [F(3,25) = 14.99, p < 0.0001; ANOVA followed by Bonferroni test; Fig. 3a]. (−)-OSU6162 prevented cocaine effects at the doses of 10 and 30 mg/kg [F(5,25) = 8.302, p < 0.0001; Fig. 3b]. In the anti-glutamatergic model, ketamine, an NMDA channel blocker, induced hyperlocomotion at the dose of 60 mg/kg [F(2,16) = 10.67, p = 0.0011; ANOVA followed by Bonferroni test; Fig. 4a]. (−)-OSU6162 prevented ketamine effects at the doses of 1 and 3 mg/kg [F(5,35) = 12.18, p < 0.0001; Fig. 4b].
Effect of cocaine on locomotion and effect of (−)-OSU6162 on cocaine-induced hyperlocomotion in mice in the open field. a Cocaine induced hyperlocomotion at the dose of 10 mg/kg (n = 7, 6, 8, 8). b (−)-OSU6162 prevented the motor hyperactivity induced by cocaine (10 mg/kg) in open field at the doses of 10 and 30 mg/kg (n = 6, 5, 5, 5, 5). ***p < 0.001 and ****p < 00001 compared with respective vehicle (Veh) groups; #p < 0.001 compared to vehicle + cocaine group; ANOVA followed by Bonferroni test. Data are presented as mean and SEM
Effect of ketamine on locomotion and effect of (−)-OSU6162 on ketamine-induced hyperlocomotion in mice in the open field. a Ketamine induced hyperlocomotion at the dose of 60 mg/kg (n = 6, 6, 7). b (−)-OSU6162 prevented the motor hyperactivity induced by ketamine (60 mg/kg) in open field at the doses of 1 and 3 mg/kg (n = 8, 7, 6, 9, 6, 5). **p < 0.01 and ****p < 0.0001 compared with respective vehicle (Veh) groups; ###p < 0.01 compared to vehicle + ketamine group. ANOVA followed by Bonferroni test. Data are presented as mean and SEM
Finally, we tested (−)-OSU6162 in a sensorimotor model predicitve of antipsychotic activity, namely the PPI disruption induced by the NMDA channel blocker, MK-801, at various prepulse intensities (Fig. 5). Two-way ANOVA followed by the Bonferroni test revealed an effect of prepulse intensity [F(2,126) = 3.932, p = 0.0221]. There was also an overall effects of treatment, which revealed that MK-801 disrupted PPI, as expected, an effect prevented by clozapine, but not by (−)-OSU6162 at any dose [F(6,126) = 23.33, p < 0.0001]. Finally, there was no interaction between factors, indicating that the drug effects were similar across different prepulse intensities [F(12126) = 0.2233, p = 0.9970].
Effect of (−)-OSU6162 and clozapine on MK-801-induced deficits in PPI in mice at the prepulse intensities of 81, 77, and 73 dB. MK-801 (0.5 mg/kg) disrupted PPI, an effect prevented by clozapine (CZP, 5 mg/kg), but not (−)-OSU6162, at all prepulse intensities (n = 7, 8, 7, 8, 7, 7, 5). *p < 0.05 compared with respective vehicle (Veh) group; #p < 0.05 compared to vehicle + MK-801 group. ANOVA followed by Bonferroni test. Data are presented as mean and SEM
Discussion
In the present study, we showed that (−)-OSU6162 was effective in inhibiting the motor hyperactivity induced by psychotomimetic drugs in mice, at doses that did not impair motor functioning. Contrary to haloperidol, (−)-OSU6162 did not induce catalepsy, suggesting low liability to provoke parkinsonism or other extrapyramidal side effects in humans. In addition, this dopamine stabilizer prevented the effects of both cocaine and ketamine (dopaminergic and anti-glutamatergic models, respectively) in the open field. This occurred at doses that did not reduce basal locomotion, indicating that the inhibition of motor hyperactivity is a specific effect, rather than secondary to motor impairment. In the sensorimotor test, however, (−)-OSU6162 failed to prevent the PPI deficits induced by MK-801. Clozapine, a positive control for this model, reversed MK-801 effects, as expected.
Our results in animal models predictive of motor side effects reproduce data from other studies (Natesan et al. 2006; Rung et al. 2008; Studer et al. 2016). (−)-OSU6162 has a favorable safety profile, as it did not impair locomotion in the open field and did not induce catalepsy, even at the highest doses. The catalepsy test has the advantages of being a simple, quick, and reliable assay, with excellent face, construct and predictive validities (Gobira et al. 2013). The pharmacological profile of (−)-OSU6162 in this test is consistent with those observed with SGAs (“atypical antipsychotics”) and TGAs, although the notion that antipsychotics can be divided in specific categories has been questioned (Leucht et al. 2013; Leucht et al. 2009). In any case, this favorable profile is particularly relevant considering that several antipsychotics may induce Parkinson-like symptoms, chorea and akathisia, which are debilitating side effects that interfere with treatment adherence (Pierre 2005).
Next, we found that (−)-OSU6162 prevents cocaine-induced hyperlocomotion. This model is based on the classical dopaminergic theory of schizophrenia, which collects pharmacological, behavioral, and neurochemical observations to suggest that schizophrenia abnormalities, particularly the positive symptoms, may result from excessive dopamine synthesis and release in the mesolimbic pathway, connecting the ventral tegmental area to the nucleus accumbens in the ventral striatum (Carlsson et al. 2004; Iversen and Iversen 2007; Kapur et al. 2005; Snyder 1972). Thus, several drugs that increase dopamine release (amphetamines) or reduce its uptake (cocaine) precipitate psychotic features in humans (Snyder 1972). In experimental animals (rats and mice), their effects consist of hyperlocomotion, which can be prevented by antipsychotics drugs (Gobira et al. 2013). An obvious limitation of this model is its predictive value for compounds that interfere with locomotor activity, yielding false positive or false negative results (Gobira et al. 2013). In the present case, there seems to be a specific effect of (−)-OSU6162, since the previous experiment showed no impairment of basal locomotion at the same doses that prevent cocaine effect. This discards any potential confounding factor in the present study.
The fact that (−)-OSU6162 prevents cocaine effects might be considered tautological, since both substances act on dopaminergic neurotransmission. Thus, we also showed that (−)-OSU6162 exerts antipsychotic-like effect in an animal model based on a different construct, the hyperlocomotion induced by the NMDA channel antagonist, ketamine. Glutamate NMDA channel hypofunction has been an alternative to the dopaminergic hypothesis of schizophrenia. In humans, NMDA channels blockers, such as ketamine and MK-801, induced psychotic features, including delusions and hallucinations, that may mimic schizophrenia symptoms more reliably than dopaminergic drugs (Carlsson et al. 2004). In addition, schizophrenia patients have misbalanced glutamatergic neurotransmission (Howes et al. 2015). Finally, these compounds induce hyperlocomotion in experimental animals, which can be prevented by antipsychotic drugs (Gobira et al. 2013). Thus, the efficacy of (−)-OSU6162 in this models reinforces the results from our previous experiments, further suggesting its efficacy as an antipsychotic compound. The reasons why different doses are required to prevent ketamine- and cocaine-induced hyperlocomotion remain unclear, but this has been commonly observed in preclinical models of antipsychotic activity (Leite et al. 2008; Moreira and Guimaraes 2005).
Considering the limitations of animal models based on motor activity, we also tested (−)-OSU6162 in PPI, a sensorimotor gating response that is preserved across species (Fendt and Koch 2013; Swerdlow et al. 2016). Moreover, PPI deficit is well described in patients diagnosed with schizophrenia and in healthy subjects or experimental animals treated with psychotomimetic drugs (Swerdlow et al. 2016). Considering the face and construct validities of this model, we hypothesized that (−)-OSU6162 prevents deficits in PPI induced by the NMDA channel blocker, MK-801. However, no effect was observed at any dose or prepulse intensity. Although the reason for this failure is unclear, it is unlikely to result from any malfunctioning of equipment or improper experimental setting, since clozapine exerted its expected effect as a positive control. Thus, it may indicate a limited efficacy of (−)-OSU6162 against sensorimotor gating deficits observed in patients with schizophrenia. We cannot contrast our findings with the literature since, to the best of our knowledge, this is the first study investigating this potential antipsychotic drug in PPI. Compounds whose mechanisms of action are apparently close to (−)-OSU6162 (“dopaminergic modulators”) include the partial agonists at the D2 receptor, such as aripiprazole, which are proposed as TGAs. However, contrary to our findings with (−)-OSU6162, aripiprazole does prevent PPI deficits induced by dopaminergic drugs as well as NMDA blockers (Auclair et al. 2006; Fejgin et al. 2007; Ishii et al. 2010; Nordquist et al. 2008). This discrepancy reinforces the proposal that (−)-OSU6162 stabilizes dopaminergic neurotransmission through mechanisms other than partial agonism (Rung et al. 2008).
Although (−)-OSU6162 failed in an animal model related to sensorimotor gating deficits in schizophrenia, the present study supports the notion that this dopaminergic stabilizer might be effective and safe to ameliorate at least some symptoms of schizophrenia. There has been a growing interest in partial agonists and “dopamine stabilizers” as alternative mechanisms to dopamine antagonism, particularly to avoid Parkinsonism and related side effect. Contrary to most antipsychotics, (−)-OSU6162 may even be useful in the treatment of neurological motor dysfunctions, including parkinsonism, chorea, and dyskinesia (Kloberg et al. 2014; Nichols et al. 2002; Tedroff et al. 1999). Thus, additional studies should evaluate the efficacy of this compound in other neuropsychiatric disorders. Moreover, since a limitation of the present study is the use of acute injection protocols, its potential therapeutic and side effects should also be evaluated after chronic treatments in different species, in which the proper dose ranges should be established. Finally, there has been very few studies in humans, which should be considered taking into account the safety of (−)-OSU6162. A case report in a patient with with Huntington’ disease and a double-blind cross-over study reported the safety and efficacy of this compound (Kloberg et al. 2014; Tedroff et al. 1999). There is also a study showing the good safety and tolerability in alcohol-dependent individuals, in which there was also a reduction in craving for this drug (Khemiri et al. 2015).
In conclusion, the monoamine stabilizer (−)-OSU6162 exerted antipsychotic-like activity in locomotor, but not sensorimotor, responses in experimental animals. This compound prevented hyperlocomotion in both dopaminergic and glutamatergic models, at doses that did not impair motor functions. (−)-OSU6162 should be further investigated as potential TGA, particularly for ameliorating agitation, hyperactivity, and related schizophrenia symptoms.
References
Auclair AL, Kleven MS, Besnard J, Depoortere R, Newman-Tancredi A (2006) Actions of novel antipsychotic agents on apomorphine-induced PPI disruption: influence of combined serotonin 5-HT1A receptor activation and dopamine D2 receptor blockade. Neuropsychopharmacology 31:1900–1909. https://doi.org/10.1038/sj.npp.1301015
Burstein ES, Carlsson ML, Owens M, Ma JN, Schiffer HH, Carlsson A, Hacksell U (2011) II. In vitro evidence that (−)-OSU6162 and (+)-OSU6162 produce their behavioral effects through 5-HT2A serotonin and D2 dopamine receptors. J Neural Transm (Vienna) 118:1523–1533. https://doi.org/10.1007/s00702-011-0701-y
Carlsson ML, Carlsson A, Nilsson M (2004) Schizophrenia: from dopamine to glutamate and back. Curr Med Chem 11:267–277
Carlsson ML, Burstein ES, Kloberg A, Hansson S, Schedwin A, Nilsson M, Rung JP, Carlsson A (2011) I. In vivo evidence for partial agonist effects of (−)-OSU6162 and (+)-OSU6162 on 5-HT2A serotonin receptors. J Neural Transm (Vienna) 118:1511–1522. https://doi.org/10.1007/s00702-011-0704-8
Demjaha A, Lappin JM, Stahl D, Patel MX, MacCabe JH, Howes OD, Heslin M, Reininghaus UA, Donoghue K, Lomas B, Charalambides M, Onyejiaka A, Fearon P, Jones P, Doody G, Morgan C, Dazzan P, Murray RM (2017) Antipsychotic treatment resistance in first-episode psychosis: prevalence, subtypes and predictors. Psychol Med 47:1981–1989. https://doi.org/10.1017/S0033291717000435
Fejgin K, Safonov S, Palsson E, Wass C, Engel JA, Svensson L, Klamer D (2007) The atypical antipsychotic, aripiprazole, blocks phencyclidine-induced disruption of prepulse inhibition in mice. Psychopharmacology 191:377–385. https://doi.org/10.1007/s00213-006-0658-y
Fendt M, Koch M (2013) Translational value of startle modulations. Cell Tissue Res 354:287–295. https://doi.org/10.1007/s00441-013-1599-5
Gardner DM, Baldessarini RJ, Waraich P (2005) Modern antipsychotic drugs: a critical overview. CMAJ 172:1703–1711. https://doi.org/10.1503/cmaj.1041064
Gobira PH, Ropke J, Aguiar DC, Crippa JA, Moreira FA (2013) Animal models for predicting the efficacy and side effects of antipsychotic drugs. Rev Bras Psiquiatr 35(Suppl 2):S132–S139. https://doi.org/10.1590/1516-4446-2013-1164
Haddad PM, Das A, Keyhani S, Chaudhry IB (2012) Antipsychotic drugs and extrapyramidal side effects in first episode psychosis: a systematic review of head-head comparisons. J Psychopharmacol 26:15–26. https://doi.org/10.1177/0269881111424929
Hartling L, Abou-Setta AM, Dursun S, Mousavi SS, Pasichnyk D, Newton AS (2012) Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med 157:498–511. https://doi.org/10.7326/0003-4819-157-7-201210020-00525
Howes O, McCutcheon R, Stone J (2015) Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 29:97–115. https://doi.org/10.1177/0269881114563634
Howes OD, McCutcheon R, Agid O, de Bartolomeis A, van Beveren NJM, Birnbaum ML, Bloomfield MAP, Bressan RA, Buchanan RW, Carpenter WT, Castle DJ, Citrome L, Daskalakis ZJ, Davidson M, Drake RJ, Dursun S, Ebdrup BH, Elkis H, Falkai P, Fleischacker WW, Gadelha A, Gaughran F, Glenthøj BY, Graff-Guerrero A, Hallak JEC, Honer WG, Kennedy J, Kinon BJ, Lawrie SM, Lee J, Leweke FM, MacCabe JH, McNabb CB, Meltzer H, Möller HJ, Nakajima S, Pantelis C, Reis Marques T, Remington G, Rossell SL, Russell BR, Siu CO, Suzuki T, Sommer IE, Taylor D, Thomas N, Üçok A, Umbricht D, Walters JTR, Kane J, Correll CU (2017) Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry 174:216–229. https://doi.org/10.1176/appi.ajp.2016.16050503
Iversen SD, Iversen LL (2007) Dopamine: 50 years in perspective. Trends Neurosci 30:188–193. https://doi.org/10.1016/j.tins.2007.03.002
Ishii D, Matsuzawa D, Kanahara N, Matsuda S, Sutoh C, Ohtsuka H, Nakazawa K, Kohno M, Hashimoto K, Iyo M, Shimizu E (2010) Effects of aripiprazole on MK-801-induced prepulse inhibition deficits and mitogen-activated protein kinase signal transduction pathway. Neurosci Lett 471:53–57. https://doi.org/10.1016/j.neulet.2010.01.010
Issy AC, Salum C, Del Bel EA (2009) Nitric oxide modulation of methylphenidate-induced disruption of prepulse inhibition in Swiss mice. Behav Brain Res 205:475–481. https://doi.org/10.1016/j.bbr.2009.08.003
Kapur S (2004) How antipsychotics become anti-"psychotic"--from dopamine to salience to psychosis. Trends Pharmacol Sci 25:402–406. https://doi.org/10.1016/j.tips.2004.06.005
Kapur S, Mizrahi R, Li M (2005) From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr Res 79:59–68. https://doi.org/10.1016/j.schres.2005.01.003
Kara E, Lin H, Svensson K, Johansson AM, Strange PG (2010) Analysis of the actions of the novel dopamine receptor-directed compounds (S)-OSU6162 and ACR16 at the D2 dopamine receptor. Br J Pharmacol 161:1343–1350. https://doi.org/10.1111/j.1476-5381.2010.01010.x
Khemiri L, Steensland P, Guterstam J, Beck O, Carlsson A, Franck J, Jayaram-Lindstrom N (2015) The effects of the monoamine stabilizer (−)-OSU6162 on craving in alcohol dependent individuals: a human laboratory study. Eur Neuropsychopharmacol 25:2240–2251. https://doi.org/10.1016/j.euroneuro.2015.09.018
Kloberg A, Constantinescu R, Nilsson MK, Carlsson ML, Carlsson A, Wahlstrom J, Haghighi S (2014) Tolerability and efficacy of the monoaminergic stabilizer (−)-OSU6162 (PNU-96391A) in Huntington's disease: a double-blind cross-over study. Acta Neuropsychiatr 26:298–306. https://doi.org/10.1017/neu.2014.16
Lin H, Saisch SG, Strange PG (2006) Assays for enhanced activity of low efficacy partial agonists at the D(2) dopamine receptor. Br J Pharmacol 149:291–299. https://doi.org/10.1038/sj.bjp.0706866
Laruelle M (2014) Schizophrenia: from dopaminergic to glutamatergic interventions. Curr Opin Pharmacol 14:97–102. https://doi.org/10.1016/j.coph.2014.01.001
Laruelle M, Kegeles LS, Abi-Dargham A (2003) Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci 1003:138–158
Leite JV, Guimaraes FS, Moreira FA (2008) Aripiprazole, an atypical antipsychotic, prevents the motor hyperactivity induced by psychotomimetics and psychostimulants in mice. Eur J Pharmacol 578:222–227. https://doi.org/10.1016/j.ejphar.2007.09.016
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (2009) Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373:31–41. https://doi.org/10.1016/S0140-6736(08)61764-X
Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. https://doi.org/10.1016/S0140-6736(13)60733-3
Lieberman JA, Stroup TS, McEvoy J, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. https://doi.org/10.1056/NEJMoa051688
Mailman RB, Murthy V (2010) Third generation antipsychotic drugs: partial agonism or receptor functional selectivity? Curr Pharm Des 16:488–501
Millan MJ, Goodwin GM, Meyer-Lindenberg A, Ove Ogren S (2015) Learning from the past and looking to the future: emerging perspectives for improving the treatment of psychiatric disorders. Eur Neuropsychopharmacol 25:599–656. https://doi.org/10.1016/j.euroneuro.2015.01.016
Moghaddam B, Javitt D (2012) From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37:4–15. https://doi.org/10.1038/npp.2011.181
Moreira FA, Guimaraes FS (2005) Cannabidiol inhibits the hyperlocomotion induced by psychotomimetic drugs in mice. Eur J Pharmacol 512:199–205. https://doi.org/10.1016/j.ejphar.2005.02.040
Natesan S, Svensson KA, Reckless GE, Nobrega JN, Barlow KB, Johansson AM, Kapur S (2006) The dopamine stabilizers (S)-(−)-(3-methanesulfonyl-phenyl)-1-propyl-piperidine [(−)-OSU6162] and 4-(3-methanesulfonylphenyl)-1-propyl-piperidine (ACR16) show high in vivo D2 receptor occupancy, antipsychotic-like efficacy, and low potential for motor side effects in the rat. J Pharmacol Exp Ther 318:810–818. https://doi.org/10.1124/jpet.106.102905
Nichols NF, Cimini MG, Haas JV, Staton BA, Tedroff J, Svensson KA (2002) PNU-96391A (OSU6162) antagonizes the development of behavioral sensitization induced by dopamine agonists in a rat model for Parkinson's disease. Neuropharmacology 43:817–824
Nordquist RE, Risterucci C, Moreau JL, von Kienlin M, Künnecke B, Maco M, Freichel C, Riemer C, Spooren W (2008) Effects of aripiprazole/OPC-14597 on motor activity, pharmacological models of psychosis, and brain activity in rats. Neuropharmacology 54:405–416. https://doi.org/10.1016/j.neuropharm.2007.10.010
Ohlsen RI, Pilowsky LS (2005) The place of partial agonism in psychiatry: recent developments. J Psychopharmacol 19:408–413. https://doi.org/10.1177/0269881105053308
van Os J, Kapur S (2009) Schizophrenia. Lancet 374:635–645. https://doi.org/10.1016/S0140-6736(09)60995-8
Pierre JM (2005) Extrapyramidal symptoms with atypical antipsychotics : incidence, prevention and management. Drug Saf 28:191–208
Rung JP, Carlsson A, Markinhuhta KR, Carlsson ML (2005) The dopaminergic stabilizers (−)-OSU6162 and ACR16 reverse (+)-MK-801-induced social withdrawal in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 29:833–839. https://doi.org/10.1016/j.pnpbp.2005.03.003
Rung JP, Rung E, Helgeson L, Johansson AM, Svensson K, Carlsson A, Carlsson ML (2008) Effects of (−)-OSU6162 and ACR16 on motor activity in rats, indicating a unique mechanism of dopaminergic stabilization. J Neural Transm (Vienna) 115:899–908. https://doi.org/10.1007/s00702-008-0038-3
Sahlholm K, Arhem P, Fuxe K, Marcellino D (2013) The dopamine stabilizers ACR16 and (−)-OSU6162 display nanomolar affinities at the sigma-1 receptor. Mol Psychiatry 18:12–14. https://doi.org/10.1038/mp.2012.3
Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988) The catalepsy test: its ups and downs. Behav Neurosci 102:748–759
Seeman P, Guan HC (2007) Dopamine partial agonist action of (−)OSU6162 is consistent with dopamine hyperactivity in psychosis. Eur J Pharmacol 557:151–153. https://doi.org/10.1016/j.ejphar.2006.11.016
Snyder SH (1972) Catecholamines in the brain as mediators of amphetamine psychosis. Arch Gen Psychiatry 27:169–179
Sonesson C, Lin CH, Hansson L, Waters N, Svensson K, Carlsson A, Smith MW, Wikstroem H (1994) Substituted (S)-phenylpiperidines and rigid congeners as preferential dopamine autoreceptor antagonists: synthesis and structure-activity relationships. J Med Chem 37:2735–2753
Studer E, Naslund J, Westman A, Carlsson A, Eriksson E (2016) The effects of the dopamine stabilizer (−)-OSU6162 on aggressive and sexual behavior in rodents. Transl Psychiatry 6:e762. https://doi.org/10.1038/tp.2016.12
Swerdlow NR, Braff DL, Geyer MA (2016) Sensorimotor gating of the startle reflex: what we said 25 years ago, what has happened since then, and what comes next. J Psychopharmacol 30:1072–1081. https://doi.org/10.1177/0269881116661075
Tamminga CA, Carlsson A (2002) Partial dopamine agonists and dopaminergic stabilizers, in the treatment of psychosis. Curr Drug Targets CNS Neurol Disord 1:141–147
Tedroff J, Ekesbo A, Sonesson C, Waters N, Carlsson A (1999) Long-lasting improvement following (−)-OSU6162 in a patient with Huntington's disease. Neurology 53:1605–1606
Viana TG, Almeida-Santos AF, Aguiar DC, Moreira FA (2013) Effects of aripiprazole, an atypical antipsychotic, on the motor alterations induced by acute ethanol administration in mice. Basic Clin Pharmacol Toxicol 112:319–324. https://doi.org/10.1111/bcpt.12036
Acknowledgements
The authors received funding from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG): Processes APQ-02064-15 (FAM) and APQ-02044-15 (ACPO) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): Processes 406122/2016-4 (FAM) and 424588/2016-1 (ACPO). FAM and ACPO are recipients of research fellowships (level 2) from CNPq.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures used in this study followed the ethical principles of animal experimentation adopted by the Ethic Committee on Animal Use of Federal University of Minas Gerais (CEUA–UFMG), and institutionally approved under protocol number 227/2013.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Antônio C. Pinheiro de Oliveira and Fabrício A. Moreira shared senior authorship
Rights and permissions
About this article
Cite this article
da Silveira, V.T., Röpke, J., Matosinhos, A.L. et al. Effects of the monoamine stabilizer (−)-OSU6162 on locomotor and sensorimotor responses predictive of antipsychotic activity. Naunyn-Schmiedeberg's Arch Pharmacol 391, 761–768 (2018). https://doi.org/10.1007/s00210-018-1500-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1500-x