Abstract
Rationale
Anxiety, a negative state of high arousal and vigilance, is especially prevalent in women, making identification of underlying mechanisms critical for developing effective therapies. With the challenge of disentangling biological and social factors in humans, animal tests can provide valuable insights, although such tests, developed in males, have unclear validity for females.
Objective
To better understand patterns of sex differences across multiple measures within two classical rodent anxiety tests.
Methods
We examined female and male adult Wistar rats (n = 15–18/group) that were single-housed in the novelty suppression of feeding test (NSFT) that involves food under a bright light in food-restricted animals, and light–dark test (LDT), which reflects innate aversion to bright light. To further validate these tests in females, we also examined the impact of 1 mg/kg diazepam.
Results
NSFT measures of the most direct interaction with food, latency to grab food and food consumed, indicated increased anxiety-like behavior in females versus males, with diazepam altering these behaviors in females but not males. Most other measures showed more similar effects of diazepam across the sexes, with some evidence of reduced anxiety-like behavior in LDT for females. Principal component analyses indicated limited relationships across behavioral factors, underscoring previous suggestions of the importance of assessing multiple measures to maximize information and ethological relevance.
Conclusions
Combining our findings and previous studies, we speculate that increased anxiety-like behavior in females manifests especially when there is a specific, life-relevant condition (e.g., food in the NSFT). Our findings also validate NSFT and LDT use in females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anxiety represents a state of high arousal and negative feelings that can result in enhanced vigilance in the absence of an immediate threat (Palanza and Parmigiani 2017). Although healthy individuals experience sporadic episodes of anxiety during life, when this state is persistent, disruptive, or disproportionate, it can be become debilitating and is considered pathological (Chisholm et al. 2016). Such anxiety and mood conditions are a major cost to society, with recent estimates finding > 700 billion US$ in lost productivity globally (Chisholm et al. 2016). Epidemiological data shows that women have a higher prevalence of anxiety disorders than men (Altemus et al. 2014; Palanza and Parmigiani 2017), but it is challenging to disentangle biological and social factors (Altemus et al. 2014). Thus, animal tests could provide valuable insights into biological mechanisms. Also, anxiety is a multi-faceted construct (Bangasser and Wicks 2017; Becker and Chartoff 2019; Palanza and Parmigiani 2017), and it is considered important to assess multiple measures to comprehensively analyze potential behavioral differences and ethological relevance of rodent tests for human conditions (Olvera-Hernandez and Fernandez-Guasti 2011; Ramos 2008).
Potential sex differences in anxiety behaviors have long been studied (Becker and Chartoff 2019; Palanza and Parmigiani 2017). Reduced female anxiety-like behavior relative to males has been observed in tests such as elevated plus maze (EPM) and open field (see Donner and Lowry 2013), while greater anxiety-like behavior versus males is seen in tasks such as shocked licking in a thirsty animal (De Jesus-Burgos et al. 2016; Johnston and File 1991) and following exposure to unconditioned predator stimuli (see Albonetti and Farabollini 1995). However, there are also mixed findings, and differences in test, strain, species, age, and other factors could contribute (see Discussion). Females also often show greater locomotion and rearing than males (Le Moene et al. 2020; Palanza and Parmigiani 2017), although lower female anxiety-like behavior can persist when accounting for locomotor differences (Johnston and File 1991; Ou et al. 2019; Scholl et al. 2019). Also, effects of the classical anxiolytic benzodiazepine (BZD) diazepam suggest that some locomotor measures may also involve anxiety-like states (e.g., Bourin and Hascoet 2003). Another often-noted concern is that anxiety-like tests have mostly been tested in males, which may lead to inaccurate interpretations when examined in females (Bangasser and Wicks 2017; Becker and Chartoff 2019; Ramos 2008). Indeed, females show more active responding than males during some anxiety-like behaviors (Olvera-Hernandez and Fernandez-Guasti 2011; Scholl et al. 2019), including darting more than freezing with fear (Bangasser and Wicks 2017), less freezing and more avoidance in a shuttle box task (Lopez-Aumatell et al. 2011), and more active defensive responses under some social stressors (Albonetti and Farabollini 1995; Fernandes et al. 1999; Palanza and Parmigiani 2017; Shepherd et al. 1992). Thus, anxiety-like states could increase activity in females under some conditions, perhaps akin to the need to judge danger near a nest, rather than restrain/reduce activity as in males (Becker and Chartoff 2019; Palanza and Parmigiani 2017). Thus, while findings overall indicate sex differences, the presence of mixed findings indicates that additional studies are needed, and several groups (e.g., Olvera-Hernandez and Fernandez-Guasti 2011; Ramos 2008) have underscored the importance of assessing multiple measures in each task in order to give maximal information about the specific aspects of anxiety-like responding that differ between the sexes.
Here, we examined single-housed animals, as social isolation is recognized as a stressful factor in both sexes for humans (Martin and Brown 2010; Mead et al. 2010) and rodents (Albrechet-Souza et al. 2020; Palanza et al. 2001). Furthermore, isolation increases anxiety-like behavior relative to group housing in females (which show less anxiety-like responding than males), with no or opposite effects in males (Palanza et al. 2001). Isolation can also increase female but not male anxiety-like behavior in EPM (Abramov et al. 2004), and increase stretch postures in female but not male mice (Palanza et al. 2001). In humans, isolation contributes to multiple mood disorders, and women report greater concern about isolation (Martin and Brown 2010; Mead et al. 2010, but see Heinrich and Gullone 2006). Thus, isolation can increase anxiety-like behavior in humans and rodents, with evidence for greater anxiety-like responses in some measures for females (see also Discussion).
Based on the previously described findings, we hypothesize here that sex differences may involve differential responses to particular aspects of anxiety-like tests. To address the need to better understand the nature of sex differences in expression of anxiety-like behavior, we examined a number of within-task measures in female and male adult Wistar rats in two classical rodent anxiety-like tests, the novelty suppression of feeding test (NSFT) and light–dark test (LDT). The NSFT measures conflict between the natural tendency to feed after food restriction and the ethologic aversion to novel, brightly lit, and central places, while the LDT measures the innate aversion to bright light. Both tests are validated by their sensitivity to chronic SSRIs which are effective treatment for many human anxiety disorders (Ramaker and Dulawa 2017), where anxiolysis after chronic (but not acute) SSRIs is seen for both NSFT (Bodnoff et al. 1989; Oh et al. 2009; Santarelli et al. 2003) and LDT (Vicente and Zangrossi 2014). Furthermore, limited studies have compared male and female responses to anxiolytics, and thus, we also examined the impact of a moderate dose of diazepam, since BZDs impact both NSFT and LDT (Bodnoff et al. 1989; Shephard and Broadhurst 1982). By assessing multiple measures within each test, we find specific sex differences in responses under control and diazepam conditions in particular measures (including direct interaction with food in NSFT), while many other measures showed limited sex differences in diazepam effects. Together, our studies provide novel and useful information regarding specific patterns of sex differences in expression of anxiety-like behavior, laying a foundation for future work to help elucidate underlying mechanisms and facilitate development of novel translational strategies.
Methods
Animals
All procedures were conducted in accordance with Guide for Care and Use of Laboratory Animals provided by National Institutes of Health and approved by Institutional Animal Care and Use Committee of University of California San Francisco. All efforts were undertaken to reduce number of animals needed and minimize pain and suffering.
All studies used 5–6-month-old rats, which were singly-housed from ~ 2 months old. Rats were maintained on 12–12-h light/dark cycle (lights off 10:00 A.M.–10:00 P.M.) with food and water available ad libitum, except during NSFT (see below). Female and male rats were offspring from a colony with heterozygous Wistar/Crh-Cre rats (Pomrenze et al. 2015) outcrossed to Wistar (Envigo), with use of equal number of wild-type Wistar and heterozygotes. Females and males were from 9 and 10 litters total, respectively, with 3.8 ± 0.2 females and 3.6 ± 0.4 males per litter (range 2–5). No other manipulations were performed on these animals except behavioral testing, with no significant differences between genotypes (not shown); thus, results are combined.
Behavior tests: General
The overall rationale for NSFT and LDT arises from rodents’ innate aversion to brightly illuminated areas (Acevedo et al. 2014; Bourin and Hascoet 2003). NSFT involves conflict between bright light and food in a food-restricted animal. The LDT has two compartments, one lit and one dark, with an opening between, and involves conflict between exploration during mild stressor and avoiding lit areas and neophobia more generally (Bourin and Hascoet 2003).
Results are from two large, approximately equal cohorts, performed March–April (38 rats, 18 females) and October–November (32 rats, 16 females), to obviate concerns about season-related differences (Lopez-Aumatell et al. 2011). Rats had balanced numbers of drug versus vehicle and male versus female run concurrently as much as possible within each cohort, to alleviate any possible litter effects. NSFT sample sizes were 15 female-saline from 9 litters, 16 female-diazepam from 9 litters, 15 male-saline from 9 litters, and 18 male-diazepam from 9 litters. Open field had only 15 female-diazepam. LDT sample sizes were 16 female-saline from 8 litters, 17 female-diazepam from 8 litters, 17 male-saline from 9 litters, and 18 male-diazepam from 10 litters. One week before starting experimental sessions, rats were gently handled, once/day for ~ 5 min, to familiarize with experimental conditions and reduce non-specific stress responses during testing. In addition, rats were acclimatized to the testing room 1 h/day for 5 days before testing, and with at least 30 min in the testing room before tests on test days. All tests were in the dark cycle (between 10:30 A.M. and 5:00 P.M.), with behavior room illuminated by ceiling red lamps. Maximum 5 rats/day were tested in NSFT-open field, and 7/day in LDT, to allow testing at approximately the same circadian period across days. Males and females were tested separately on alternate days. Due to some technical failures with the camera and other factors, only one day’s experiments were measured for some animals. Each behavioral apparatus was cleaned between animals with 0.025% bleach and allowed 5 min to air dry.
Finally, we were more interested in NSFT, with the specific point of conflict (food versus light), but one limitation is that we did not counterbalance NSFT and LDT tests across rats, and we cannot rule out that NSFT testing impacted LDT.
Novelty-suppressed feeding test (NSFT) and open field
Rats received 3 days of food restriction (Blasco-Serra et al. 2017). Food intake was first averaged across five 24-h periods in each individual, which was later used to give rats 80% normal intake in two food restriction days, then 20% normal intake the third day. NSFT testing was the following day in a square arena (60 × 60 × 40 cm, black plastic) with a bright light shining at the center, targeting ~ 3 g food on a 3-inch circular white paper (160 lux at center, < 60 lux along chamber edges) (Gobinath et al. 2018; Olivier et al. 2008). After saline/diazepam injection (see Reagents), animals were placed in the corner and allowed 15 min to explore, filmed by video camera.
After NSFT, the rat was returned to home cage for 5 min, and then placed in open field (the same NSFT arena except with dim red light and no food). Rats were placed in the center and movement video-recorded for 10 min. Total distance and average velocity were determined with EthoVision XT10 (Noldus, Netherlands).
After open field, rats were returned to home cage and allowed ad libitum consumption, with intake level measured after 30 min.
Light–dark test (LDT)
One week after NSFT, rats were exposed to the LDT apparatus (Amodeo et al. 2018; Vicente and Zangrossi 2014), with two equal-sized (41 × 41 × 41 cm) chambers, one black (with black lid) and one white (with 100 lux light and clear lid), connected by an 8 × 8 cm opening between chambers at floor level. After saline/diazepam injection (see Reagents), rat was placed in middle of the lit compartment facing away from the doorway and allowed to explore for 10 min, with sessions video-recorded.
NSFT and LDT measures
NSFT and LDT analyses were performed blinded and scored by eye (Bourin and Hascoet 2003). In NSFT, latency to grab food (Blasco-Serra et al. 2017) is widely measured (often called latency to feed) and validated as a measure of anxiety-like behavior by anxiolysis after chronic SSRIs or acute diazepam (Bodnoff et al. 1989; Santarelli et al. 2003; Shephard and Broadhurst 1982). We used latency to grab food instead of latency to feed since many animals moved to the center, grabbed the food, then dragged it to the edge where feeding occurred. We also examined total food consumed during test, where lower intake, indicating greater anxiety-like response, can be increased by diazepam (Shephard and Broadhurst 1982). Furthermore, we measured home cage intake after NSFT testing, since this is often used as a control for ability to eat in non-anxiety-provoking conditions, as it shows no changes by anxiolytics (Blasco-Serra et al. 2017; Santarelli et al. 2003). In addition, we assessed time in center (central 20 × 20 cm), and number of approaches to center (of which > 90% did not involve grabbing food), which could be reduced under greater light-induced anxiety-like response. Finally, non-test-day intake, averaged across 5 days, was assessed before the food restriction period.
In LDT, latency to move into dark chamber, latency to re-enter lit chamber, and total time in lit chamber are considered to reflect anxiety-like behavior through avoidance of light (Acevedo et al. 2014; Amodeo et al. 2018). Entry into a given chamber was indicated by placing four paws in a compartment (Acevedo et al. 2014). In addition, number of transitions and rearings were measured and interpreted as activity and exploration, although they can also reflect anxiety-like behavior (Acevedo et al. 2014; Griebel et al. 1997). Finally, we examined stretched postures at the door between chambers, which have been considered risk assessment responses (Griebel et al. 1997; Shepherd et al. 1992).
Reagents
Rats were injected i.p. 30 min before testing sessions with vehicle (saline) or 1 mg/kg diazepam (Hospira, INC, Lake Forest, IL), considered a moderate dose that provides anxiolytic-like effects while minimizing locomotor effects (Chaouloff et al. 1997; Griebel et al. 1997; Olvera-Hernandez and Fernandez-Guasti 2011).
Statistics and analyses
Our primary analyses used two-way analysis of variance (ANOVA) tests, with sex and drug treatment as independent, between-subject factors, using SPSS v27. Post hoc effects were determined in GraphPad Prism using t-tests, or Kolmogorov–Smirnov (KS, indicated where used) for non-normal data. Bonferroni correction for 6 possible post hoc multiple comparisons yielded a significance threshold of p < 0.0083. All data are shown as mean ± standard error of the mean. Principal component analyses (PCA) were performed separately for the seven NSFT and six LDT measures in SAS v9.4 utilizing the correlation matrix (Kosobud et al. 2015), using data from animals where all measures were acquired.
It would also be quite interesting to compare within-animal baseline responses in the NSFT with those in the LDT. However, in our design, animals received a single NSFT test, with vehicle or diazepam, and a single LDT test, with vehicle or diazepam, but animals were randomized for vehicle/diazepam in NSFT then separately randomized for vehicle/diazepam in LDT. Thus, fewer rats received vehicle in both NSFT and LDT, and thus, the correlation of NSFT and LDT behavior was insufficiently powered. This limitation could be addressed in future studies where no animals receive diazepam.
We also note it would be optimal to test multiple diazepam doses, and in a within-animal design. Unfortunately, our preliminary results found that repeated testing of NSFT within an animal changed behavior across repeated testing (also seen with LDT). With similar effects for Elevated Plus Maze, many groups only use a single EPM test in a given animal (with or without experimental intervention) (e.g., Gonzalez and File, 1997; Pereira et al. 1999). The need for a single NSFT (or LDT) test is part of the reason we tested a single diazepam dose, picking a more moderate dose which has been widely examined (see above), allowing us to retain large sample sizes per group.
Results
Figure 1 shows a timeline schematic for behavioral tasks. Data were primarily analyzed with two-way ANOVA, with sex and drug treatment as the two between-subject factors.
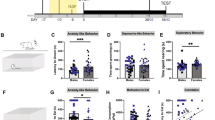
Timeline schematic for behavioral tasks. All rats were 5–6 months old, after single housing since ~ 2 months old, and thus potential effects of differences in animal age should have limited impact for our findings. During the testing period, 30 min after diazepam or vehicle injection, rats were tested in NSFT for 15 min. Rats were removed to home cage for 5 min, then placed in open field (OField) for 10 min, then returned to the home cage for 30-min feeding assessment. One week later, 30 min after diazepam or saline injection, rats were tested in LDT for 10 min. DZP, diazepam; sal, saline
Novelty suppression of feeding task
NSFT involves conflict between bright light and drive to eat in a food-restricted animal. Interestingly, two measures more directly related to interaction with food showed significant sex differences and sex-drug interactions. First, latency to grab food, where longer latency can indicate greater anxiety-like behavior (see Methods), showed significant effects of drug and a sex-drug interaction (Fig. 2A) (sex: F(1,64) = 0.560, p = 0.457; drug: F(1,64) = 23.153, p < 0.001; interaction: F(1,64) = 4.959, p = 0.030). Diazepam significantly reduced latency to grab food in females (p < 0.001 KS) but not males (p = 0.067 KS), and latency in saline-treated animals was slower in females versus males (p = 0.003 KS). Females thus exhibited increased anxiety-like behavior under saline, expressed as delayed approach to grab food, and greater diazepam impact.
Significant interactions of sex and drug in NFST for measures most directly related to food interaction. A Latency to initially grab food was significantly longer under saline in females than males, and significantly reduced by diazepam in females but not males. B Food consumed during the test period under saline was significantly lower in females than males, and increased by diazepam in females but not males. DZP, diazepam; Sal, saline vehicle. *** indicates p < 0.001 for diazepam within a particular sex; ##, ### indicate p < 0.01, p < 0.001 across sexes for a given treatment condition (saline or drug)
Another measure of food interaction, food consumed during test, showed a similar pattern (Fig. 2B), with significant effects of sex, drug, and sex-drug interaction (sex: F(1,64) = 13.437, p = 0.001; drug: F(1,64) = 13.966, p < 0.001; interaction: F(1,64) = 4.130, p = 0.047). Females ate significantly less food than males under vehicle conditions (p < 0.001), and diazepam increased food consumed in females (p < 0.001), with no effect on males (p = 0.293). Thus, similar to latency to grab food, females displayed greater food intake-related anxiety-like behavior, and diazepam only impacted female consumption.
In contrast to latency to grab food and food consumed during test, other NSFT measures indicated more similar patterns in females and males. Time in center had only a significant effect of drug (Fig. 3A) (sex: F(1,64) = 0.997, p = 0.322; drug: F(1,64) = 20.947, p < 0.001; interaction: F(1,64) = 0.013, p = 0.911), with a significant post hoc effect of diazepam in both sexes (female: p < 0.001; male: p = 0.008). Thus, female and male rats had similar anxiety-like behavior, and a similar diazepam reduction, in the total time spent in the brightly lit center of the NSFT apparatus.
Putative anxiety-like NFST measures with more distal interaction with food showed less clear sex differences. A Total time spent in the brightly lit center was similar under saline in females and males, and was similarly and significantly enhanced by diazepam in both sexes. B Number of approaches to the center was not significantly different between sexes under saline or diazepam, although diazepam only significantly reduced number of approaches in males. DZP, diazepam; Sal, saline vehicle. **, *** indicate p < 0.01, p < 0.001 for diazepam within a particular sex
A related pattern was observed for number of approaches to center (where animals move to center but largely do not touch the food, which is > 90% of approaches). There was a significant effect only of drug (Fig. 3B) (sex: F(1,64) = 2.483, p = 0.120; drug: F(1,64) = 18.695, p < 0.001; interaction: F(1,64) = 2.863, p = 0.096), with a significant post hoc effect of diazepam in males (male: p < 0.001) with a trend in females (p = 0.061). Thus, females and males had similar number of center approaches under saline, with an overall diazepam effect that was significant in males.
Since many studies observe greater locomotion in females (see Introduction), distance travelled in the open field was assessed after NSFT (Fig. 1, see Methods). Females traveled more than males (Fig. 4A), with significant main effects of sex and drug (sex: F(1,63) = 64.138, p < 0.001; drug: F(1,63) = 4.805, p = 0.032; interaction: F(1,63) = 0.831, p = 0.366). Post hoc revealed no effect of diazepam in females (p = 0.1024) or males (p = 0.173), but significant sex differences under saline (p < 0.001) and diazepam (p < 0.001). The limited impact of diazepam on locomotion suggests that diazepam impacts on other, putative anxiety-like measures were unlikely to simply reflect locomotor effects (see Discussion). Females also had slower velocity (Fig. 4B), with a significant effect of sex (sex: F(1,63) = 23.295, p < 0.001; drug: F(1,63) = 0.081, p = 0.777; interaction: F(1,63) = 1.640, p = 0.205). However, it is important to note that, since open field was tested after NSFT, open field results could be impacted by carry-forward effects from NSFT testing. We consider this less likely, since diazepam showed small effects in open field, unlike what was observed in NSFT, especially food-related measures.
Overall similar locomotion across sexes, although home cage intake on test day after NSFT showed sex effects. A Total distance traveled in the open field was significantly greater in females under both saline and diazepam. B Velocity in open field was slower in females under saline, with a similar trend under diazepam. C Home cage intake after the NSFT test showed similar responding as intake during the test (Fig. 2B), with less intake in saline females, and diazepam enhancement of intake in females but not males. DZP, diazepam; Sal, saline vehicle. *** indicates p < 0.001 for diazepam within a particular sex; ### indicates p < 0.001 across sexes for a given treatment condition (saline or drug)
Many NSFT studies examine home cage intake after the NSFT test, where consumption often recovers in the more familiar context. Unexpectedly, home cage intake (Fig. 4C) showed a similar pattern as NSFT intake (Fig. 2B), with reduced intake levels and diazepam effects only in females, and significant main effects of sex, drug, and interaction (sex: F(1,64) = 25.143, p < 0.001; drug: F(1,64) = 5.448, p = 0.023; interaction: F(1,64) = 6.023, p = 0.017; post hoc p < 0.001 diazepam effect in females, p < 0.001 male versus female saline). While the reason is unclear, we speculate that anxiety-like states experienced during NSFT carried forward into the home cage (although this seemed not to be apparent in the preceding open field). Another possibility is that there were systematic differences in body weight or food consumption levels in females destined to be tested with diazepam versus vehicle. Arguing against this, body weight and home cage intake before NSFT test (see Methods) (Online Resource 1A,B) only showed significant effects of sex (weight: [sex: F(1,68) = 564.19, p < 0.001; drug: F(1,68) = 3.845, p = 0.055; interaction: F(1,68) = 0.176, p = 0.676]; consumption: [sex: F(1,68) = 203.17, p < 0.001; drug: F(1,68) = 2.255, p = 0.138; interaction: F(1,68) = 0.073, p = 0.788]), and females are widely seen to weigh less than males and thus also eat less. Thus, these results suggest that reduced home cage intake after the NSFT test did not reflect differences in basic consummatory drives across rats.
Light–dark task
One week after NSFT, rats were examined in LDT. Rats were placed in the lit chamber (see Methods), and the first measure was the latency to enter the dark chamber (i.e., to move from the lit chamber, where the rat is first placed, into the dark side), and shorter latency is taken to indicate increased anxiety-like behavior (see Methods). This latency to enter the dark chamber demonstrated a significant effect of sex, drug, and interaction (sex: F(1,68) = 8.851, p = 0.004; drug: F(1,68) = 7.844, p = 0.007; interaction: F(1,68) = 26.074, p < 0.001) (Fig. 5A). Females exhibited a trend for less anxiety-like behavior than males (p = 0.044), with diazepam reducing anxiety-like response (increasing latency) in males (p < 0.001). There was a seemingly paradoxical effect in females, where diazepam tended to shorten this latency (p = 0.022), addressed further in the Discussion. Overall, there were significant sex differences in latency to enter the dark chamber, with shorter latency in males and different diazepam effects across sexes.
Behavioral measures in LDT overall suggest reduced female anxiety-like responding relative to males. A Latency to enter the dark chamber was slowed by DZP in males but not females. B–E Latency to re-enter the lit chamber (B), time in the lit chamber (C), transitions between the lit and dark chambers (D), and rearing (E) had significant effects of sex and drug but no interaction (see Results). DZP, diazepam; Sal, saline vehicle. ** indicates p < 0.01 for diazepam within males
A different pattern was observed for several other anxiety-like LDT measures. Both latency to re-enter the lit chamber (Fig. 5B) and total time in the lit chamber (Fig. 5C) revealed a significant effect of sex and drug but no interaction (latency to re-enter light: [sex: F(1,68) = 8.088, p = 0.006; drug: F(1,68) = 6.728, p = 0.012; interaction: F(1,68) = 0.002, p = 0.962]; total time in light: [sex: F(1,68) = 14.216, p < 0.001; drug: F(1,68) = 9.544, p = 0.003; interaction: F(1,68) = 0.019, p = 0.892]). Thus, females exhibited less anxiety-like behavior than males in these measures, with no clear sex differences in diazepam effects.
Several LDT measures have been considered to indicate locomotor activity, including number of transitions between the two chambers (Fig. 5D) and number of rears (Fig. 5E). Both demonstrated a significant effect of sex and drug but no interaction (transitions: [sex: F(1,68) = 14.590, p < 0.001; drug: F(1,68) = 10.280, p = 0.002; interaction: F(1,68) = 2.056, p = 0.157]; rearing: [sex: F(1,68) = 13.752, p < 0.001; drug: F(1,68) = 12.588, p = 0.001; interaction: F(1,68) = 2.008, p = 0.161]). These results agree with our (Fig. 4) and others’ work suggesting greater locomotion in females, while the overall impact of diazepam suggests some aspects of anxiety in these measures. Finally, we examined stretch responses (see Methods) which showed a different pattern from other measures (Online Resource 2), with significant effect of sex (sex: F(1,68) = 5.151, p = 0.027; drug: F(1,68) = 0.748, p = 0.390; interaction: F(1,68) = 2.539, p = 0.116). These results are less consistent with other behaviors examined, and will require future work to understand.
Relationship between anxiety-like measures
Given our sex-specific and -general patterns in anxiety-like behavior and diazepam effects, understanding possible relationships between behavioral measures could provide useful information. For example, some measures might better capture overall anxiety-like patterns, while others could relate more to locomotion. Therefore, we performed correlations (Online Resources 3 and 4) and PCA separately on NSFT measures (Table 1) and LDT measures (Table 2). Correlations face the challenge of determining significance with the great many possible comparisons, but overall suggest both separation and overlap of putative anxiety and locomotor measures (Online Resources 3 and 4). In addition, the first principle component (PC1) for each paradigm accounted for only a modest proportion of the variance (28.4% for NSFT, 32.7% for LDT). In addition, while certain measures were a priori considered to reflect locomotion rather than anxiety-like behavior (open field velocity and distance, transitions and rearing in LDT), these putative locomotor factors did not clearly segregate relative to other, more traditional anxiety-like measures. This lack of clear clustering across measures, combined with the low variability accounted for by PC1, suggests the necessity of examining all behavioral measures and a potential non-linear relationship between them, as indicated by interaction effects in only particular measures reported above. Thus, as suggested by other groups, our results validate the suggestion that it is important to determine multiple measures from each task examined to maximize ethological relevance (see Introduction).
In addition, PC1 and PC2 estimated from NSFT data both demonstrated significant effects of sex and drug (all p < 0.007), with PC1 revealing a sex-drug interaction (p = 0.017; see Online Resources). These PCs, accounting for 51.8% of the variability in all NSFT measures, largely separated the sex and drug groups (Online Resources 5A). Conversely, PC1 from the LDT measures revealed only a significant effect of sex (p < 0.001), and PC2 only an interaction effect (p < 0.001; see Online Resources 5B), despite both PCs combined accounting for a similar proportion of variability (~ 52%) as NSFT. These results are consistent with the strong sex-drug interaction seen in particular NSFT measures, latency to grab food, and food eaten during the NSFFT test (Fig. 2A, B), while LDT measures showed some sex differences (Fig. 5) but overall demonstrated more sex-similar diazepam effects.
Discussion
Anxiety disorders are highly prevalent, with women at greater risk of developing such conditions. Understanding sex-specific and -general mechanisms that promote anxiety, important for treatment development, is made challenging due to hard-to-disambiguate biological and social/cultural contributions in humans. Thus, animal tests can provide valuable insights into biological sex differences. However, while sex-related anxiety-like patterns have been broadly studied, there remain a number of mixed results, which could be resolved by more in-depth examination of different behavioral measures within each anxiety-like test, as well as use of larger sample sizes. Thus, we examined anxiety-like behaviors in single-housed female and male adult rats in two widely used tests, NSFT and LDT, which are validated by their sensitivity to chronic SSRIs and BZDs (see Introduction). Interestingly, NSFT measures more directly related to food interaction, initial latency to grab food and food consumed in the NSFT test, indicated greater anxiety-like behavior under control conditions in females relative to males, and where diazepam produced anxiolytic-like effects in females but not males. In contrast, other NSFT measures more distal to food interaction showed limited sex differences. In the LDT, where bright light evokes conflict but has no localized anxiety-provoking stimulus, most measures suggested reduced anxiety-like behavior in females and general diazepam efficacy; one exception was rearing, often considered a locomotor response but which was greater under saline in females but reduced by diazepam only in females. Thus, our results indicated less female anxiety-like behavior and similar sex effects of diazepam in several measures, except for strong sex-by-drug interactions and greater female anxiety-like responses in NSFT measures most directly related to food interaction. Together with previous studies, we speculate that sex differences in anxiety-like patterns may primarily reflect greater female responding under conditions involving a specific, life-relevant stimulus (discussed below).
As noted in the Introduction, sex differences in anxiety-like behavior have been widely studied, where both consistent and mixed results have been observed across putative anxiety-like tests. While females can show less anxiety-like behavior in EPM and open field (see Donner and Lowry 2013), greater anxiety-like behavior is seen in tasks such as shocked licking (De Jesus-Burgos et al. 2016; Johnston and File 1991) and some predator responses (see Albonetti and Farabollini 1995), and with some diverse results regarding primary reactivity to aversive stimuli (Johnston and File 1991; Olvera-Hernandez and Fernandez-Guasti 2011). However, there are also mixed findings (see below). Females also often show greater locomotion and rearing, including velocity differences (Belviranli et al. 2012), although BZD modulation of some locomotor measures (LDT transitions and rearing, open field distance) suggests the presence of anxiety-like contributions to locomotion (e.g., Bourin and Hascoet 2003). In addition, there are likely sex differences in the nature of responding under anxiety, where females can show more active responding (see Introduction). Here, the slower velocity in females, despite greater distance traveled, may be consistent with greater caution even with greater total exploration. Thus, anxiety-like states could increase activity in females under some conditions, perhaps akin to the need to judge danger near a nest, rather than restrain/reduce activity as in males (Becker and Chartoff 2019; Palanza and Parmigiani 2017).
We addressed these different challenges in several ways. First, we examined a number of measures within each task, a strategy which has been suggested to be important to maximize information gained and ethological relevance, and provide clearer insight into the nature of sex and other differences (Olvera-Hernandez and Fernandez-Guasti 2011; Ramos 2008). Also, to assess whether particular responses reflected anxiety-like behavior, we utilized a moderate dose of diazepam, which should provide some anxiolytic-like effects while minimizing locomotor effects (Chaouloff et al. 1997; Griebel et al. 1997; Olvera-Hernandez and Fernandez-Guasti 2011). In this regard, several reviews have noted that most anxiety-like tests were developed and validated in males, making their interpretation in females less certain. Here, most factors examined showed diazepam efficacy, which provides useful evidence that many NSFT and LDT measures can be used to interpret female anxiety-like behavior. One important consideration when interpreting our diazepam findings is that, taken overall, diazepam seems to have stronger impacts for conditions exhibiting higher levels of anxiety-like behavior. This interpretation might be a strength of our findings, since they would imply that baseline sex differences in different measures do reflect anxiety-related differences, validating use of these tasks in females (as noted above), although our results would suggest that the tasks did not generate high levels in anxiety in all measures in males (although we note that other measures, such as time in NSFT center, show strong diazepam effects in both sexes). Another caveat for NSFT food measures is that BZDs can promote hyperphagia (Pittman et al. 2012), which might lead to the increased food intake in the NSFT chamber and home cage in females; however, our results would suggest that any such diazepam effects would be sex-specific (since intake did not increase in males). Furthermore, we note that chronic SSRIs are effective against many human anxiety disorders (Ramaker and Dulawa 2017), and anxiolysis after chronic (but not acute) SSRIs in rodents is considered to provide validation for human relevance. In this regard, both NSFT (Bodnoff et al. 1989; Oh et al. 2009; Santarelli et al. 2003) and LDT (Vicente and Zangrossi 2014) show such chronic SSRI anxiolysis, supporting that tests we examined tap into substrates relevant to human anxiety. Importantly, chronic SSRIs do not alter locomotion (Homberg et al. 2011; Vicente and Zangrossi 2014), in agreement with a lack of diazepam effect in our open field locomotion measure. Also, differences in diazepam pharmacokinetics could contribute to observed sex differences, but some measures we examined show equivalent effects of diazepam across the sexes (e.g., time in center of NSFT).
Since we found sex differences in anxiety-like responding and diazepam effects only in particular measures, especially NSFT factors most directly related to food acquisition and consumption, it is important to relate our findings to those in previous studies. Indeed, some of our findings concur with previous work. One study in adult rats (Olivier et al. 2008) found a longer latency to eat in females under group-housed conditions (although it did not report consumption level). In contrast, other groups found different results, but there were substantial methodological differences: one study found no sex differences in latency to feed (Gobinath et al. 2018), although the method did not utilize the bright overhead light over the food, while another work found no sex differences in several measures at baseline or with diazepam (Shephard and Broadhurst 1982), although there were several methodological differences (including use of a 6–18-h dark–light cycle). In younger animals, NSFT studies find that females eat less food and have fewer entries in the center, although with no differences in latency to eat (Amodeo et al. 2018; Miragaia et al. 2018). Thus, while there are some mixed findings in the literature, methods closest to ours with adult rats (Olivier et al. 2008) find greater female anxiety-like responses (expressed as latency to eat), similar to our findings. One other challenge is that many previous studies report a limited number of NSFT measures.
Similar to NSFT, several aspects of our LDT results were also similar to those previously reported. One group (Kokras et al. 2020) found that adult female rats spend more time in the lit chamber and have more inter-chamber transitions, and other studies (Hughes 2011; Ramos 2008) also show greater interaction with the lit chamber in adult female rats. However, further studies find little sex difference in LDT (Amodeo et al. 2018; Fleming et al. 2019), although these used younger adults (~ P75) rather than somewhat older adults in our work here and other findings (Hughes 2011; Kokras et al. 2020). Also, as discussed below, the differences relate to anxiety-like behaviors are often not apparent or of a different pattern in younger animals (e.g., Albrechet-Souza et al. 2020). Other methodological differences could also contribute to differential results, although methods in Amodeo et al. (2018) are very similar to those used here except animal age.
Our studies used socially isolated animals, since isolation is known to induce stress in humans and rodents, and can enhance anxiety-like and related states (see above). Some sex differences have been reported (see Introduction), and isolation increases rearing in isolated females more than males (Dalla et al. 2005), consistent with our LDT findings. Interestingly, greater anxiety-like behavior after social stressors (including isolation) in adult females has been linked to sex-specific inflammatory mechanisms (Slavich and Sacher 2019). It is also interesting that women can report greater concern about social isolation (Martin and Brown 2010; Mead et al. 2010), and isolation can promote depression (Heinrich and Gullone 2006), which is also more prevalent in women and often co-morbid with anxiety (Altemus et al. 2014; Baratta et al. 2018; Palanza and Parmigiani 2017). Thus, isolation can cause mood-related disruptions in humans and rodents. We also note that some mixed findings in isolation studies may reflect use of adolescents (e.g., Du Preez et al. 2020; Pisu et al. 2016), such as where isolation has limited effects in LDT (Amodeo et al. 2018; Fleming et al. 2019; Martin and Brown 2010). However, our single housing results agree with group-housed 4 + -month-old females showing less anxiety-like behavior than males in LDT (Hughes 2011; Kokras et al. 2020). We also note that part of our reason for using single housing is that the present findings are meant to undergird our future studies of stress interactions with alcohol, where single housing is widely used (e.g., Wegner et al. 2019). Nonetheless, taken together with previous studies, our studies concur that isolated adult females have greater anxiety-like responding in some measures.
We speculate that our results, together with previous findings, provide new insights into sex-specific and -general patterns of anxiety-like behavior. Here, NSFT measures most directly related to food interaction, latency to first grab food and food intake in test, were the main factors showing sex-drug interactions. In both, females displayed significantly greater anxiety-like behavior under control conditions and diazepam only significantly impacted females. In contrast, other NSFT measures were similar across sexes under control and diazepam. One possibility is that, in females, anxiety-like behavior is more pronounced in the presence of a specific stimulus of high motivational relevance (like food in NSFT). In possible support, females versus males show increased anxiety-like behavior to lick-paired shocks in water-restricted animals (De Jesus-Burgos et al. 2016; Olvera-Hernandez and Fernandez-Guasti 2011). Similarly, females show quicker latency to bury a shocking probe than males, and lower-dose diazepam slows latency-to-bury in females but not males (Olvera-Hernandez and Fernandez-Guasti 2011), similar to our NSFT findings, while more basic measures (number of shocks tolerated and diazepam enhancement of shock tolerance) do not differ between sexes. In addition, diazepam reduces stretches in response to predator odor more in females than males (Shepherd et al. 1992). Also interesting are the lack of sex differences in a social task when a conspecific is confined in a cage, but clear sex differences when direct physical interaction is possible (see Le Moene et al. 2020). Thus, greater female anxiety-like behavior may primarily occur in relation to specific, life-relevant stimuli (food when food restricted; imminent dangers). In contrast, responding here was more similar across sexes for behaviors more distal to the specific motivational focus in NSFT, and in the LDT which lacks a localized anxiety-provoking stimulus. One exception was the first action in LDT, movement from lit to dark chamber; this could be interpreted as increased anxiety-like behavior in females, which delayed movement from the light as the situation was assessed, perhaps akin to longer avoidance reactions in human females (Sheynin et al. 2014). LDT rearing and transitions and diazepam reduction were also greater in females, which may reflect greater anxiety-like exploring in females. Taken together, our and previous findings suggest that sex differences in anxiety-like behavior may in part reflect different strategies to most salient anxiety-provoking stimuli; considerable future work will be required to fully assess such possibilities.
To better understand the potential relationship between different measures examined, we performed correlations between measures, and PCA across measures, within each anxiety-related task. PC weightings did not reveal patterns delineating locomotor from anxiety-like behaviors, and no particular behavioral domain was central to explaining overall behavior in either test, congruent with a previous analysis (Fernandes et al. 1999). Simpler correlation analyses of NSFT/open field measures (Online Resource 3) and LDT measures (Online Resource 4) also provided mixed findings, where some putative locomotor measures did dissociate from anxiety measures, while others did not. For example, in the NSFT, greater time to grab food (a putative indicator of greater anxiety) did not correlate with level of open field locomotion (dissociating anxiety from locomotion), and did correlate with less time in center and less food intake (both indicators of greater anxiety), but also correlated with more approaches, a measure sometimes considered to be a locomotor measure in NSFT (Online Resource 3). One limitation is that we measured open field after NSFT, and stress during NSFT could increase anxiety in open field; indeed, we were most interested in NSFT, and did not want open field testing to impact subsequent NSFT behavior. Also, at present, we measure anxiety-like states indirectly, through behavior changes, and thus all anxiety-like behaviors intrinsically have motor components. Such considerations highlight the challenges of examining multiple putative anxiety-related tasks within the same individual, and the general need for future work to better assess and understand mechanisms underlying different aspects of behavior in anxiety-like and locomotor tasks. However, we note that our PCAs did confirm the overall results for each task, where NSFT in particular showed strong effects of sex, drug, and sex-drug interaction, congruent with specific behavioral measures closest to food interaction (latency to grab and food intake) which showed the strongest sex-drug patterns. Thus, taken together, these findings underscore the previously suggested importance of measuring multiple behaviors within a given test (Olvera-Hernandez and Fernandez-Guasti 2011; Ramos 2008), especially when seeking to understand diverse mechanisms that likely contribute to sex differences in anxiety-like behaviors, and the need for continued work in this direction.
One central future question relates to the molecular/circuit mechanisms that underlie sex differences in anxiety, once there is a clear understanding of the actual sex-similar and -different patterns in expression of anxiety-like responses. It is clear there are likely a number of sex differences in neuronal signaling mechanisms which could contribute, including CRF, opiates and dopamine (Bangasser and Wicks, 2017; Becker and Chartoff 2019) Our future studies should also examine possible estrous cycle influences. Many studies show reduced anxiety-like behavior in proestrus (Amodeo et al. 2018; Miragaia et al. 2018; Palanza et al. 2001), although other groups find no estrous influence in EPM or open field (Henricks et al. 2017; Scholl et al. 2019). Locomotion can also vary across estrous cycle (e.g., Roman and Arborelius 2009), although with inconsistent findings (Jaric et al. 2019; Keeley et al. 2015). Also, lower shocked licking in females under Vogel conflict can be independent of estrous stage (De Jesus-Burgos et al. 2016). Here, we examined a large sample size of females, tested across several days, in a partial, although likely incomplete, attempt to sample across estrous stages, but estrous-related effects represent an important future direction given anxiety differences across the menstrual cycle in women (Altemus et al. 2014).
Taken together, the present studies provide new context for understanding sex-specific and -general anxiety-like behavioral expression, including the speculation that female anxiety-like response differences may occur for specific, most salient anxiety-provoking stimuli. Our findings also provide strong foundation for examining sex differences in stress- and/or alcohol-related increases in anxiety-like behaviors (Becker and Chartoff 2019).
References
Abramov U, Raud S, Koks S, Innos J, Kurrikoff K, Matsui T, Vasar E (2004) Targeted mutation of CCK(2) receptor gene antagonises behavioural changes induced by social isolation in female, but not in male mice. Behav Brain Res 155:1–11
Acevedo MB, Nizhnikov ME, Molina JC, Pautassi RM (2014) Relationship between ethanol-induced activity and anxiolysis in the open field, elevated plus maze, light-dark box, and ethanol intake in adolescent rats. Behav Brain Res 265:203–215
Albonetti ME, Farabollini F (1995) Effects of single restraint on the defensive behavior of male and female rats. Physiol Behav 57:431–437
Albrechet-Souza L, Schratz CL, Gilpin NW (2020) Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol Sex Differ 11:27–38
Altemus M, Sarvaiya N, Neill EC (2014) Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35:320–330
Amodeo LR, Wills DN, Sanchez-Alavez M, Nguyen W, Conti B, Ehlers CL (2018) Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol 73:57–66
Bangasser DA, Wicks B (2017) Sex-specific mechanisms for responding to stress. J Neurosci Res 95:75–82
Baratta MV, Leslie NR, Fallon IP, Dolzani SD, Chun LE, Tamalunas AM, Watkins LR, Maier SF (2018) Behavioural and neural sequelae of stressor exposure are not modulated by controllability in females. Eur J Neurosci 47:959–967
Becker JB, Chartoff E (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharm 44:166–183
Belviranli M, Atalik KE, Okudan N, Gokbel H (2012) Age and sex affect spatial and emotional behaviors in rats: the role of repeated elevated plus maze test. Neuroscience 227:1–9
Blasco-Serra A, Gonzalez-Soler EM, Cervera-Ferri A, Teruel-Marti V, Valverde-Navarro AA (2017) A standardization of the novelty-suppressed feeding test protocol in rats. Neurosci Lett 658:73–78
Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ (1989) A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharm 97:277–279
Bourin M, Hascoet M (2003) The mouse light/dark box test. Eur J Pharmacol 463:55–65
Chaouloff F, Durand M, Mormede P (1997) Anxiety- and activity-related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behav Brain Res 85:27–35
Chisholm D, Sweeny K, Sheehan P, Rasmussen B, Smit F, Cuijpers P, Saxena S (2016) Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry 3:415–424
Dalla C, Antoniou K, Drossopoulou G, Xagoraris M, Kokras N, Sfikakis A, Papadopoulou-Daifoti Z (2005) Chronic mild stress impact: are females more vulnerable? Neuroscience 135:703–714
De Jesus-Burgos MI, Gonzalez-Garcia S, Cruz-Santa Y, Perez-Acevedo NL (2016) Amygdalar activation of group I metabotropic glutamate receptors produces anti- and pro-conflict effects depending upon animal sex in a sexually dimorphic conditioned conflict-based anxiety model. Behav Brain Res 302:200–212
Donner NC, Lowry CA (2013) Sex differences in anxiety and emotional behavior. Pflugers Arch 465:601–626
Du Preez A, Law T, Onorato D, Lim YM, Eiben P, Musaelyan K, Egeland M, Hye A, Zunszain PA, Thuret S, Pariante CM, Fernandes C (2020) The type of stress matters: repeated injection and permanent social isolation stress in male mice have a differential effect on anxiety- and depressive-like behaviours, and associated biological alterations. Transl Psychiatry 10:325–242
Fernandes C, Gonzalez MI, Wilson CA, File SE (1999) Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav 64:731–738
Fleming W, Jones Q, Chandra U, Saini A, Walker D, Francis R, Ocampo G, Kuhn C (2019) Withdrawal from brief repeated alcohol treatment in adolescent and adult male and female rats. Alcohol Clin Exp Res 43:204–211
Gobinath AR, Wong S, Chow C, Lieblich SE, Barr AM, Galea LAM (2018) Maternal exercise increases but concurrent maternal fluoxetine prevents the increase in hippocampal neurogenesis of adult offspring. Psychoneuroendo 91:186–197
Gonzalez LE, File SE (1997) A five minute experience in the elevated plus-maze alters the state of the benzodiazepine receptor in the dorsal raphe nucleus. J Neurosci 17:1505–1511
Griebel G, Perrault G, Sanger DJ (1997) CCK receptor antagonists in animal models of anxiety: comparison between exploration tests, conflict procedures and a model based on defensive behaviours. Behav Pharmacol 8:549–560
Heinrich LM, Gullone E (2006) The clinical significance of loneliness: a literature review. Clin Psychol Rev 26:695–718
Henricks AM, Berger AL, Lugo JM, Baxter-Potter LN, Bieniasz KV, Petrie G, Sticht MA, Hill MN, McLaughlin RJ (2017) Sex- and hormone-dependent alterations in alcohol withdrawal-induced anxiety and corticolimbic endocannabinoid signaling. Neuropharm 124:121–133
Homberg JR, Olivier JD, Blom T, Arentsen T, van Brunschot C, Schipper P, Korte-Bouws G, van Luijtelaar G, Reneman L (2011) Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One 6:e16646–e16656
Hughes RN (2011) Adult anxiety-related behavior of rats following consumption during late adolescence of alcohol alone and in combination with caffeine. Alcohol 45:365–372
Jaric I, Rocks D, Cham H, Herchek A, Kundakovic M (2019) Sex and estrous cycle effects on anxiety- and depression-related phenotypes in a two-hit developmental stress model. Front Mol Neurosci 12:74–89
Johnston AL, File SE (1991) Sex differences in animal tests of anxiety. Physiol Behav 49:245–250
Keeley RJ, Bye C, Trow J, McDonald RJ (2015) Strain and sex differences in brain and behaviour of adult rats: learning and memory, anxiety and volumetric estimates. Behav Brain Res 288:118–131
Kokras N, Dioli C, Paravatou R, Sotiropoulos MG, Delis F, Antoniou K, Calogeropoulou T, Charalampopoulos I, Gravanis A, Dalla C (2020) Psychoactive properties of BNN27, a novel neurosteroid derivate, in male and female rats. Psychopharm 237:2435–2449
Kosobud AE, Wetherill L, Plawecki MH, Kareken DA, Liang T, Nurnberger JL, Windisch K, Xuei X, Edenberg HJ, Foroud TM, O’Connor SJ (2015) Adaptation of subjective responses to alcohol is affected by an interaction of GABRA2 genotype and recent drinking. Alcohol Clin Exp Res 39:1148–1157
Le Moene O, Ramirez-Renteria ML, Agmo A (2020) Male and female immediate fear reaction to white noise in a semi-natural environment: a detailed behavioural analysis of the role of sex and oestrogen receptors. J Neuroendocrinol 32:e12902–e12921
Lopez-Aumatell R, Martinez-Membrives E, Vicens-Costa E, Canete T, Blazquez G, Mont-Cardona C, Johannesson M, Flint J, Tobena A, Fernandez-Teruel A (2011) Effects of environmental and physiological covariates on sex differences in unconditioned and conditioned anxiety and fear in a large sample of genetically heterogeneous (N/Nih-HS) rats. Behav Brain Funct 7:48–63
Martin AL, Brown RE (2010) The lonely mouse: verification of a separation-induced model of depression in female mice. Behav Brain Res 207:196–207
Mead H, Andres E, Katch H, Siegel B, Regenstein M (2010) Gender differences in psychosocial issues affecting low-income, underserved patients’ ability to manage cardiovascular disease. Womens Health Issues 20:308–315
Miragaia AS, de Oliveira Wertheimer GS, Consoli AC, Cabbia R, Longo BM, Girardi CEN, Suchecki D (2018) Maternal deprivation increases anxiety- and depressive-like behaviors in an age-dependent fashion and reduces neuropeptide Y expression in the amygdala and hippocampus of male and female young adult rats. Front Behav Neurosci 12:159–176
Oh JE, Zupan B, Gross S, Toth M (2009) Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharm 34:2197–2207
Olivier JD, Van Der Hart MG, Van Swelm RP, Dederen PJ, Homberg JR, Cremers T, Deen PM, Cuppen E, Cools AR, Ellenbroek BA (2008) A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience 152:573–584
Olvera-Hernandez S, Fernandez-Guasti A (2011) Sex differences in the burying behavior test in middle-aged rats: effects of diazepam. Pharmacol Biochem Behav 99:532–539
Ou C, Dringenberg HC, Soutar CN (2019) Is hippocampal theta frequency related to individual and sex differences in anxiety-like behaviour? An analysis in male and female Long-Evans rats. Behav Brain Res 364:366–373
Palanza P, Gioiosa L, Parmigiani S (2001) Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav 73:411–420
Palanza P, Parmigiani S (2017) How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci Biobehav Rev 76:134–143
Pereira JK, Vieira RJ, Konishi CT, Ribeiro RA, Frussa-Filho R (1999) The phenomenon of “one-trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze is abolished by the introduction of a motivational conflict situation. Life Sci 65:PL101–7.
Pisu MG, Garau A, Boero G, Biggio F, Pibiri V, Dore R, Locci V, Paci E, Porcu P, Serra M (2016) Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience 320:172–182
Pittman DW, McGinnis MR, Richardson LM, Miller EJ, Alimohamed ML, Baird JP (2012) Multiple processes underlie benzodiazepine-mediated increases in the consumption of accepted and avoided stimuli. Chem Senses 37:431–444
Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De Guglielmo G, Crawford E, Janak PH, George O, Rice KC, Messing RO (2015) A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci 9:487–501
Ramaker MJ, Dulawa SC (2017) Identifying fast-onset antidepressants using rodent models. Mol Psychiatry 22:656–665
Ramos A (2008) Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci 29:493–498
Roman E, Arborelius L (2009) Male but not female Wistar rats show increased anxiety-like behaviour in response to bright light in the defensive withdrawal test. Behav Brain Res 202:303–307
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809
Scholl JL, Afzal A, Fox LC, Watt MJ, Forster GL (2019) Sex differences in anxiety-like behaviors in rats. Physiol Behav 211:112670–112678
Shephard RA, Broadhurst PL (1982) Effects of diazepam and picrotoxin on hyponeophagia in rats. Neuropharm 21:771–773
Shepherd JK, Flores T, Rodgers RJ, Blanchard RJ, Blanchard DC (1992) The anxiety/defense test battery: influence of gender and ritanserin treatment on antipredator defensive behavior. Physiol Behav 51:277–285
Sheynin J, Beck KD, Pang KC, Servatius RJ, Shikari S, Ostovich J, Myers CE (2014) Behaviourally inhibited temperament and female sex, two vulnerability factors for anxiety disorders, facilitate conditioned avoidance (also) in humans. Behav Processes 103:228–235
Slavich GM, Sacher J (2019) Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharm 236:3063–3079
Vicente MA, Zangrossi H Jr (2014) Involvement of 5-HT2C and 5-HT1A receptors of the basolateral nucleus of the amygdala in the anxiolytic effect of chronic antidepressant treatment. Neuropharm 79:127–135
Wegner SA, Hu B, De Oliveira Sergio T, Darevsky D, Kwok CC, Lei K, Hopf FW (2019) A novel NMDA receptor-based intervention to suppress compulsion-like alcohol drinking. Neuropharm 157:107681–107688
Acknowledgements
Thanks to Molly Sazer-Hopf for assistance with Fig. 1.
Funding
Work was supported by National Institutes of Health (R01 AA024109 to FWH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Oliveira Sergio, T., Wetherill, L., Kwok, C. et al. Sex differences in specific aspects of two animal tests of anxiety-like behavior. Psychopharmacology 238, 2775–2787 (2021). https://doi.org/10.1007/s00213-021-05893-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05893-w