Abstract
Animal models have been vital to recent advances in experimental neuroscience, including the modeling of common human brain disorders such as anxiety, depression, and schizophrenia. As mice express robust anxiety-like behaviors when exposed to stressors (e.g., novelty, bright light, or social confrontation), these phenotypes have clear utility in testing the effects of psychotropic drugs. Of specific interest is the extent to which mouse models can be used for the screening of new anxiolytic drugs and verification of their possible applications in humans. To address this problem, the present chapter will review different experimental models of mouse anxiety and discuss their utility for testing anxiolytic and anxiogenic drugs. Detailed protocols will be provided for these paradigms, and possible confounds will be addressed accordingly.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Animal models are widely used for simulating human brain disorders and for providing insight into their neurobiological mechanisms [1–4]. The latter is of great interest in the current neuroscientific community, given the increasing use of laboratory animals for screening various classes of psychotropic drugs [5, 6]. The use of mice has been particularly beneficial, since fine-tuned manipulations of selected genes have led to new animal models relevant to drug discovery [3, 4, 7, 8].
It is important to understand, however, that any animal experiment in the laboratory is an artificial situation, and it may be biologically different from the natural behavior of the animal. Thus, it is crucial to correctly interpret the animal behavior observed in an experiment in order to identify parallels with specific human brain disorders. Although there are many other conceptual and methodological limitations of working with mice, this species shows much promise for future psychopharmacological research.
In order for animal models to be useful, researchers must follow certain practices and methods which will optimize the translatability of data from animal models to human affective disorders. Here, we will present a broad review of some reliable methods of analyzing mouse anxiety, and their utility for screening for anxiolytic therapeutic agents. All these tests are of a complex nature and we would suggest that the reader explore each system to better understand the variables and sublets of each test. We will also discuss how these protocols can be applied correctly in order to avoid confounding experimental data.
2 Materials
2.1 Animals
-
1.
Various inbred, selectively bred (for specific behavioral/physiological phenotypes ), and genetically modified (mutant or transgenic ) mice may be used, and some searchable online databases, such as Mouse Phenome Project (www.jax.org/phenome) or Mouse Genome Informatics (www.informatics.jax.org/), may provide appropriate strains for studying mouse anxiety. We recommend using most of the inbred strains listed in the Tier 1 list of the Mouse Phenome Project database, especially C57BL/6J, A/J, and 129S1/SvImJ mice (see http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=docs/pristrains for details) (see Note 1 ).
-
2.
Generally, several different models of anxiety that target different domains (e.g., locomotion/exploration, risk assessment, defensive responses) are necessary in order to more fully characterize drug effects or a mutant mouse phenotype. The use of a single model, or only models targeting one particular behavioral domain, may not be sufficient.
-
3.
Researchers should also take other factors like age, weight, sex, stage of estrous cycle, diet, and housing situation into account when designing experiments (see Note 2 ).
2.2 Housing
-
1.
If mice are obtained from a commercial vendor or another laboratory, allow at least 1 week acclimation from shipping stress . In most cases, a much longer time (e.g., 1 month) may be required for a better acclimation.
-
2.
Housing animals in groups will help avoid social isolation stress/anxiety, but keeping groups small enough (e.g., not more than 5 animals per cage) will be necessary to avoid overcrowding stress. While overcrowding of mice may cause significant levels of stress, single housing is equally detrimental to experimental models. For example, social isolation may lead to altered neurobiology, increased basal anxiety, reduced exploration, and a profound vulnerability to depression-like behaviors [9, 10].
-
3.
The room in which mice are housed should be kept at approximately 21 °C, on a 12/12-h light cycle. As mice are nocturnal, the light cycle may be inverted if spontaneous activity measures are needed.
-
4.
Food and water should be freely available, unless the intake is being controlled for experimental purposes.
-
5.
Utilize plastic, solid-floored cages with sufficient space for mice to exercise and fully rear up. Note that enrichment items, such as cardboard tunnels, can improve general welfare but may also affect experimental outcomes or increase territorial aggression.
2.3 Drugs
-
1.
All experimental protocols described here are compatible with drug testing. Researchers may choose from various antidepressants , anxiogenics, anxiolytics, or other psychotropic drugs, administered with a vehicle (e.g., saline). A typical experiment may include one or several drug-treated groups (e.g., several doses or several pretreatment times) compared to a vehicle-treated group of mice. Usually (unless stated otherwise), 10 animals per experimental group will be needed, also providing adequate statistical power (see further). However, if the effects of the drugs are particularly robust, a smaller n (e.g., n = 7–8) may suffice. For mild effects, a larger number of animals (n = 15–16) may be required.
2.4 Observations , Video Recording, and General Procedures
-
1.
Video tracking software:
Ethovision, Noldus, Nijmegen, Netherlands.
Videotrack system, Viewpoint, Lyon, France.
Loco-, Maze- and Top Scan, Clever Sys Inc, Reston, VA, USA.
-
2.
Photobeam-based activity monitoring:
Columbus Instruments, Columbus, OH, USA.
Coulbourn Instruments, Whitehall, PA, USA.
-
3.
Vibration-based activity monitoring:
Laboras/Metris, Hoofddorp, Netherlands.
Bioseb, Vitrolles, France.
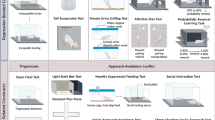
2.5 Requirements for Experimental Models
-
1.
Elevated Plus Maze (EPM) :
Elevated maze with two open and two closed arms in the shape of a plus, made of steel, fiberboard, or Plexiglas (either transparent or painted matte black), see Table 1. Arms are typically 30 cm long and 5 cm wide. The apparatus is usually elevated 40–60 cm on sturdy legs [1, 9, 15].
Table 1 Selected commercial suppliers of behavioral equipment for anxiety research -
2.
Open Field .
Enclosed 50 × 30 cm wood, plastic, or Plexiglas arena, marked into 10-cm squares (Table 1). Gray or black arenas are typically used. If an arena is not available, a large animal cage marked into squares with indelible ink may be used [10, 16].
-
3.
Marble Burying Test .
Woodchip bedding (e.g., aspen chips), up to 20 marbles (15 mm in diameter). Animal cages (e.g., large cage 30 × 20 cm for 20 marbles, smaller cages for 6–8 marbles) [17–20].
-
4.
Defensive Shock-Prod Burying test.
Familiar test cage or home cage with plentiful bedding and a hole in the wall 2 cm above bedding [6, 21].
Electrical probe connected to a shock source.
Ruler for measuring depth to which prod is buried. Optional: Large (e.g., 10 cm) object associated with shock.
-
5.
Grooming Analysis Algorithm.
Small (e.g., 20 × 20 × 30 cm) transparent observation box.
Stressors to induce grooming: e.g., novel environment, predator exposure, bright light, or other means of artificially inducing grooming (e.g., water mist).
Optional: video camera for subsequent frame-by-frame analysis [8].
-
6.
Startle Response .
Observation box (similar to the open field test).
Conditioned stimulus: e.g., a light, paired with a footshock, Table 1. Startle stimulus, such as an air puff or loud noise [1, 7, 22].
-
7.
Social Interaction Test .
Low-anxiety version: Test apparatus (similar to the open field test) familiar to the animals, with low illumination.
Mid-low anxiety version: Familiar test apparatus with high illumination.
Mid-high anxiety version: Unfamiliar test apparatus with low illumination.
High-anxiety version: Unfamiliar test apparatus with high illumination [1].
-
8.
Suok Test .
Test apparatus: 2.6-m aluminum tube, 2 cm in diameter (marked into 10-cm segments with indelible ink) with fixed 50 × 50 × 1 cm Plexiglas side walls to prevent escape, elevated 20 cm from a cushioned floor.
Optional (the light-dark version of the test): several 60-W light bulbs suspended 40 cm above one half of the test apparatus [23, 24].
-
9.
Light-Dark Box Test .
Test apparatus: a 2-compartment box, 30 × 30 × 30 cm each; with one black, and one transparent brightly illuminated boxes, separated by a sliding door [5].
-
10.
Stress-Induced Hyperthermia (SIH) .
Oiled rectal thermometer with rounded tip, up to 3 mm thick:
Surgilube sterile surgical lubricant by Fougera & Co. (Melville, NY, USA),
K-Y lubricant by Johnson & Johnson (Waltham, MA, USA),
MLT1404 rectal probe for mice by Adinstruments, Inc (Colorado Springs, CO, USA).
Cage or box (as in the open field test) to which mice can be transferred [25].
-
11.
Hole-Board Test .
Test apparatus (similar to the open field test) with hole-board insert (Table 1). The floor has four or more identical holes approximately 3 cm in diameter [26].
-
12.
Rat Exposure Test .
Medium (e.g., 40 × 30 × 30 cm) transparent observation box, with a wire mesh separating the two halves of the box.
Small (e.g., 8 × 8 × 12 cm) black Plexiglas box, serving as the starting placement (home chamber for the mouse).
Transparent Plexiglas tube (e.g., 4.5 cm in diameter, 13 cm in length) connecting the small black box to the medium transparent box [27].
-
13.
Novel Object Test .
Test apparatus similar to the open field test (see above).
Novel objects: e.g., Mega Bloks structures [28].
3 Methods
3.1 Drug Administration
-
1.
Common routes of injection include systemic [intraperitoneal (i.p.), intramuscular (i.m.), intravenous (i.v.), per oral (p.o.), subcutaneous (s.c.)] and local [intracerebral (i.c.), intranasal (i.n.), or intracerebroventricular (i.c.v)]. Continuous drug infusion (using osmotic pumps , such as Alzet pumps) at a constant rate may be used to improve the availability of the drug, and implantable depots can be used for s.c. drug administration to achieve a lasting therapeutic effect. Though not as commonly used in anxiety research, i.n. drug administration is a rapid, noninvasive method for drug delivery [11]. By this method, drugs can be administered either by pipetting small (6-μl) drops of a drug solution into each nostril once per minute [11] or by placing a 10-μl drop of a drug to be inhaled on the end of the snout [12]. As i.n. administration delivers the drug to the brain directly via the olfactory nerve and olfactory epithelium, this method may be favored for administration of drugs that are rapidly metabolized when given systemically or have difficulty crossing the blood–brain barrier [13]. For example in rats, i.n. dopamine has been shown to decrease grooming activity and increase locomotor activity in the open field at one tenth of the dose needed to observe these effects when the drug is administered i.p. [13]; also see data on its antidepressant effects [14].
-
2.
Route of administration, dose, and pretreatment time vary depending on strain sensitivity and the drug being used.
3.2 Observations, Video Recording, and General Procedures
-
1.
A computer , digital camera mounted above the test apparatus, and video tracking software will aid researchers in the collection of accurate behavioral data.
-
2.
Alternative methods of behavioral tracking include photobeam-based or vibration-based activity monitoring.
-
3.
In addition to automated tracking, an observer with a timer and data sheet to tally behaviors will allow comparison of data if video tracking is unreliable due to poor detectability (from poor angle or bad contrast; e.g., if fur color matches the background).
-
4.
Observers must refrain from making noise or movement, as their presence may alter animal behavior. Assess intra- and inter-rater reliability for consistency.
-
5.
Allow the animals at least 1 h acclimation after their transfer from the animal holding room to the experimental room.
-
6.
Mice should be introduced to the testing environment during their normal waking cycle, to prevent possible confounds. When performing ethological analysis as part of a battery of tests, consider how effects of these tests (such as habituation) may confound the mouse performance and drug sensitivity.
-
7.
After each testing session, clean the equipment (e.g., with a 30 % ethanol solution) to eliminate olfactory cues.
3.3 Data Analysis
-
1.
Behavioral data may be analyzed with the Mann-Whitney U-test for comparing two groups (parametric Student’s t-test may be used only if data are normally distributed), or analysis of variance (ANOVA) for multiple groups, followed by an appropriate post hoc test.
-
2.
Some experiments may require one-way ANOVA with repeated measures, while for more complex studies (e.g., those including treatment, genotype, sex, and/or stress) n-way ANOVA may be used.
-
3.
Analysis of statistical power is becoming particularly important in animal research. Effect size (the difference between means of the two groups) in neurobehavioral research can be small (0.2), moderate (0.5), or large (0.8). Statistical power is the probability of finding statistical significance for a true hypothesis, and its common value used in behavioral research is 0.8. Factors that can affect statistical power include the experimental design (independent/dependent groups), 1- or 2-tailed hypotheses, statistical test chosen, effect size, and sample size. In order to determine an effect size, the researcher may rely on effect size reported in similar studies, or can decide on it based on the goals of the study (e.g., use large effect size for robust phenotypes, and small effect size for less profound differences). The researcher can then use statistical power-analyzing software to determine the sample size required for the level of power decided upon. The choice of sample size, based on power calculations, is increasingly important for Institutional research ethics committees, to prove that neither too few nor too many subjects are used in the proposed research.
3.4 Elevated Plus Maze (EPM) Test
Possessing good face-, construct-, and predictive validity, the EPM is a reliable and pharmacologically sensitive paradigm based on the conflict between innate rodent desire to explore and the fear of open, elevated areas [3, 5]. Anxious mice generally have a lower ratio of open arm entries to total arm entries, and display fewer explorative measures such as rears, wall leans, or head dips [3, 5]. Anxiety also increases EPM freezing and stretch-attend postures. After administration of anxiolytic drugs (e.g., diazepam, chlordiazepoxide, ethanol), mice generally display more exploratory behaviors, a greater number of open arm entries, and an increased duration of time spent on open arms [9, 15]. Anxiogenic drugs (e.g., pentylenetetrazole, picrotoxin) produce the opposite behavioral effects in this model.
-
1.
Place rodent on the central platform of the EPM facing either an open arm or a closed arm consistently at the start of each trial (see Notes 1 – 3 ).
-
2.
The open arm, closed arm, and total (open + closed arm) activity can be recorded for 5–10 min using a video tracking system, while the researchers simultaneously document the number of arm entries (all four paws are on the arm) and time spent on each open arm (see Notes 4 and 5 ).
3.5 Open Field Test (OFT)
This test is based on the balance between the animal’s natural drive to explore novelty and its aversion to open illuminated areas. Measuring exploratory behaviors and generalized motor activity, the open field test is simple and the most frequently used model of mouse anxiety [2]. In general, anxious mice exhibit more freezing, less time spent and a lower percentage of ambulation in the center of the arena (thigmotaxis), and fewer exploratory behaviors (see Note 6 ). Anxiolytic drugs generally increase exploration and reduce freezing and thigmotaxis [16].
-
1.
After the apparatus is divided into central and peripheral zones, mice are placed consistently in a corner, or the center of the open field arena, and allowed to explore for 5–10 min.
-
2.
Behavioral measurements can be recorded automatically with appropriate software, and include: time spent in the central area, distance traveled in the center as a ratio of total distance traveled, ambulation duration, time spent immobile (freezing), defecation score, and vertical activity such as rearing and wall leans [16], (see Notes 7 and 8 ).
3.6 Marble Burying Test
While not a direct model of anxiety per se, this simple test represents a pharmacologically sensitive method assessing digging activity—a species-typical response to anxiogenic stimuli [17, 19, 30]. Digging behavior is attenuated by low (nonsedative) doses of anxiolytic benzodiazepines and other ligands [18, 20]. Control mice can be expected to bury roughly 75 % of marbles, whereas drug-treated mice show a marked decrease in digging activity [31, 32]. Mouse strains with high basal anxiety levels (e.g., 129S1/SvImJ) should be avoided when testing anxiogenic drugs, to avoid a ceiling effect. Likewise, mice with very low basal anxiety may show poor results when examining the effects of anxiolytic drugs (see Note 9 ).
-
1.
Cages should be filled with wood chip bedding approximately 5 cm deep. The bedding must be flattened to create an even surface. Use the same volume (e.g., 300 ml per 26 × 16 cm cage) of bedding in each cage. Although wood chips and shavings are most commonly used, sawdust has also been used with similar effectiveness in this model [33, 34]. There are many suppliers of these types of bedding, including Shavings-Direct (www.shavings-direct.com), Doctors Foster and Smith (www.drsfostersmith.com), and local pet stores (e.g., Petsmart, www.petsmart.com). The important thing is to be consistent within experiments.
-
2.
Mouse cage dimensions vary, but this test has been used effectively with 26 × 16 cm (for 6–8 marbles), or 30 × 20 cm (for 20 marbles) [18]. Marbles should be placed on the surface of the bedding in a regular pattern, roughly 4 cm apart.
-
3.
Place one mouse in each cage. After 30 min, count the number of buried marbles. Any marble covered 2/3 of its depth with bedding is considered “buried” [18]. Alternatively, count fully covered (1/1) and partially covered (2/3) marbles separately, also calculating the sum of the latter two categories (see Notes 9 – 11 ).
-
4.
Use a new clean cage with fresh bedding for each animal. Wash the marbles with ethanol after each test.
3.7 Defensive (Shock-Prod) Burying Test
Similar to the marble burying test, this paradigm is another pharmacologically sensitive method to assess rodent anxiety. Mice usually bury noxious stimuli posing an immediate threat (e.g., electrified shock-prod). The test has pharmacological validity, as benzodiazepines and the serotonergic anxiolytics potently suppress shock-prod burying in a dose-dependent manner, whereas anxiogenic drugs have been proven to increase this behavior [6].
-
1.
In a standard-sized mouse cage (see above) with bedding 5 cm deep, insert a wire-wrapped prod (6–7 cm long) through a hole 2 cm above the bedding surface.
-
2.
After the initial contact with the bare wires and the subsequent shock, record the behavior of the animal for 10–15 min. Behavioral measures of activity may include: prod-directed burying, burying latency, height of pile at prod base, prod contacts (number, duration), prod contact latency, and stretch-attend postures directed at the prod [6] (see Note 12 ).
3.8 Grooming Analysis Algorithm
Anxious mice tend to display a disorganized behavioral sequencing of grooming (higher percentage of incorrect transitions, more interrupted bouts) and a longer duration of this behavior. In contrast, anxiolytic benzodiazepines normalize mouse grooming sequencing by significantly reducing interrupted bouts and incorrect transitions [8] (see Notes 13 and 14 ).
-
1.
Induce grooming through exposure to novelty or a stressor. Alternatively, mist the animal with water using a spray bottle. Place the animal in a small transparent observation box for 5 or 10 min.
-
2.
If using a video camera, begin recording. With a stopwatch, record cumulative measures of grooming activity, such as: latency to onset, time spent grooming, and total number of bouts. A new bout takes place after an interruption of greater than 6 s; bouts containing pauses of less than 6 s are deemed “interrupted.” Additionally, record the patterning of each bout using the following scale: 0—no grooming, 1—paw licking, 2—nose/face/head wash, 3—body grooming, 4—leg grooming, 5—tail/genitals grooming.
-
3.
There are several types of incorrect transitions, including skipped (e.g., 1–4, 3–5), reversed (e.g., 3–2, 5–3), prematurely terminated (e.g., 3–0, 4–0), and incorrectly initiated (e.g., 0–3, 0–5) transitions. Calculate the percentage of interrupted bouts and the percentage of incorrect transitions; see [8] for details (see Notes 15 and 16 ).
3.9 Startle Response
The startle response test pairs a conditioned stimulus (sound, light) with a footshock to induce an anxiogenic “startle” response in mice. While the sensitivity of this test to many drugs is yet to be established, benzodiazepine and serotonergic anxiolytics have been effective in reducing the startle response [1]. Since this model seems to be unaffected by motor phenotypes, activity levels, or neurological deficits, this test (unlike many other anxiety models discussed here) allows researchers to study mouse anxiety without these confounding factors (see Note 17 ).
-
1.
In a conditioning trial, a conditioned stimulus (usually a light: e.g., 15 W) is paired with a footshock. The timing of the conditioned stimulus and foots.hock can be controlled by the data acquisition software for consistency [7].
-
2.
In a separate trial 24 h later, the animals are presented with a startle stimulus (e.g., loud noise 70–80 db, or air puff) and their activity is recorded as a baseline. The startle stimulus can be presented in 4 blocks of 5 startles each, with 30–35 s between each startle stimulus [7]. Peak and amplitude of the startle response can be recorded (e.g., using a piezoelectric accelerometer) and digitized [22].
-
3.
24 h later in testing trials, the conditioned stimulus is displayed immediately prior to the startle stimulus, and the observed response is compared to the baseline startle response. Stimuli should be presented when the animal is quiet and inactive [7] (see Notes 18 and 19 ).
3.10 Social Interaction Test
The social interaction test is a useful drug-sensitive approach to assessing anxiety in mice, subjected to the apparatus similar to the OFT. There are four testing conditions which introduce varying levels of stress: (1) familiar test apparatus and low illumination; (2) familiar test apparatus and high illumination; (3) unfamiliar test apparatus and low illumination; and (4) unfamiliar test apparatus and high illumination. The level of anxiety across these conditions ranges from low to high, respectively. Overall, the duration and frequency of social interactions negatively correlate with anxiety. Because this test successfully isolates levels of anxiety (high vs. low) in the subjects, it has been used for pharmacological screening of both anxiolytic and anxiogenic drugs in their effectiveness for increasing or decreasing social interaction, respectively [1].
-
1.
The test environment should be the same in all conditions, except for the test apparatus (familiar or unfamiliar) and the lighting (low or high illumination).
-
2.
Introduce the two mice into the test environment for 5 or 10 min, recording the duration and frequency of all social interactions (e.g., sniffing, following, chasing, touching, and biting). All behavioral endpoints mentioned should be recorded, including the frequency (number of bouts) and duration (cumulative of all bouts per trial). The best way would be to use a video tracking system with either automatic or manual scoring, although it is possible for the observers to record these behaviors manually in real time.
-
3.
After obtaining baseline data for each condition, administer an anxiolytic or anxiogenic drug to the mice. The same test (step 2) can be conducted and analyzed relative to baseline data. Alternatively, compare drug-treated with saline-treated groups (only one animal in the interacting pair receives the drug) (see Notes 20 – 22 ).
3.11 Suok Test
The Suok test simultaneously examines anxiety, vestibular, and neuromuscular deficits by combining an unstable rod with novelty. To analyze anxiety, the threats of height, loss of balance, and novelty are presented and animal exploration is recorded. Anxiolytic or anxiogenic drugs will increase or decrease animal exploration, respectively. Risk assessment and vegetative behaviors are generally higher in anxious mice. The model is also sensitive to anxiety-evoked balancing deficits, since administration of anxiogenic drugs increases the number of falls and missteps, while anxiolytics generally improve balancing [23, 24]. A light-dark modification of the test may also be employed, as the illuminated environment will represent an additional stressor. We recommend reviewing the Mouse Phenome and Mouse Genome Informatics Databases for examples of anxious mouse strains, mice displaying low motor or vertical activity, hyperactive mice, and mice with sensory deficits or mouse strains with vestibular difficulties. Researchers should easily find mice that fit their specific experimental needs.
-
1.
Place individual mice in the center of the apparatus facing either end (or, in the light-dark modification, orient the animal facing the dark end) (see Note 23 ).
-
2.
Standing approximately 2 m away from the apparatus, record the following behavioral measures (for 5–10 min per animal): horizontal exploration activity (latency to leave central zone, number of segments visited with four paws, distance traveled, number of stops, time spent immobile, average inter-stop distance, number of stops near border separating light-dark areas of the apparatus), vertical exploration (number of vertical rears or wall leans), directed exploration (head dips, side looks), risk assessment behavior (stretch-attend postures), vegetative responses (number of defecation boli and urination spots), and vestibular/motor indices (number and latency of hind-leg slips and falls from rod). If the animal falls, replace it in the same position from where it fell (see Notes 24 and 25 ).
3.12 Light-Dark Box Test
This ethological model of anxiety measures the activity and time spent in brightly lit vs. dark compartments of the apparatus, and is based on the animal’s innate desire to explore novel areas [5, 16]. Anxious mice exhibit a profound preference for the dark area and display fewer exploratory behaviors (e.g., horizontal activity, vertical rears, or wall leans) in the light. Increased duration of time spent in the light area and more exploratory behaviors can be seen following anxiolytic drug administration.
-
1.
Place one animal into the dark compartment of the box for 5 min for acclimation
-
2.
Lift the shutter to allow the mouse to move freely between the dark and light compartments for 5 min.
-
3.
Measure the latency to initial transition into the light box. Record the duration of time spent in each compartment, the number of transitions between them, and the distance traveled in each box. Additional indices may be vertical rearing, wall leans (in the light compartment), and the number of defecation boli (see Note 26 ).
3.13 Stress-Induced Hyperthermia (SIH)
This test relies on the evolutionarily important role of hyperthermia, a rise in body temperature upon encountering stressful stimuli which occurs across many species, including humans. In mouse SIH test, the insertion of a rectal thermometer records a 0.5–1.5 °C increase in body temperature (see Note 27 ). SIH is reduced or prevented by different anxiolytic drugs ; however, it seems to be unable to detect anxiogenic and antidepressant effects [25].
-
1.
Animals should be put in individual cages the day before testing to avoid effects of acute isolation stress (see Note 28 ).
-
2.
Baseline body temperature should be recorded. To test mouse rectal temperature, carefully insert a probe with a rounded tip (up to 3 mm thick) after dipping it in any kind of oil for lubrication. For example, use surgilube sterile surgical lubricant by Fougera & Co. (Melville, NY, USA), K-Y lubricant by Johnson & Johnson (Waltham, MA, USA), and MLT1404 rectal probe for mice by Adinstruments, Inc (Colorado Springs, CO, USA). The probe should be inserted consistently, approximately 2–2.5 cm for 10 s (see Note 29 ).
-
3.
Present the mouse with a stressor, such as a novel cage, and document the change in internal temperature (see Note 30 ).
-
4.
After testing, mice may be re-socialized in grouped housing . They may be retested after in 1-week intervals. Typically, 10–15 mice per group are sufficient to observe significant effects [38] (see Note 31 ).
-
5.
It is also possible to use an implanted temperature sensor to monitor temperature remotely, and without using this type of invasive measurement. For example, microchip transponders (ELAMS, BioMedic Data Systems, Inc., Seaford, DE, USA) have been shown to reliably monitor temperature without significant difference from rectal temperature measurements [39].
3.14 Hole-Board Test
Conceptually similar to the open field test (OFT), the hole-board test focuses on specific head dipping behaviors. Head dipping, an indication of directed exploration, can be vigorously affected by various drug classes, including anxiolytic and anxiogenic drugs . Due to its short duration and quantifiable behavioral measures, this test is a readily available method for the testing of classic or novel drugs [26]. Place mice individually in hole-board apparatus and record behavior for 5–15 min, documenting traditional exploratory behaviors (as in the OFT, see above) and the number of head dips (see Notes 7 , 8 , and 32 ).
3.15 Rat Exposure Test
This test utilizes the natural defensive “avoidance” behavioral response of mice to signs of potential danger, such as a natural predator (e.g., rat ). Defensive behaviors include stretch-attend posture, stretch approach, freezing, burying, and hiding, and are measured as a function of risk assessment. This test has proven useful to determine strain differences in defensive behaviors and relative levels of anxiety in response to predators (see Note 33 ). Additionally, the defensive behaviors measured are sensitive to anxiolytics, making this paradigm useful in pharmacological screening [27].
-
1.
Introduce the mouse into the small black box, which will serve as a “home chamber” (safe environment). The Plexiglas tunnel should allow free movement between the home chamber and the observation box.
-
2.
On the first 3 days of testing, allow the mouse to explore the observation box for 10 min to become familiar with the environment. In these sessions, there should be no rat present.
-
3.
On the fourth day, insert the mouse into the home chamber and the rat into the observation box for 10 min. The rat should be placed in the opposite side of the cage, isolated from the mouse by the wire mesh.
-
4.
In every testing session, record the number of stretch-attend postures, stretch approaches, freezes, and number of times the mouse retreats to the home chamber. Also, measure the amount of time spent in the home chamber and observation box, as well as time in contact with the wire mesh.
3.16 Novel Object Test
This model investigates the approach-avoidance behaviors of mice in response to novel stimuli. Typically, mice tend to explore a novel object longer than a familiar one, and prior exposure to a stimulus increases consecutive approach behavior and decreases avoidance behavior. This robust behavior, as well as the simplicity of this model, makes this test particularly useful for measuring anxiety in a battery of tests [28]. Anxiolytics have been shown to increase exploratory behavior of mice in novel environments [41], suggesting that the use of anxiolytics would similarly increase this behavior with novel objects.
-
1.
On Day 1, introduce the mouse into the test apparatus, allowing it to explore the environment for 30–60 min.
-
2.
On testing day, insert the novel object into the center of the testing apparatus prior to introducing the mouse. Record the frequency and duration of exploratory behavior, such as approaches, sniffing, physical contacts (e.g., touching, licking, biting), wall leans, vertical rears, head dipping, and time spent near the novel object, for 10–30 min. Also record amount of avoidance behavior as time spent in the perimeter.
-
3.
Any video tracking system (see Table 3 for details) may be useful for measuring amount of movement and position within the test apparatus. Conversely, the apparatus may be sectioned off, and duration in each section can be recorded, comparing the perimeter sections to the novel object section [28] (see Note 34 ).
Table 2 Potential combinations of emotional and cognitive phenotypes [29] Table 3 Examples of video tracking manufacturers
4 Notes
-
1.
As genetic background greatly influences behavioral phenotypes in mice, comparative studies must take this into account. The use of inbred mice substantially decreases within-subject variation, and also provides valuable insight into the neurobiological mechanisms that modulate specific phenotypes. The most updated detailed nomenclature for mouse strains should be used, and mice should be obtained from certified vendors or other reliable sources to ensure comparability of results between laboratories. In contrast, outbred mice do not seem to present similar benefits, and therefore, may yield more confounded results.
-
2.
Age, gender, and strain differences affect Elevated Plus Maze (EPM) performance. Young females generally spend less time on the open arms than males, although this varies with the estrous cycle. Pro-estrus rodents spend significantly more time on open arms than di-estrus females (or male mice) [9].
-
3.
All experimental procedures (including handling, housing , husbandry , and drug treatment) must be conducted in accordance with national and institutional guidelines for the care and use of laboratory animals.
-
4.
Since lighting can affect behavior in the maze, make sure it is consistent on all arms. Red light in a darkened room is preferable, as mice cannot see red light. To avoid excessive freezing, testing environment should be kept quiet without disruptions. If the mouse freezes for more than 30 % of the total test time, researchers should note of this abnormality, but continue testing. In case of unexpected or loud noises or other disruptions, the data should be discarded from analyses.
-
5.
As some mice may fall off an open arm of the EPM , the data from these animals must be excluded from further analyses.
-
6.
In some cases, reduced anxiety can be mistaken for hyperactivity. A minute-by-minute analysis of exploration and activity may aid in distinguishing these two different domains [29].
-
7.
It is important not to misinterpret reduced locomotion due to high habituation as an anxiogenic response (see Table 2 for details). The mouse learning/memory phenotypes should be assessed in separate tests [29]. If mice have poor habituation, this may result in increased “exploration” that should not be misinterpreted as decreased anxiety [29]. Consider testing mouse cognitive functions in a separate study.
-
8.
Mice with altered motor, vestibular, neurological, memory , or depression domains may need additional screening before use in the OFT. Variable aged ranges may be used, but all mice should be tested at the same age in a comparative experiment.
-
9.
Some strain differences are apparent in digging behaviors . Slow or inactive mouse strains (e.g., 129S1/SvImJ) may be replaced with more active strains (e.g., C57BL/6J) to achieve recordable amounts of burying data. It has been observed that younger mice (2–4 months old) tend to show enhanced digging behaviors as opposed to mice over 1 year old [18].
-
10.
If mice continue to display low burying activity, it may be useful to assess the environment for confounding factors. Unnecessary noise or stress should be eliminated and mice should be undisturbed throughout the experiment. Testing on cage-cleaning days may also cause mice to be less responsive to the new bedding [18].
-
11.
Some strains with low burying/digging activity may require a longer (e.g., 45–60 min) testing time that may help reveal their phenotype .
-
12.
Troubleshooting is the same as in the marble burying test .
-
13.
In choosing a proper mouse strain for testing, it is important to consider possible motor and sensory deficits. For example, C57BL/6J strain is widely used as it has relatively no deficits in these domains and is sensitive to drug and behavioral testing .
-
14.
Abnormally high grooming activity may be due to a strain-specific compulsive-like phenotype (consider using a more appropriate strain), or due to unintended stress in the animal facility (which may be assuaged by improved husbandry or enrichment).
-
15.
High baseline or transfer anxiety may lead to unusually low-grooming activity. This may be alleviated by using smaller observation boxes and dimmer lighting, as well as by improving handling techniques and lengthening acclimation time. Reduced grooming activity may also be due to a strain-specific low-grooming phenotype (e.g., due to abnormal neurological/vestibular/motor phenotype) or overall inactivity of the stain being tested.
-
16.
Detection of different stages of grooming behavior may sometimes be difficult. If using a video camera, replaying in slow motion will make the detection of transitions and interruptions much easier.
-
17.
If the startle stimulus is auditory, some mouse strains may be insensitive to this test because of hearing deficits which can also be age related (e.g., C57BL/6J mice have a progressive hearing with onset after 10 months of age). To rule out this possibility, mice should be tested for hearing problems. If the mouse strain shows abnormally poor hearing, consider using a physical startle stimulus (e.g., air puff or bright light) or a different strain.
-
18.
If the mouse does not show a heightened response to the startle stimulus in the testing trials, it may have cognitive deficits. Memory should be examined in separate, specific tests to ensure accurate data interpretation.
-
19.
C57BL/10J and FVB/NJ strains have high startle responses and 129S1/SvImJ mice have low startle responses, whereas BALB/cJ and C57BL/6J strains have more moderate responses [35]. Some animals may show an abnormally high startle response as a result of brain pathological overexcitation, and this abnormality should be investigated further. Also, consider baseline brain activity as well when administering drugs. For example, due to the floor/ceiling effect, anxiogenic drugs can be tested on mice with a low baseline startle response , whereas anxiolytics would yield clearer results if tested on mice with high baseline responses. Review literature for drug efficacy and concentrations.
-
20.
Some mice display particularly high levels of social interaction, including FVB and C57BL/6J [36]. Certain strains may be more likely to engage in social interaction because of their high sociability phenotype (which may be unrelated to their anxiety or emotionality profile per se). In this case, consider using other strains for this test. Low levels of social interaction may occur with the spontaneous deletion of the Dtnbp1 gene, leading to social withdrawal [37], or in some inbred mouse strains, for example, A/J, BALBcByJ, and BTBR T+ tf/J mice [36].
-
21.
Thus, the use of some strains should be avoided as their autism-like behavior may prevent the relevance of this test as a model of anxiety. In performing the social interaction test for screening anxiolytic and anxiogenic drugs , it is suggested that the same two mice are not re-introduced into the same environment together, as this may eliminate the social novelty of the condition, and will affect their test performance.
-
22.
Mice with abnormally poor or abnormally good cognitive abilities may produce aberrant behavior in this test (e.g., increased or decreased social interaction, respectively). To rule out this possibility, consider testing mice in some additional memory paradigms. Memory tests, such as the Morris Water Maze and OFT habituation, may be performed to assess cognitive functions in any abnormally behaving mouse.
-
23.
Low motor or vertical activity may be a strain-specific phenotype. Inactive strains will produce less activity overall, and may not be suitable for this model. Likewise, hyperactive strains generally display less non-horizontal exploration and may have difficulties with balance. A narrower apparatus will encourage the animal to show less horizontal activity, enabling it to focus on other behavioral responses . Differences in mouse size should also be addressed. Use animals of similar size, age, and weight to accurately compare between groups.
-
24.
If the mouse displays abnormally high transfer anxiety, gently support it for approximately 5 s to facilitate a solid grip. If the animal continues to display high transfer anxiety, exclude it from the experiment. A dimly lit experimental room may help reduce anxiety.
-
25.
Some strains have difficulties balancing on an aluminum rod, and a more textured surface (e.g., wood) may help stabilize the animal. Increasing the diameter of the rod is another possible solution. If mice continue to struggle with balance or motor abilities, assess motor and vestibular functions separately as these behaviors may be due to a neuromuscular or motor coordination problem unrelated to vestibular deficits or anxiety.
-
26.
Certain strains of mice may be less inclined to explore the test environment, such as mice with anxiety- or depression-like phenotypes (see Mouse Phenome and Mouse Genome Informatics Databases for details). Allow a longer acclimation and/or test time (e.g., 10 or 15 min) to reduce this factor. Some mouse strains (e.g., many albino mice) display visual deficits, and may not be a suitable model for this test. Consider other mouse strains for the light-dark box testing .
-
27.
While most strains respond consistently to this paradigm due to its independence from motor activity, specific mouse strains (e.g., FVB/N) have considerably higher baseline body temperatures, and should be avoided in this model [25]. However, this procedure has been shown to effectively induce hyperthermia to varying degrees in all inbred and outbred strains tested [40], and is also an effective indicator of stress in genetically modified animals [38].
-
28.
In the group-housed mice, the last mice to be tested show an increase in body temperature (compared with the first mice) due to anticipatory anxiety. Therefore, animals should be tested individually, with at least 10 mice in each experimental group [25].
-
29.
Temperature measurements should be performed at the same time due to circadian rhythm. Baseline body temperature is significantly higher during the night. If testing occurs during the dark phase, there may be an interference with the amplitude of hyperthermia when the stressor is presented [25].
-
30.
Mice should be kept undisturbed before the experiment with proper handling and opening of cages to ensure accurate results.
-
31.
The above protocol may be modified for the testing of anxiolytic drugs . Sixty minutes prior to the first temperature measurement, inject the mouse with the desired drug. The first temperature measurement serves as an acute stressor, and is followed after 10 min with a second temperature measurement [38].
-
32.
Certain drugs (e.g., ethanol) are known to be strain-dependent in their effects and may not produce consistent results in the hole-board test . Many commonly used drugs (e.g., fluoxetine) have pronounced dose-dependent effects on head dipping behavior , and therefore, dosing should be carefully considered; see review in [26].
-
33.
Certain mouse strains (e.g., 129S1/SvImJ or BALB/cJ) may not be useful in this test due to their hypoactivity and/or high anxiety phenotypes. If the mouse tested is very inactive and anxious, it may not even leave the home chamber, and this test will not work. In this case, use a milder stressor, such as an anesthetized rat, a toy rat , or rat odor. However, it may also be recommended to use a different mouse strain. Although this test is very useful for comparing defensive behaviors between mouse strains, some strains are not suitable for this test. For example, mice with sensory deficits (e.g., poor vision or olfaction) or with particular cognitive problems (e.g., poor working memory ) will not provide reliable data in this paradigm. As mentioned above, it may help to check online mouse databases for selecting an appropriate mouse strain.
-
34.
Similar to some other previously described tests, mouse strains with sensory or cognitive deficits may not provide reliable data in this model. In addition, some mice can exhibit strong neophobia, which would also confound behavioral data. Test mice prior to this experiment to screen for such defects, and consider using alternate strains and/or extending the observation time.
References
Warnick JE, Sufka KJ (2008) Animal models of anxiety: examining their validity, utility, and ethical characteristics. In: Kalueff AV, LaPorte JL (eds) Behavioral models in stress research. Nova Biomedical Books, New York, pp 55–71
Flint J (2003) Animal models of anxiety and their molecular dissection. Semin Cell Dev Biol 14:37–42
Ohl F (2005) Animal models of anxiety. Handb Exp Pharmacol 1:35–69
Sousa N, Almeida OF, Wotjak CT (2006) A hitchhiker’s guide to behavioral analysis in laboratory rodents. Genes Brain Behav 5(Suppl 2):5–24
Borsini F, Podhorna J, Marazziti D (2002) Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 163:121–141
De Boer SF, Koolhaas JM (2003) Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol 463:145–161
Falls WA, Carlson S, Turner JG, Willott JF (1997) Fear-potentiated startle in two strains of inbred mice. Behav Neurosci 111:855–861
Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P (2007) Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc 2:2538–2544
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2:322–328
Deacon RM (2006) Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc 1:936–946
Martinez JA, Francis G, Liu W, Pradzinsky N, Fine J et al (2008) Intranasal delivery of insulin and a nitric oxide synthase inhibitor in an experimental model of amyotrophic lateral sclerosis. Neuroscience 157(4):908–925
Ito N, Nagai T, Oikawa T, Yamada H, Hanawa T (2008) Antidepressant-like effect of l-perillaldehyde in stress-induced depression-like model mice through regulation of the olfactory nervous system. Evid Based Complement Alternat Med 2011:512697
De Souza Silva M, Topic B, Huston J, Mattern C (2008) Intranasal dopamine application increases dopaminergic activity in the neostriatum and nucleus accumbens and enhances motor activity in the open field. Synapse 62:176–184
Buddenberg TE, Topic B, Mahlberg ED, de Souza Silva MA, Huston JP et al (2008) Behavioral actions of intranasal application of dopamine: effects on forced swimming, elevated plus-maze and open field parameters. Neuropsychobiology 57:70–79
Hagenbuch N, Feldon J, Yee BK (2006) Use of the elevated plus-maze test with opaque or transparent walls in the detection of mouse strain differences and the anxiolytic effects of diazepam. Behav Pharmacol 17:31–41
Karl T, Duffy L, Herzog H (2008) Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci 28:173–180
Archer T, Fredriksson A, Lewander T, Soderberg U (1987) Marble burying and spontaneous motor activity in mice: interactions over days and the effect of diazepam. Scand J Psychol 28:242–249
Deacon RM (2006) Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc 1:122–124
Nicolas LB, Kolb Y, Prinssen EP (2006) A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur J Pharmacol 547:106–115
Njung'e K, Handley SL (1991) Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav 38:63–67
Mikics E, Baranyi J, Haller J (2008) Rats exposed to traumatic stress bury unfamiliar objects—a novel measure of hyper-vigilance in PTSD models? Physiol Behav 94:341–348
Halberstadt AL, Geyer MA (2008) Habituation and sensitization of acoustic startle: opposite influences of dopamine D(1) and D(2)-family receptors. Neurobiol Learn Mem 92(2):243–248
Kalueff AV, Keisala T, Minasyan A, Kumar SR, LaPorte JL et al (2008) The regular and light-dark Suok tests of anxiety and sensorimotor integration: utility for behavioral characterization in laboratory rodents. Nat Protoc 3:129–136
Kalueff AV, Tuohimaa P (2005) The Suok (“ropewalking”) murine test of anxiety. Brain Res Brain Res Protoc 14:87–99
Bouwknecht JA, Olivier B, Paylor RE (2007) The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev 31:41–59
Kliethermes CL, Crabbe JC (2006) Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacol Biochem Behav 85:57–65
Yang M, Augustsson H, Markham CM, Hubbard DT, Webster D et al (2004) The rat exposure test: a model of mouse defensive behaviors. Physiol Behav 81:465–473
Powell SB, Geyer MA, Gallagher D, Paulus MP (2004) The balance between approach and avoidance behaviors in a novel object exploration paradigm in mice. Behav Brain Res 152:341–349
Kalueff AV, Murphy DL (2007) The importance of cognitive phenotypes in experimental modeling of animal anxiety and depression. Neural Plasticity 2007:52087
Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL (1986) Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol 126:223–229
Bruins Slot LA, Bardin L, Auclair AL, Depoortere R, Newman-Tancredi A (2008) Effects of antipsychotics and reference monoaminergic ligands on marble burying behavior in mice. Behav Pharmacol 19:145–152
Bespalov AY, van Gaalen MM, Sukhotina IA, Wicke K, Mezler M et al (2008) Behavioral characterization of the mGlu group II/III receptor antagonist, LY-341495, in animal models of anxiety and depression. Eur J Pharmacol 592(1-3):96–102
Gordon CJ (2004) Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68
Li X, Morrow D, Witkin JM (2006) Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci 78:1933–1939
Paylor R, Crawley JN (1997) Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 132:169–180
Bolivar VJ, Walters SR, Phoenix JL (2007) Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res 176:21–26
Feng YQ, Zhou ZY, He X, Wang H, Guo XL et al (2008) Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res 106(2-3):218–228
Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R et al (2003) Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol 463:117–132
Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, VanStrik R (1998) A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Laboratory Animals 32(3):260–269
Bouwknecht JA, Paylor R (2002) Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res 136:489–501
Klebaur JE, Bardo MT (1999) The effects of anxiolytic drugs on novelty-induced place preference. Behav Brain Res 101:51–57
Acknowledgements
This work was supported by the NARSAD YI Award to AVK, and by Stress Physiology and Research Center (SPaRC) of Georgetown University Medical School. AVK is the President of the International Stress and Behavior Society (ISBS, www.stressandbehavior.com). He is supported by Guangdong Ocean University, St. Petersburg State University (internal grant 1.38.201.2014) and Ural Federal University (Government of Russian Federation Act 211, contract 02-A03.21.0006).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Hart, P.C. et al. (2016). Experimental Models of Anxiety for Drug Discovery and Brain Research. In: Proetzel, G., Wiles, M. (eds) Mouse Models for Drug Discovery. Methods in Molecular Biology, vol 1438. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3661-8_16
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3661-8_16
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3659-5
Online ISBN: 978-1-4939-3661-8
eBook Packages: Springer Protocols