Abstract
Rationale
Emerging data support a role for GABAB receptors in anxiety. GABAB receptors are comprised of a heterodimeric complex of GABAB1 and GABAB2 receptor subunits. The predominant neuronal GABAB1 receptor isoforms are GABAB(1a) and GABAB(1b). Recent findings indicate specific roles for these isoforms in conditioned fear responses, although their influence on behavior in tests of unconditioned anxiety is unknown.
Objective
The aim of this study was to examine the role of the GABAB(1) isoforms in unconditioned anxiety.
Materials and methods
Mice deficient in the GABAB(1a) or GABAB(1b) receptor isoforms were examined in a battery of anxiety tests.
Results
In most tests, genotype did not significantly affect anxious behavior, including the elevated plus maze, marble burying, and stress-induced hypothermia tests. Corticosterone and adrenocorticotropic hormone levels were similarly unaffected by genotype. Female, but not male, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice showed increased anxiety relative to wild-type controls in the elevated zero maze. In the staircase test, male \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice defecated more than male \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice, although no other test parameter was influenced by genotype. In the light–dark box, female \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice spent less time in the light compartment compared to the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) females, whereas male \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice made fewer light–dark transitions than \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) males.
Conclusions
Specific roles for either GABAB(1) isoform in unconditioned anxiety were not explicit. This differs from their contribution in conditioned anxiety and from the anxious phenotype of GABAB1 and GABAB2 subunit knockout mice. The findings suggest that the GABAB(1) isoforms have specific relevance for anxiety with a cognitive component, rather than for innate anxiety per se.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
G-protein coupled γ-aminobutyric acid (GABA) receptors, GABAB receptors, are heterodimers comprised of GABAB(1) and GABAB(2) subunits. They are expressed both as presynaptic heteroreceptors and also postsynaptically, where they respectively modulate neuronal excitability. Heteroreceptors modulate the release of (excitatory) neurotransmitters, mainly via actions on presynaptic Ca+2 channels, and postsynaptic GABAB receptors activate slow inhibitory postsynaptic potentials via the activation of inwardly rectifying K+ channels. GABAB receptors also function as autoreceptors on interneurons. Additionally, GABAB receptors are negatively coupled to adenylyl cyclase, through which they influence downstream molecular pathways (Bettler et al. 2004; Couve et al. 2000).
There is a growing body of evidence indicating that GABAB receptors play a critical role in anxiety (Cryan and Kaupmann 2005; Pilc and Nowak 2005). The classic GABAB receptor agonist, baclofen, has shown anxiolytic activity in some clinical settings. Baclofen reduced anxiety in posttraumatic stress disorder patients (Drake et al. 2003), in alcoholics after alcohol withdrawal (Addolorato et al. 2002, 2006; Ameisen 2005; Flannery et al. 2004), in panic disorder (Breslow et al. 1989), and in patients suffering from acute spinal trauma (Hinderer 1990). Baclofen has also demonstrated anxiolytic effects in several preclinical studies including ultrasonic vocalization in rat pups (Nastiti et al. 1991), punished drinking (Shephard et al. 1992), elevated plus maze (Andrews and File 1993; but see Dalvi and Rodgers 1996), and in the social interaction and elevated plus maze tests after withdrawal of dependent rats from either diazepam or alcohol (File et al. 1991a,b, 1992). GABAB receptor-positive modulators such as GS39783 also demonstrate an anxiolytic profile in both rats and mice in a wide range of tests (Cryan et al. 2004).
Perhaps the strongest preclinical evidence to date for a role of GABAB receptors in anxiety was demonstrated by the phenotype of GABAB receptor-deficient mice. Deletion of either the GABAB(1) or GABAB(2) receptor subunits results in a complete loss of typical GABAB functions and induces a highly anxious phenotype in mice in exploratory-based tests of anxiety (Mombereau et al. 2004a,b).
The GABAB(1) subunit is predominantly expressed as one of two isoforms: GABAB(1a) or GABAB(1b), both of which are transcribed from different promoters in the Gabbr1 gene (Steiger et al. 2004) and heterodimerize with the GABAB(2) subunit to form functional receptors (Bettler et al. 2004). The primary sequence of the GABAB(1a) isoform differs from that of the GABAB(1b) isoform by the inclusion of short consensus repeats (or “sushi domains”) at the N terminus (Blein et al. 2004). Currently, there are no pharmacological tools available to dissect the physiological roles of these isoforms. Recently, however, the generation of mice deficient in either the GABAB(1a) or GABAB(1b) isoforms has facilitated the demonstration of specific and differential roles for the GABAB(1) isoforms. The two isoforms localize differentially: the GABAB(1a) isoform was predominantly expressed as a presynaptic heteroreceptor in the hippocampus (Vigot et al. 2006) and lateral amygdala (Shaban et al. 2006). In contrast, the GABAB(1b) isoform was predominantly located postsynaptically in these structures (Shaban et al. 2006; Vigot et al. 2006). Both the GABAB(1a) and GABAB(1b) isoforms also fulfilled autoreceptor functions in the hippocampus and amgydala (Shaban et al. 2006; Vigot et al. 2006), although in the apical dendrites of layer 5 cortical neurons, the GABAB(1a) isoform was shown to be the predominant autoreceptor (Perez-Garci et al. 2006). The GABAB(1) isoforms also have differential impacts on neurophysiological processes, such as LTP in the hippocampus (Vigot et al. 2006) and amygdala (Shaban et al. 2006). Finally, deletion of either isoform demonstrates a divergence of behavioral phenotypes in conditioned aversive learning and memory paradigms (Jacobson et al. 2006b; Shaban et al. 2006). Specifically, in a conditioned fear paradigm \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice showed generalized freezing to both paired and unpaired tones (Shaban et al. 2006). In a conditioned taste aversion paradigm (CTA), \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice failed to acquire an aversion to a saccharin solution when paired with a lithium chloride-induced malaise, while \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice acquired CTA, but demonstrated a profound failure in the extinction of this aversion (Jacobson et al. 2006b).
It is, therefore, well-established that GABAB receptors play a role in anxiety and that the GABAB(1) subunit isoforms are functionally relevant molecular variants of the GABAB receptor, which convey differential behavioral responses in tests of aversive learning and memory. However, the influence of the two GABAB(1) isoforms on behavior in tests of unconditioned anxiety is currently unknown. The aim of the present study, therefore, was to determine the influence of the different GABAB(1) subunit isoforms on innate anxiety-related behaviors, using mice deficient in either the GABAB(1a) or GABAB(1b) isoforms.
Materials and methods
Animals
The generation of wild-type, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \), and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice as used in the present studies has been described previously (Vigot et al. 2006). Briefly, a knock-in point mutation strategy was adopted, whereby GABAB(1a) and GABAB(1b) initiation codons were converted to stop codons by targeted insertion of a floxed neo-cassette. All mutant and wild-type mice were maintained on a pure inbred BALB/c genetic background. \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice used in the present experiments were derived from homozygous breeding of siblings originating from the founding heterozygotic mice (F3–4). Homozygous wild-type controls for the GABAB(1) isoform mutant mice were derived from mating together wild-type siblings generated from \( {\text{GABA}}^{{ + \mathord{\left/ {\vphantom { + - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and \( {\text{GABA}}^{{ + \mathord{\left/ {\vphantom { + - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) heterozygous breedings (F3–4). The breeding strategy was applied in accordance with the recommendations proposed by The Jackson Laboratory to obviate genetic drift and the formation of substrains (http://jaxmice.jax.org/geneticquality/guidelines.html).
Male and female mice were investigated in separate experiments for all tests performed, with the exception of the determination of corticosterone and adrenocorticotropic hormone (ACTH) levels, in which only male mice were used. All mice were singly housed after achieving at least 8 weeks of age, with the exception of those used in the marble-burying test, that were housed in groups of two to four mice per cage. Mice were housed in macrolon cages with sawdust bedding, tissue paper nesting materials and one red, triangular, polycarbonate Mouse House® (Nalgene) per cage. Housing was at a constant room temperature of 22–24°C in a 12-h light:dark cycle with lights on at 6:00–6:30 a.m. Food pellets and tap water were available ad libitum (except during experimentation). Mice were allowed to settle for a minimum of 1 week after single housing before testing began. All mice were 8 weeks of age or older at testing. All mice were drug-naïve, and mice used in the stress-induced hyperthermia (SIH), light–dark box, marble-burying tests, and in the corticosterone and ACTH assessment were experimentally naïve. Mice used in the staircase test were the same as those from the SIH test, with an interval of approximately 3 and 5 weeks between tests for the male and female mice, respectively. Mice used in the marble-burying test were singly house immediately after the test and subsequently tested in the elevated plus maze after an interval of approximately 5 weeks and 1 week for male and female mice, respectively. Mice tested with the elevated zero maze had been previously exposed to a 5-min handling period approximately 1 week before testing, during which basic sensory and sensorimotor characteristics of the mutant mice and wild-type controls were examined (Jacobson et al. 2006a). All animal experiments were conducted during the light phase. All animal experiments were conducted in accordance with Swiss ethics guidelines and approved by the Veterinary Authority of Basel Stadt, Switzerland.
Stress-induced hyperthermia
The SIH test is an ideal inclusion in an anxiety test battery as it is not unduly influenced by alterations in locomotor activity and is a translational model across strains and species (including mice and humans; see Bouwknecht et al. 2006). The test procedure for SIH was adapted from that reported by Van der Heyden et al. (1997) and was conducted essentially as described by Cryan et al. (2003), with the exception that mice in the present study were already singly housed before the time of SIH testing. Two SIH experiments were conducted, one with male and one with female mice. Age-matched (within gender), experimentally naive male and female wild-type, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \), and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (mean age: 10 ± 0.4 weeks for males and 13 ± 0.3 weeks for females, n = 10 per sex and genotype) were rehoused in new cages overnight in the testing room. They had free access to water and food, but were without nesting materials or Mouse Houses®. The following day, rectal temperature was measured twice in each mouse i.e., at t = 0 min (T 1) and t = +15 min (T 2). The first measurement of temperature serves as the stressor and results in a rapid hyperthermic response. The difference in temperature (T 2 − T 1) was considered to reflect the SIH. Time points were based on previous experiments that showed that a T 2 − T 1 interval of 15 min was optimal in terms of SIH (Spooren et al. 2002). Rectal temperature was measured to the nearest 0.1°C by an ELLAB instruments thermometer (Copenhagen, Denmark Model DM 852), by inserting a lubricated thermistor probe model PRA-22002-A (ELLAB), 2.2 mm in diameter, 20 mm into the rectum. The mouse was handheld at the base of the tail during this determination and the thermistor probe was left in place for 15 s.
Staircase test
The staircase test in mice (Simiand et al. 1984) allows the distinction between locomotor vs anxiolytic responses by comparing the ratio of steps climbed to rearings performed. Anxiolysis is thus interpreted when reductions in rearing are accompanied by an unchanged, or an increased, number of steps climbed. The test shows pharmacological selectivity for anxiolytics and, indeed, locomotor stimulants tend to decrease both parameters in the test (Simiand et al. 1984). Additionally, the number of fecal boli and urination spots made by the mice are easily quantifiable in this test and provide a further indicator of stress responses (Cryan et al. 2003; Gray and Lalljee 1974).
The test was carried out essentially as previously described (Cryan et al. 2003; Simiand et al. 1984). The apparatus comprised an enclosed staircase with five steps made of gray plastic. Each step was 2.5 cm in height, 7.5 cm in length, and 11 cm in width, such that the staircase rose to a height of 12.5 cm at the top step. The apparatus was 45 cm in length, and surrounded by walls 12.5 cm height at one end and 25 cm at the other. Mutant and wild-type mice utilized in the staircase test in the present study were the same animals as those for the SIH test, with the exception that of the wild-type female mice, only eight rather than ten mice were used. Experiments with male and female mice were conducted separately. Animals were moved in their home cages to the testing room at least 1 h before testing started. Mice were placed on the bottom level facing away from the stairs. The number of steps ascended and rearings made in a 3-min period were observed. The apparatus was wiped with a wet paper towel and dried between animals.
Light–dark box
The light–dark box test is a conflict-based anxiety test whereby the tendency to explore a novel environment contrasts against the natural aversion of mice to brightly lit spaces (Crawley 2000). The test was included in the present study as the most reliable indictors of anxiety, such as light–dark transitions and time spent in the light (in that order; see Crawley and Davis 1982; Holmes et al. 2002), are expressed as passive avoidance behaviors, thus increasing the range of behaviors used in the present study to evaluate anxiety. The test was carried out similarly to that previously described (Cryan et al. 2003; Holmes et al. 2002; Mombereau et al. 2004a). The apparatus consisted of a Plexiglas enclosure (44 × 21 × 21 cm) divided into two compartments (one light and one dark) by a partition in which there was a small opening (12 × 5 cm) at the floor level. The light compartment was open-roofed, with walls of transparent Plexiglas and was brightly illuminated by a 60-W desk lamp overhead (approximately 1,000 lx). The smaller, dark compartment (14 cm width) was closed-roofed and was constructed of black Plexiglas. Male and female mice were investigated in separate experiments. Age-matched, experimentally naive wild-type, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \), and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (mean age: 12 ± 0.4 weeks, n = 10–11 per sex and genotype) were individually placed in the center of the light compartment, facing away from the partition and allowed to explore the apparatus freely for 10 min. The apparatus was cleaned thoroughly between subjects. The number of light–dark transitions, time spent in the light compartment, and latency to enter the dark were recorded by a trained observer.
Marble-burying test
The marble-burying test was similar to that previously described (Broekkamp et al. 1986; Spooren et al. 2000). This test was included, as animals that are more anxious must engage in active behaviors (defensive marble burying) as opposed to passive behaviors utilized to avoid anxiogenic stimuli in the light–dark box and elevated mazes. Male and female mice were investigated in separate experiments. Mice were group-housed, age-matched (within gender), experimentally naive male and female wild-type, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \), and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (males: mean age 15.2 ± 0.4 weeks, wild-type n = 14, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) n = 19, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) n = 26; females: mean age 20.6 ± 0.6, wild-type n = 23, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) n = 16, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) n = 35). Mice were placed individually in small cages (26 × 20 × 14 cm), in which ten marbles had been equally distributed on top of a 5-cm deep bed of sawdust, and a wire lid was placed on top of the cage. Mice were left undisturbed for 30 min, after which the number of buried marbles (i.e., those covered by sawdust three-quarters or more) was counted.
Elevated plus maze
The elevated plus maze is a widely used test for assessing anxiety behavior in mice (Crawley 2000; Holmes 2001; Rodgers 1997). Similar to the light–dark box, the elevated plus maze is based on the conflict between exploratory drive and avoidance of the innately aversive open arms and in the expression of passive behaviors that are employed to avoid the more anxiogenic areas of the mazes. However, the tests differ both in the nature of the anxiogenic stimuli (heights vs a brightly lit space) and in the starting place of the mice (choice point vs maximally anxiogenic area; Crawley 2000; Rodgers 1997). As such, results between the two tests can be expected to vary; therefore, the inclusion of both tests in the present study provides a more comprehensive test battery. Separate experiments were conducted with male and female mice. Mice were the same animals as those utilized in the marble-burying test, with the exception that of the female \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice, only 34, rather than 35, mice were used. The elevated plus maze was carried out as described previously (Cryan et al. 2003; Rodgers et al. 1997). The apparatus comprised two open arms (30 × 5 cm) and two enclosed arms (30 × 5 × 15 cm), which extended from a common central platform (5 × 5 cm). The configuration formed the shape of a plus sign, with like arms arranged opposite one another, and the apparatus was elevated 60 cm above floor level on a central pedestal. The maze floor was made of black Plexiglas, while the side and end walls of the enclosed arms were made from clear Plexiglas. Grip on the edges of open arms was facilitated by inclusion of a small raised edge (0.25 cm) around their perimeter. Animals were transported from the holding room to the laboratory at least 1 h before testing. Mice were placed onto the central platform facing an enclosed arm. A 6-min trial was performed and, between subjects, the maze was thoroughly cleaned. Direct registrations were made by an observer sitting close to the maze using the following conventional parameters: number of open and closed arm entries (arm entry defined as all four paws entering an arm) and time spent on open arms (excluding the central platform).
Elevated zero maze
The elevated zero maze is an ideal complementary test to the elevated plus maze. It eliminates the central area of the plus maze and provides a continuous circular track to facilitate exploration, while maintaining end point parameters similar in construction to that of the elevated plus maze (Lee and Rodgers 1990; Shepherd et al. 1994). Furthermore, we have previously shown that mice lacking the GABAB(1) subunit jumped off the maze in a panic-like response (Mombereau et al. 2004a). It was therefore of interest to assess the influence of GABAB(1) receptor isoform deletion on mice in this test. The elevated zero maze test was conduced as previously described (Cryan et al. 2004). Male and female mice were investigated in separate experiments. Age-matched (within gender), experimentally naive male and female wild-type, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \), and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (mean age: 21.2 ± 1.4 weeks for males and 23.8 ± 0.8 weeks for females, n = 11–12 for each sex and genotype) were used. Mice had been individually handled for about 5 min each 1 week before testing in the elevated zero maze. The apparatus was a 5.5-cm wide circular track constructed of gray Plexiglas with an inside diameter of 34 cm, a midtrack circumference of approximately 121 cm, and was elevated 40 cm above the floor. It consisted of two open quadrants with a raised, 2-mm edge and two closed quadrants with walls 11 cm high. Mice were placed in one of the closed quadrants designated as the starting quadrant and were allowed to investigate the zero maze for a period of 5 min. During this time, an observer scored mice on several anxiety-related variables as identified in previous studies (Cryan et al. 2004; Shepherd et al. 1994; Tarantino et al. 2000). These included time spent in both open and closed quadrants, number of transitions between quadrants, latency to leave the closed quadrant, stretch-attend postures (SAP, elongated body posture with at least snout over open/closed divide) into the open quadrant, rearing, and head dips over the sides of the open quadrants.
Corticosterone and ACTH
To investigate the effects of deletion of the GABAB(1a) or GABAB(1b) isoforms on hypothalamic–pituitary–adrenal (HPA) axis activity, corticosterone and adrenocortico tropic hormone (ACTH) levels at 0700–0800 hours were measured in trunk blood of male, experimentally naïve wild-type (n = 9), \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) (n = 9), and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) (n = 6) mice [mean age (±SEM) 15.6 ± 0.6 weeks]. Home cages for each individual mouse were removed from the housing room one at a time to another room where mice were decapitated immediately on arrival and trunk blood collected into EDTA-treated 1.5-ml tubes (Milian S.A., Meyrin, Switzerland). Blood samples were kept on ice for up to about 20 min until centrifugation at 10,000 rpm for 15 min in a refrigerated centrifuge (4°C). The plasma fraction was collected and stored at −80°C until subsequent analysis for corticosterone and ACTH. Plasma corticosterone and ACTH concentrations were measured using commercially available radioimmunoassay kits (ICN Biomedicals, Costa Mesa, CA, USA).
Statistical analyses
As studies which were carried out in both male and female mice were conducted in independent experiments, data for each sex were analyzed separately. Corticosterone levels the number of steps taken in the staircase test and head-dips and SAPs in the elevated zero maze were analyzed using one-way analysis of variance (ANOVA). Parameters measured within the SIH, other staircase test parameters, light–dark box, elevated plus maze, marble burying, the remaining elevated zero maze parameters, and ACTH data were analyzed using Kruskal–Wallis one-way ANOVA on ranks, followed by Dunn’s Method post hoc comparisons, where indicated, by significant ANOVA factors.
Results
Stress-induced hyperthermia
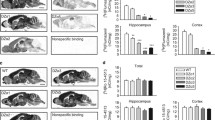
SIH was not influenced significantly by genotype in either of the experiments using male or female mice (Fig. 1a,b; H = 4.25, P = 0.12 and H = 1.64, P = 0.44 for males and females, respectively). In the male mice, the first temperature recording, T 1, tended to be higher for the \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}\) mice (Fig. 1a; H = 5.27, P = 0.072), although in the female mice this trend was not apparent (Fig. 1b; H = 3.582, P = 0.17). The second temperature recording, T 2, was not significantly influenced by genotype in either the male or female mice (Fig. 1a,b; H = 2.24, P = 0.33 and H = 3.40, P = 0.18 for male and female mice, respectively).
Stress-induced hyperthermia (SIH). Genotype did not significantly influence SIH or rectal temperature at the two time points measured in experiments with a male (n = 10 per genotype) or b female (n = 10 per genotype) wild-type (WT), \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \)(1a −/−), or \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) (1b −/−) mice. Bars represent means+SEM. T 1 First rectal temperature recording, T 2 second rectal temperature recording taken 15 min later, ΔT temperature difference between T 1 and T 2
Staircase test
The number of steps taken, incidence of rearing, and the ratio of steps to rears in the staircase test were not significantly affected by genotype in either of the experiments using male or female mice (males: steps F 2,29 = 0.26, P = 0.772; rears H = 0.96, P = 0.62; ratio H = 0.88, P = 0.64; females: steps F 2,27 = 0.996, P = 0.38; rears H = 3.61, P = 0.17; ratio H = 1.25, P = 0.536; Fig. 2a,b).
Staircase test. Deletion of GABAB(1a) or GABAB(1b) receptor subunit isoforms did not influence the number of ascending steps taken (Steps), incidence of rearing (Rears), the ratio between these two parameters (Ratio steps: rears), or the amount of urination in the staircase test in experiments with a male (n = 10 per genotype) and b female (n = 10 per genotype) mice, although male \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice defecated more than \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) male mice. Bars represent means+SEM. The symbol ## is used to denote P < 0.01 for \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) vs \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \). WT Wild-type, 1a −/− \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \), 1b −/− \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \)
With regard to physiological indicators of stress (Fig. 2a,b), genotype significantly influenced the number of fecal boli produced by male mice during the test (H = 14.13, P < 0.001). Post hoc comparisons demonstrated that the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice defecated more than \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice (P < 0.01), although neither mutant strain differed from the wild-types in this regard (P > 0.05). Fecal boli production was not significantly affected by genotype in the female mice, as was also the case for the number of urine spots produced by male or female mice (females: fecal boli H = 2.37, P = 0.31; males urination score H = 3.73, P = 0.16; females urination score H = 3.74, P = 0.154).
Light–dark box
In the experiment with male mice, three wild-type mice, one \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and one \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mouse made no transitions during the test—all of their time in the test was spent in the light compartment in which they were initially placed, and the latency to enter the dark side was therefore greater than the 600-s duration of the test. As a consequence, absolute data values for latency for these mice were not determined. Furthermore, data from these mice may have introduced bias in other test measures such as time in the light compartment and light–dark transitions; therefore, these mice were excluded from statistical analysis.
Genotype influenced the number of light–dark transitions in male mice (H = 6.48, P = 0.039; Fig. 3a). Post hoc comparisons revealed that male \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice conducted significantly less light–dark transitions than \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice (P < 0.05), although neither mutant differed from the wild-type controls in this regard. The latency to enter the dark compartment and the total time spent in the light compartment were not significantly influenced by genotype in the male mice (latency: H = 1.55, P = 0.46; time in light: H = 2.26, P = 0.32; Fig. 3a).
Light–dark box. In male mice (a), \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) (1b −/−) mice made fewer transitions between the light and dark compartments (Transitions) relative to the \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) (1a −/−) mice (n = 10 per genotype). In a separate experiment with female mice (b), \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice spent more time in the light compartment (Time in light) than \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) mice (n = 10–11 per genotype). Genotype did not influence the time take for mice of either sex to initially enter the dark compartment (Latency). Bars represent means+SEM. The symbol # denotes P < 0.05 for \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) vs \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \). WT Wild-type
In the experiment with female mice, some mice also failed to enter the dark compartment (two wild-type, one \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and two \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice) and were therefore excluded from statistical analysis. The number of light–dark transitions made by female mice was not significantly affected by genotype (H = 1.99, P = 0.37, Fig. 3b). However, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) female mice spent more time in the light compartment than \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) female mice (P < 0.05, Fig. 3b), although neither mutant genotype differed significantly from the wild-types (H = 6.58, P = 0.037; Fig. 3b). As for the experiment conducted with the male mice, latency to enter the dark compartment was not significantly affected by genotype in the female mice (H = 0.71, P = 0.70).
Marble-burying test
The number of marbles buried in the experiments with either male or female mice were not significantly affected by genotype (H = 0.33, P = 0.85 and H = 2.49, P = 0.29 for males and females, respectively; Table 1).
Elevated plus maze
Genotype did not significantly influence any of the parameters measured either in the elevated plus maze in the experiments involving the male or the female mice. The number of open arm entries for male (H = 0.71, P = 0.70) or female mice (H = 2.62, P = 0.27) was not affected by genotype, nor were closed arm entries for male or female mice (H = 0.91, P = 0.63; H = 0.71, P = 0.70 for males and females respectively), nor the total number of arm entries (H = 0.87, P = 0.65; H = 1.67, P = 0.44 for males and females, respectively). With regard to total arm entries, some mice made only one or no arm entries during the experiment. In female mice, this was the case for 4 of the 23 wild-type, 1 of the 16 \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \), and 9 of the 35 \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice. In the experiment with male mice, all of the wild-type and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice made more than one arm entry, while 2 of the 26 \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) males made one or no arm entries. The ratio of the number of open to closed arm entries was not affected by genotype in the experiments with either sex (H = 2.16, P = 0.34 and H = 0.87, P = 0.65 for males and females, respectively). Finally, the amount of time spent on the open arms by the mice in each respective experiment was not significantly affected by genotype (H = 2.35, P = 0.31 and H = 2.05, P = 0.36 for males and females, respectively; Table 2).
Elevated zero maze
In the experiment with male mice, the latency to enter the open side, time spent on the open side, the number of head dips over the side of the open areas, and number of rears were not significantly affected by genotype (H = 1.98, P = 0.371; H = 0.62, P = 0.733; F 2,34 = 1.75, P = 0.19 and H = 1.18, P = 0.55, respectively). There was a tendency for genotype to influence the distance travelled in the maze (line crossings: H = 5.50, P = 0.064) and the number of SAPs performed by the mice (F 2,34 = 2.577, P = 0.092; Fig. 4a).
Elevated zero maze. In male mice (a), deletion of either the GABAB(1a) or GABAB(1b) isoforms did not significantly influence the time taken to enter an open-sided quadrant (Latency), time spent in the open-sided quadrants (Time in open), ambulation in the maze (Line crossings), or the ethological parameters: incidence of dipping the head over the open sides (Head dip), rearing (Rears), and the number of stretch-attend postures (SAP) performed into the open quadrants (WT n = 12, 1a−/− n = 11, 1b−/− n = 12). In a separate experiment with female mice (b), genotype influenced all elevated zero maze parameters measured with the exception of SAPs (WT n = 12, 1a−/− n = 12, 1b−/− n = 12). Bars represent means+SEM. A single asterisk denotes P < 0.05, while two asterisks denote P < 0.01, and three asterisks denotes P < 0.01 vs wild-type (WT). 1a −/− \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}\), 1b −/− \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}\)
In the experiment with female mice, genotype significantly influenced the majority of parameters measured in the elevated zero maze. The latency to enter the open-sided area of the maze (H = 11.39, P < 0.01) was significantly longer in both \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice than in the wild-type mice. Both mutant strains also performed significantly less head dips over the maze open sides (F 2,35 = 21.07, P < 0.001) and rearings (H = 13.52, P = 0.001) than the wild type mice. Genotype also influenced the time spent on the open-sided areas (H = 11.60, P < 0.01) and the number of line crossings (H = 9.09, P < 0.05), and post hoc comparisons revealed a significant difference between \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice and wild-types in these parameters. The mutants did not differ significantly from each other in post hoc comparisons of the aforementioned parameters. The only parameter measured in the elevated zero maze that was not significantly influenced by genotype in the female mice was the number of SAPs (F 2,35 = 0.80, P = 0.46; Fig. 4b).
Basal corticosterone and ACTH
Mean plasma corticosterone appeared to be slightly elevated in the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice compared to the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (Fig. 5a); however, the factor “genotype” did not achieve statistical significance (F 2,12 = 1.91, P = 0.17). Two data points were removed from the ACTH data set as statistical outliers: one from the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice (922 pg/ml) and one from the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (1,176 pg/ml). The genotype did not significantly influence basal ACTH levels (Fig. 5b; H = 1.93, P = 0.38).
Hypothalamic–pituitary–adrenal axis characteristics. Deletion of GABAB(1a) or GABAB(1b) isoforms did not significantly influence plasma corticosterone (a) or ACTH (b) of male mice as measured within 1 h of the start of the circadian light phase (wild-type n = 9, \(\text{GABA}_{\text{B}\left( {1\text{a}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) n = 9, \(\text{GABA}_{\text{B}\left( {1\text{b}} \right)}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }} \) n = 6). Bars represent means+SEM
Discussion
The present study aimed to determine the influence of GABAB(1) subunit isoform deletion on innate, unconditioned anxiety-related behaviors in mice. To this end, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \), \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \), and wild-type control mice were investigated in a comprehensive battery of anxiety tests that employed a number of different types of experimental end points including autonomic, passive and active avoidance and ethological parameters. The test battery approach has been advocated for the detection of genuine differences in anxiety phenotypes in mutant mice, as reliance on fewer tests may give rise to erroneous interpretations depending on the specific idiosyncrasies of individual tests or mutations (Cryan and Holmes 2005). Overall, the present study demonstrated that the constitutive genetic deficiency of either the GABAB(1a) or GABAB(1b) isoform did not, for the most part, result in an alteration of innate, unconditioned anxiety (Table 3).
No anxiety-related phenotype was detected for either the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) or \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice, in experiments with either gender, in the SIH, marble-burying, and elevated plus maze paradigms. Furthermore, in male mice at least, genotype did not influence the levels of HPA axis hormones (Table 3). In the staircase, light–dark box, and elevated zero maze tests, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice showed some minor differences in behavior when compared with \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice. However, given that neither mutant differed from the control wild-type mice in these measures or, for that matter, in the majority of the response parameters measured in these tests, one cannot place too much credence on such subtle effects with relation to anxiety-related behaviors.
Deletion of either GABAB(1) subunit isoforms significantly influenced a number of parameters in the elevated zero maze in female mice. Locomotor activity can influence aspects of performance in exploratory-based tests of anxiety (Cryan and Holmes 2005); for example, a hyperactive phenotype may falsely suggest anxiolysis, and likewise, hypoactivity anxiogenesis. \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (both females and males) were previously demonstrated to be hyperactive in a novel environment, while the distance traveled by \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice was not different to that of the wild-type controls (Jacobson et al. 2006a). However, these observations are at odds with the reductions in exploratory activity by both mutant strains relative to the wild-types in the elevated zero maze in the present study, as indicated by an increased latency to enter the open-sided area in both female mutant genotypes and by the reduced line crossings made by the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) females. Furthermore, the ethological measures of head-dipping and rearing were also reduced in both \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) female mice in this test. Reductions in the parameters of latency to enter an innately aversive area, head-dipping over the edges of elevated apparatuses, and rearing have been interpreted as heightened anxious responses in various apparatuses, including the elevated zero maze (Belzung 1999; Homanics et al. 1999; Rodgers 1997; Rodgers and Johnson 1995; Shepherd et al. 1994). The elevated zero maze has been considered as a more sensitive test than the elevated plus maze, mainly due to the elimination of the central square of the elevated plus maze, which can produce difficulties in data interpretation (Lee and Rodgers 1990; Shepherd et al. 1994), and to the facilitation of locomotor exploration given by the circular track of the zero maze, thus eliminating corners in which the mice may barricade themselves (Rodgers 1997; Shepherd et al. 1994).Therefore, the present findings suggest a subtle, sex-specific role for the GABAB(1) isoforms in innate anxiety. It should be noted, however, that in the light–dark box test, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) female mice spent an increased amount of time in the light side relative to the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) female mice, which is an anxiolytic-like response. Together with the data from other anxiety paradigms where no robust anxiety phenotype was observed, these results indicate that the influence of GABAB(1) isoforms on anxiety in female mice is most likely highly dependent on the environment.
Failure to enter the dark compartment of the light–dark box was shown by a small number of both male and female mice, and occurred to a reasonably similar level in wild-type and mutant mice. The proportion of mice of each genotype making 0 or 1 total arm entries in the elevated plus maze was also similar to that of the light–dark box, although only in female mice. These behaviors may reflect “freezing”, neophobic avoidance of newly discovered compartments, or failure to explore extensively enough to find additional compartments (Rodgers 1997) and, thus, may indicate anxiety in these animals. As genotype did not significantly influence these behaviors, they may be a consequence of the background strain. The BALB/c substrains have previously been reported as anxious in comparison to other mouse strains (Belzung 1999; Belzung and Griebel 2001; Cryan and Holmes 2005; Griebel et al. 2000). It could therefore also be posited that innate anxiety may have been near-maximal in the background strain for the GABAB(1) isoform mutant mice; thus, further increases in anxiety induced by the genetic manipulations may be difficult to detect (Crawley et al. 1997). However, this was certainly not the case with either of the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( 1 \right)}}} \) or \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( 2 \right)}}} \) mice, both of which were also maintained on a BALB/c background (Gassmann et al. 2004; Schuler et al. 2001) and both of which show profoundly increased anxiety relative to wild-type controls in exploratory anxiety tests (Mombereau et al. 2004a,b, 2005). \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( 1 \right)}}} \) mice were substantially more anxious than wild-types in the light–dark box (Mombereau et al. 2004a,b) and staircase tests (Mombereau et al. 2004a), while in the elevated zero maze, all \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( 1 \right)}}} \) mice jumped from the maze, a response indicating heightened flight or panic behavior (Mombereau et al. 2004a). This clearly indicates that the tests used in the present study are suitable to detect increases in anxious behaviors in the BALB/c strain.
The anxious phenotype of the aforementioned \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( 1 \right)}}} \) mice differs considerably from that of the specific GABAB(1) isoform-deficient mice in the present study. Both \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( 1 \right)}}} \) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( 2 \right)}}} \) mice show spontaneous seizures, hyperalgesia, hyperlocomotion, memory impairments, and significantly elevated exploratory anxiety (Gassmann et al. 2004; Mombereau et al. 2004a,b, 2005; Schuler et al. 2001). In these mutant mice, classical GABAB receptor agonist responses are abolished (Gassmann et al. 2004; Kaupmann et al. 2003; Schuler et al. 2001). In contrast, there is only partial GABAB receptor loss in the GABAB(1a) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (Vigot et al. 2006), and some residual agonist-induced function remains, albeit blunted, in both the \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice (Jacobson et al. 2006a; Perez-Garci et al. 2006; Shaban et al. 2006; Vigot et al. 2006). Together with the lack of overt anxious phenotype of the GABAB(1) isoform mutant mice in the present study, these findings demonstrate that at least some heterodimeric GABAB receptor function is essential for the prevention of an increase in anxiety behavior and, furthermore, that the presence of either the GABAB(1a) or GABAB(1b) isoforms can fulfill this task.
The indistinct innate anxiety phenotype of \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) and \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice demonstrated in the present study contrasts with their reported phenotypes in aversive taste memory (Jacobson et al. 2006b) and conditioned freezing paradigms (Shaban et al. 2006). \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice did not acquire a CTA to a saccharin solution paired with LiCl-induced malaise, while \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{b}}} \right)}}} \) mice acquire CTA well, but were profoundly impaired in the extinction of this aversion (Jacobson et al. 2006b). In conditioned freezing, \( {\text{GABA}}^{{ - \mathord{\left/ {\vphantom { - - }} \right. \kern-\nulldelimiterspace} - }}_{{{\text{B}}{\left( {1{\text{a}}} \right)}}} \) mice show subsequent generalized freezing to sound cues irrespective of whether they were paired with high-intensity (0.9 mA) foot shocks during conditioning (Shaban et al. 2006). In theory, it should not necessarily be expected that conditioned and unconditioned tests of fear and anxiety should demonstrate similar phenotypes or, indeed, even to produce similar phenotypes within each of these categories (Rodgers 1997). Certainly, dissociations between aversive conditioning and exploratory anxiety has also been reported for other mutant mice, such as forebrain-selective glycine transporter 1-deficient mice (Yee et al. 2006) and 5-HT1A receptor knockout mice (Klemenhagen et al. 2006), although it should be noted that in each of these studies only one unconditioned anxiety paradigm was utilized. However, the differentiation between conditioned and unconditioned responses appears particularly clearly delineated with GABAB(1) isoform-deficient mice. CTA is well-known as an aversive, associative learning and memory paradigm (Akirav 2006; Akirav et al. 2006; Berman and Dudai 2001; Bermudez-Rattoni 2004; Lamprecht et al. 1997; Welzl et al. 2001) and has recently been applied to the study of anxiety disorders associated with altered emotional learning (Cryan and Holmes 2005; Guitton and Dudai 2004; Yasoshima and Yamamoto 2005). Conditioned freezing in rodents is thought to model emotive cognition aspects of human anxiety disorders such as posttraumatic stress disorder and panic disorder (Barad 2005; Cryan and Holmes 2005; Delgado et al. 2006; Ledgerwood et al. 2005; Ressler et al. 2004). It should also be noted that the GABAB(1a) isoform has been demonstrated as necessary for both hippocampal (Vigot et al. 2006) and amygdala (Shaban et al. 2006) long-term potentiation, and the ability to discriminate successfully between novel and familiar objects in a mouse object recognition paradigm—a task which requires the presence of an intact hippocampus (Broadbent et al. 2004; Clark et al. 2000). Together, these findings suggest that the GABAB(1a) and GABAB(1b) isoforms of the GABAB(1) subunit have specific relevance for anxiety with a cognitive component, rather than for innate anxiety per se. Indeed, it remains possible that the specific and differential deficiencies in emotive learning and memory in these mutant mice could be a product predominantly of cognitive impairments, rather than one of emotive processing in itself.
In conclusion, previous studies have demonstrated that genetic ablation of all functional GABAB receptors results in increases in unconditioned anxiety behavior (Cryan and Kaupmann 2005). Results of the present study suggest that this not due to the specific loss of either one of the predominant GABAB(1) subunit isoforms and that the role for the isoforms in innate anxiety is relatively indistinct. In contrast, these isoforms appear to be more explicitly involved in anxious behaviors that are associated with a cognitive component.
References
Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G (2002) Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol 37:504–508
Addolorato G, Leggio L, Abenavoli L, Agabio R, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G (2006) Baclofen in the treatment of alcohol withdrawal syndrome: a comparative study vs diazepam. Am J Med 119(276)e13–e18
Akirav I (2006) NMDA partial agonist reverses blocking of extinction of aversive memory by GABA(A) agonist in the amygdala. Neuropsychopharmacology (in press)
Akirav I, Khatsrinov V, Vouimba RM, Merhav M, Ferreira G, Rosenblum K, Maroun M (2006) Extinction of conditioned taste aversion depends on functional protein synthesis but not on NMDA receptor activation in the ventromedial prefrontal cortex. Learn Mem 13:254–258
Ameisen O (2005) Complete and prolonged suppression of symptoms and consequences of alcohol-dependence using high-dose baclofen: a self-case report of a physician. Alcohol Alcohol 40:147–150
Andrews N, File SE (1993) Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol 235:109–112
Barad M (2005) Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol 15:710–715
Belzung C (1999) Measuring rodent exploratory behavior. In: Gerlai RT, Crusio WE (eds) Handbook of molecular-genetic techniques for brain and behavioral research (Techniques in the behavioral and neural sciences). Elsevier, Amsterdam, The Netherlands, pp 739–749
Belzung C, Griebel G (2001) Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125:141–149
Berman DE, Dudai Y (2001) Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291:2417–2419
Bermudez-Rattoni F (2004) Molecular mechanisms of taste-recognition memory. Nat Rev Neurosci 5:209–217
Bettler B, Kaupmann K, Mosbacher J, Gassmann M (2004) Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev 84:835–867
Blein S, Ginham R, Uhrin D, Smith BO, Soares DC, Veltel S, McIlhinney RA, White JH, Barlow PN (2004) Structural analysis of the complement control protein (CCP) modules of GABA(B) receptor 1a: only one of the two CCP modules is compactly folded. J Biol Chem 279:48292–48306
Bouwknecht JA, Olivier B, Paylor RE (2006) The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev (in press)
Breslow MF, Fankhauser MP, Potter RL, Meredith KE, Misiaszek J, Hope DG Jr (1989) Role of gamma-aminobutyric acid in antipanic drug efficacy. Am J Psychiatry 146:353–356
Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA 101:14515–14520
Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL (1986) Major tranquillizers can be distinguished from minor tranquilizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol 126:223–229
Clark RE, Zola SM, Squire LR (2000) Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20:8853–8860
Couve A, Moss SJ, Pangalos MN (2000) GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci 16:296–312
Crawley JN (2000) What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. Wiley, New York
Crawley JN, Davis LG (1982) Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res Bull 8:609–612
Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790
Cryan JF, Kaupmann K (2005) Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci 26:36–43
Cryan JF, Kelly PH, Neijt HC, Sansig G, Flor PJ, van Der Putten H (2003) Antidepressant and anxiolytic-like effects in mice lacking the group III metabotropic glutamate receptor mGluR7. Eur J Neurosci 17:2409–2417
Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, Froestl W, Bettler B, Kaupmann K, Spooren WP (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 310:952–963
Dalvi A, Rodgers RJ (1996) GABAergic influences on plus-maze behaviour in mice. Psychopharmacology (Berl) 128:380–397
Delgado MR, Olsson A, Phelps EA (2006) Extending animal models of fear conditioning to humans. Biol Psychol 73:39–48
Drake RG, Davis LL, Cates ME, Jewell ME, Ambrose SM, Lowe JS (2003) Baclofen treatment for chronic posttraumatic stress disorder. Ann Pharmacother 37:1177–1181
File SE, Mabbutt PS, Andrews N (1991a) Diazepam withdrawal responses measured in the social interaction test of anxiety and their reversal by baclofen. Psychopharmacology (Berl) 104:62–66
File SE, Zharkovsky A, Gulati K (1991b) Effects of baclofen and nitrendipine on ethanol withdrawal responses in the rat. Neuropharmacology 30:183–190
File SE, Zharkovsky A, Hitchcott PK (1992) Effects of nitrendipine, chlordiazepoxide, flumazenil and baclofen on the increased anxiety resulting from alcohol withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 16:87–93
Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A (2004) Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res 28:1517–1523
Gassmann M, Shaban H, Vigot R, Sansig G, Haller C, Barbieri S, Humeau Y, Schuler V, Muller M, Kinzel B, Klebs K, Schmutz M, Froestl W, Heid J, Kelly PH, Gentry C, Jaton AL, Van der Putten H, Mombereau C, Lecourtier L, Mosbacher J, Cryan JF, Fritschy JM, Luthi A, Kaupmann K, Bettler B (2004) Redistribution of GABAB(1) protein and atypical GABAB responses in GABAB(2)-deficient mice. J Neurosci 24:6086–6097
Gray JA, Lalljee B (1974) Sex differences in emotional behaviour in the rat: correlation between open-field defecation and active avoidance. Anim Behav 22:856–861
Griebel G, Belzung C, Perrault G, Sanger DJ (2000) Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 148:164–170
Guitton MJ, Dudai Y (2004) Anxiety-like state associates with taste to produce conditioned taste aversion. Biol Psychiatry 56:901–904
Hinderer SR (1990) The supraspinal anxiolytic effect of baclofen for spasticity reduction. Am J Phys Med Rehabil 69:254–258
Holmes A (2001) Targeted gene mutation approaches to the study of anxiety-like behavior in mice. Neurosci Biobehav Rev 25:261–273
Holmes A, Yang RJ, Crawley JN (2002) Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci 18:151–165
Homanics GE, Quinlan JJ, Firestone LL (1999) Pharmacologic and behavioral responses of inbred C57BL/6J and strain 129/SvJ mouse lines. Pharmacol Biochem Behav 63:21–26
Jacobson L, Bettler B, Kaupmann K, Cryan JF (2006a) GABAB(1) receptor subunit isoforms exert a differential influence on baseline but not GABAB receptor agonist-induced changes in mice. J Pharmacol Exp Ther (in press)
Jacobson LH, Kelly PH, Bettler B, Kaupmann K, Cryan JF (2006b) GABA(B(1)) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories. J Neurosci 26:8800–8803
Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der Putten H, Mosbacher J, Brauner-Osborne H, Waldmeier P, Bettler B (2003) Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci 18:2722–2730
Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT (2006) Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology 31:101–111
Lamprecht R, Hazvi S, Dudai Y (1997) cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci 17:8443–8450
Ledgerwood L, Richardson R, Cranney J (2005) D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry 57:841–847
Lee C, Rodgers RJ (1990) Antinociceptive effects of elevated plus-maze exposure: influence of opiate receptor manipulations. Psychopharmacology (Berl) 102:507–513
Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF (2004a) Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology 29:1050–1062
Mombereau C, Kaupmann K, van der Putten H, Cryan JF (2004b) Altered response to benzodiazepine anxiolytics in mice lacking GABA B(1) receptors. Eur J Pharmacol 497:119–120
Mombereau C, Kaupmann K, Gassmann M, Bettler B, van der Putten H, Cryan JF (2005) Altered anxiety and depression-related behaviour in mice lacking GABAB(2) receptor subunits. Neuroreport 16:307–310
Nastiti K, Benton D, Brain PF (1991) The effects of compounds acting at the benzodiazepine receptor complex on the ultrasonic calling of mouse pups. Behav Pharmacol 2:121–128
Perez-Garci E, Gassmann M, Bettler B, Larkum ME (2006) The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron 50:603–616
Pilc A, Nowak G (2005) GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors. Drugs Today (Barc) 41:755–766
Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M (2004) Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144
Rodgers RJ (1997) Animal models of ‘anxiety’: where next? Behav Pharmacol 8:477–496 (discussion 497–504)
Rodgers RJ, Johnson NJ (1995) Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav 52:297–303
Rodgers RJ, Cutler MG, Jackson JE (1997) Behavioural effects in mice of subchronic chlordiazepoxide, maprotiline and fluvoxamine. II. The elevated plus-maze. Pharmacol Biochem Behav 57:127–136
Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B (2001) Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)). Neuron 31:47–58
Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Luthi A (2006) Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci 9(8):1028–1035
Shephard RA, Wedlock P, Wilson NE (1992) Direct evidence for mediation of an anticonflict effect of baclofen by GABAb receptors. Pharmacol Biochem Behav 41:651–653
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT (1994) Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 116:56–64
Simiand J, Keane PE, Morre M (1984) The staircase test in mice: a simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacology (Berl) 84:48–53
Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C (2000) Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther 295:1267–1275
Spooren WP, Schoeffter P, Gasparini F, Kuhn R, Gentsch C (2002) Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608). Eur J Pharmacol 435:161–170
Steiger JL, Bandyopadhyay S, Farb DH, Russek SJ (2004) cAMP response element-binding protein, activating transcription factor-4, and upstream stimulatory factor differentially control hippocampal GABABR1a and GABABR1b subunit gene expression through alternative promoters. J Neurosci 24:6115–6126
Tarantino LM, Gould TJ, Druhan JP, Bucan M (2000) Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome 11:555–564
Van der Heyden JA, Zethof TJ, Olivier B (1997) Stress-induced hyperthermia in singly housed mice. Physiol Behav 62:463–470
Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Muller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B (2006) Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron 50:589–601
Welzl H, D’Adamo P, Lipp HP (2001) Conditioned taste aversion as a learning and memory paradigm. Behav Brain Res 125:205–213
Yasoshima Y, Yamamoto T (2005) Effects of midazolam on the expression of conditioned taste aversion in rats. Brain Res 1043:115–123
Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Mohler H, Boison D (2006) Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci 26:3169–3181
Acknowledgements
This work was supported by the National Institutes of Mental Health/National Institute on Drug Abuse grant U01 MH69062 (LHJ, KK, JFC) and the Swiss Science Foundation (3100–067100.01, BB). The authors thank Dr. Christopher Pryce for critical reading of the manuscript. We thank Christine Hunn and Hugo Bürki for their excellent technical assistance. We would also like to thank Dr. Doncho Uzunov and Christian Kohler for endocrine measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobson, L.H., Bettler, B., Kaupmann, K. et al. Behavioral evaluation of mice deficient in GABAB(1) receptor isoforms in tests of unconditioned anxiety. Psychopharmacology 190, 541–553 (2007). https://doi.org/10.1007/s00213-006-0631-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0631-9